Preparation Of Gushukang (GSK) Granules for In Vivo and In Vitro Experiments
Summary
This article provides a detailed protocol for preparing a working solution of Gushukang granules for animal studies and GSK granule containing serum for in vitro experiments. This protocol can be applied to pharmacological investigations of herbal medicines as well as prescriptions for both in vivo and in vitro experiments.
Abstract
Traditional Chinese herbal medicine plays a role as an alternative method in treating many diseases, such as postmenopausal osteoporosis (POP). Gushukang (GSK) granules, a marketed prescription in China, have bone-protective effects in treating POP. Before administration to the body, one standard preparation procedure is commonly required, which aims to promote the release of active constituents from raw herbs and enhance the pharmacological effects as well as therapeutic outcomes. This study proposes a detailed protocol for using GSK granules in in vivo and in vitro experimental assays. The authors first provide a detailed protocol to calculate the animal-appropriate dosages of granules for in vivo investigation: weighing, dissolving, storage, and administration. Second, this article describes protocols for micro-CT scanning and the measurement of bone parameters. Sample preparation, protocols for running the micro-CT machine and quantification of bone parameters were evaluated. Third, serum-containing GSK granules are prepared, and drug-containing serum is extracted for in vitro osteoclastogenesis and osteoblastogenesis. GSK granules were intragastrically administered twice per day to rats for three consecutive days. Blood was then collected, centrifuged, inactivated, and filtered. Finally, serum was diluted and used for performing osteoclastogenesis and osteoblastogenesis. The protocol described here can be considered a reference for pharmacological investigations of herbal prescription medicines, such as granules.
Introduction
Traditional Chinese medicine (TCM) is one of the important complementary and alternative approaches to treat osteoporosis1,2. Water decoction is the basic and most commonly used form of the formula3. However, drawbacks also exist: bad taste, inconvenience for carriage, short shelf life and inconsistent protocols, limiting the uses as well as the curative effects. To avoid the above disadvantages as well as to pursue better effects, granules were developed and have been widely used4. Although many studies have explored the pharmacological mechanisms of one or more effective components from the granules5,6,7, the exact mechanisms and underlying pharmacological processes are still difficult to identify. This is because too many effective components from one granule may simultaneously exert similar or opposed effects4. Therefore, the development of one standard protocol to prepare the granules before delivering to the body not only would have a great impact on the therapeutic outcomes but is also required for both in vivo and in vitro assays.
Moreover, the curative effects of granules in the clinic are difficult to confirm and exactly identify using in vitro or ex vivo studies, which creates a challenge because the pharmacological mechanisms are too complex. To resolve this, the preparation of drug-containing serum was first proposed by Tashino in 1980s8. From then on, numerous researchers applied drug-containing serum to herbal medicine, including granules9,10,11. Currently, the choice of drug-containing serum for in vitro investigations is regarded as one strategy that closely mimics physiological conditions.
Gushukang (GSK) granules were developed to treat postmenopausal osteoporosis (POP) based on clinical practice in light of the theory of TCM. GSK granules prevent bone loss in ovariectomized (OVX) mice in vivo, inhibit osteoclastic bone resorption, and stimulate osteoblastic bone formation4. Consequently, Li et al.12 found that GSK granules have bone protective effects in OVX mice by enhancing the activities of calcium receptor to stimulate bone formation. To confirm the bone-protective effects as well as the pharmacological effects of GSK granules, the authors here provide a detailed procedure for preparation of working solutions and drug (GSK granule)-containing serum. Moreover, this article describes the application of GSK granules in an OVX-induced osteoporotic mouse model and GSK granule-containing serum for in vitro osteoclastogenesis/osteoblastogenesis.
GSK granules are composed of several herbs13,14 and can be completely dissolved in saline easily. Therefore, saline serves as the vehicle. Sham-operated mice (Sham) and OVX mice were administered the same volume of saline as the granule-administered mice. The equivalent doses of GSK granules for the mouse were calculated based on the Meeh-Rubner equation15. This equation not only has the advantage of obtaining safe dosages but also guarantees pharmacological effects15. The three dosages of GSK granules were generated as the follows: (1) GSKL: OVX + low-dose GSK granules, 2 g/kg/day. (2) GSKM: OVX + medium-dose GSK granules, 4 g/kg/day. (3) GSKH: OVX + high-dose GSK granules, 8 g/kg/day. Mice in the GSKL, GSKM and GSKH groups were intragastrically administered GSK granules. Calcium carbonate (600 mg/tablet) with vitamin D3 (125 international unit/tablet), for example, in a mature and marketed product (e.g., Caltrate [CAL]) for treating and preventing osteoporosis, was used as a positive control.
Protocol
All of the experimental procedures were performed with the approval of Institutional Animal Care and Use Committee of the Shanghai University of TCM (SZY201604005).
1. Preparation and administration of GSK working solution
- Calculate the equivalent doses of GSK granules for mouse.
- Calculate body surface based on the Meeh-Rubner equation15: body surface = K x (body weight2/3)/1000, where the K values are 10.6 for human and 9.1 for mouse. Assuming a human body weight of 70 kg, then human body surface (m2) = 10.6 x (702/3)/1000 = 1.8 m2. Assuming a mouse body weight of 20 g (0.02 kg; e.g., 1 month old, female, C57/BL6), then mouse body surface (m2) = 9.1 x (0.022/3)/1000 = 0.0067 m2.
- Based on the calculated body surface, calculate the body transform ratio for human and mouse. Human: 70 kg/1.8 m2 = 39. Mouse: 0.02 kg/0.0067 m2 = 3. GSK granule = 20 g/70 kg x 39/3 = 3.72 g/kg ≈ 4 g/kg.
- Based on a body weight of 20 g per mouse, calculate the equivalent dosage for mouse: 4 g/kg x 0.02 kg = 0.08 g.
- Calculate three equivalent doses of GSK granules based on 20 mice per group and an intervention lasting for 3 months (90 days): (1) GSKL (OVX + low-dose GSK granules [2 g/kg/day]): 0.04 g mouse/day x 20 mice x 90 days = 72 g. (2) GSKM (OVX + medium-dose GSK granules [4 g/kg/day]): 0.08 g mouse/day x 20 mice x 90 days = 144 g. (3) GSKH (OVX + high-dose GSK granules [8 g/kg/day]): 0.12 g mouse/day x 20 mice x 90 days = 216 g.
NOTE: Prepare an additional 20% of GSK granules in practice to offset the loss.
- Calculate the volume of GSK granule per mouse based on the body weight15: e.g., volume (V) = 0.24 mL/mouse/day.
NOTE: The volume for intragastric administration for mouse is 0.12 mL/10 g. - Weigh 10-days’ worth of three doses of GSK granules. Weigh 8 g, 16 g, and 24 g of GSK granules and serve as GSKL, GSKM, and GSKH, respectively.
- Calculate the equivalent dose of calcium carbonate with vitamin D3 (CAL) for mouse based on the Meeh-Rubner equation15 as in steps 1.1.1 and 1.1.2: CAL dosage = 2 tablet/70 kg x 39/3 = 0.372 tablet/kg ≈ 0.4 tablet/kg.
- Based on a body weight of 20 g per mouse (e.g., 1 month old, female, C57/BL6), calculate the equivalent dosage of CAL for mouse: 0.4 tablet/kg x 0.02 kg = 0.008 tablet. Then calculate the equivalent dose of CAL based on 20 mice per group and an intervention lasting for 3 months (90 days): 0.008 tablet x 20 x 90 = 14.4 tablets. Weigh 10-days’ worth of CAL (1.6 tablets).
- Dissolution
- Place 8 g of GSK granules into a 50 mL tube. Add 48 mL of saline and shake tube to completely dissolve.
NOTE: The standard for complete dissolution is the absence of sediment. Complete dissolution can be further confirmed if a gavage needle can draw up the working solution and then expel it smoothly. - Repeat step 1.5.1 with 16 g and 24 g of GSK granules.
- Place 1.6 tablets (10-days’ worth) of CAL into a 50 mL tube. Add 48 mL of saline and shake tube to completely dissolve.
NOTE: The working solutions can be stored at -4 °C and prepared every 10 days.
- Place 8 g of GSK granules into a 50 mL tube. Add 48 mL of saline and shake tube to completely dissolve.
- Intragastric administration
- Grasp the back of the mouse (1 month old, female, C57/BL6) with the mouse facing forward and ensure that it stays firmly in that position. Keep the mouse calm for 2−3 min before administration.
NOTE: Ensure that the researcher can clearly see the front of the mouse. Wear gloves to prevent mouse bites, particularly for new researchers. - Place the gavage needle (size: #12, 40 mm) in the working solution of GSK granules and draw 0.24 mL of the working solution.
- Put the gavage needle into the mouse through one side of its mouth until the gavage needle reaches the stomach.
NOTE: To confirm the gavage needle has reached the stomach: (1) The gavage needle encounters the feeling of resistance. Meanwhile, the mouse shows the action of swallowing before the gavage needle passes the physical narrowing of the esophagus. (2) Inject approximately 0.5 mL of the working solution into the mouse and wait for 1 min. If there is no solution coming out of the mouse, this means that the gavage needle has reached the stomach. - Inject the working solution of GSK granule (0.24 mL/mouse) into the stomach and then draw out the gavage needle. Return the mouse into its cage.
- Repeat step 1.6.4 with the CAL solution and inject 0.24 mL of CAL solution per mouse.
NOTE: The volume of CAL solution is calculated as in step 1.2.
- Grasp the back of the mouse (1 month old, female, C57/BL6) with the mouse facing forward and ensure that it stays firmly in that position. Keep the mouse calm for 2−3 min before administration.
2. Micro-CT scanning
- Tibia harvesting and preparation
- Intraperitoneally anesthetize mouse with 300 mL/100 g of 80 mg/kg ketamine the day following the 90 day’s intervention. Use a needle pinch of the toes to confirm whether the mouse is completely anesthetized. No response indicates successful anesthesia. Then kill the mouse with cervical dislocation.
- Fix the mouse with both the arms and legs on foam with tacks.
- Cut off the skin with scissors (size: 8.5 cm) and tweezers (size: 10 cm) of the legs from the proximal to the distal end and then harvest tibias.
- Immediately put the tibias into 70% ethyl alcohol and wash for 3 times.
- Wrap the left tibia of the mouse with sponge foam and put it into a sample tube (35 mm diameter, 140 mm length).
NOTE: The long axis of the specimen should be along with that of the sample tube. Ensure the proximal end of the tibia points upwards. - Running the micro-CT 80 scan machine
- Start the micro-CT 80 scan machine at room temperature.
- Set the sample tube into micro-CT 80 and start cross-section scanning with the following scanning parameters: pixel size 15.6 μm, tube voltage 55 kV, tube current 72 μA, integration time 200 ms, spatial resolution 15.6 μm, pixel resolution 15.6 μm, and image matrix 2048 x 2048.
NOTE: The cancellous bone is distinguished from the cortical bone by pre-scanning. The scan area of the tibia is defined as the cancellous bone area from 5 mm below the tibial plateau to the distal end.
- Quantification of bone parameter
- After completing cross-section scanning, obtain the images of the left tibias.
- Set the density threshold to 245−1000. Use the micro-CT evaluation program V6.6 to measure the following bone parameters: bone mineral densities (BMD), bone volume over total volume (BV/TV), trabecular bone number (Tb.N), trabecular bone thickness (Tb.Th), as well as bone trabecular bone separation (Tb.Sp).
3. Preparation of blood serum for in vitro experiments
- Calculation
- Based on a rat body weight of 0.2 kg (1 month old, female, Sprague-Dawley), calculate the dosage of GSK granule: human dosage/day x body weight of human x K/body weight of rat = 20 g/70 kg/day x 70 kg x K (K = 0.018) /0.2 kg = 2 g/kg/day.
NOTE: K is the pharmacological transformation coefficient between human and mouse15 (K = 0.018). - Repeat step 3.1.1 and calculate the following dosages.
- Calculate dosage of GSKL: 10 g/70 kg/day x 70 kg x K/0.2 kg = 1 g/kg/day.
- Calculate dosage of GSKM: 20 g/70 kg/day x 70 kg x K/0.2 kg = 2 g/kg/day.
- Calculate dosage of GSKL: 40 g/70 kg/day x 70 kg x K/0.2 kg = 4 g/kg/day.
- Calculate dosage of CAL: 2 tablet /70 kg/day x 70 kg x K/0.2 kg = 0.2 tablet/kg/day.
- Calculate the total dosage of GSK granule and CAL.
- Calculate the total dosage for GSKL: 1 g/kg/day x 0.2 kg x 6 rats x 3 days = 3.6 g.
- Calculate the total dosage for GSKM: 2 g/kg/day x 0.2 kg x 6 rats x 3 days = 7.2 g.
- Calculate the total dosage for GSKH: 4 g/kg/day x 0.2 kg x 6 rats x 3 days = 14.4 g.
- Calculate the CAL dosage = 0.2 tablet/kg/day x 0.2 kg x 6 rats x 3 days = 0.72 tablet.
NOTE: A total of 10 mL of GSK granule-containing serum is needed to prepare 100 mL culture medium (20% GSK granule-containing serum). Each rat (6 rats/group) is expected to provide 1.5−2 mL of GSK granule-containing serum after centrifugation.
- Calculate the volume of GSK granules applied per rat based on body weight15: e.g., volume (V) = 2 mL/rat/day.
NOTE: The volume for intragastric administration for rat is 0.1 mL/10 g.
- Based on a rat body weight of 0.2 kg (1 month old, female, Sprague-Dawley), calculate the dosage of GSK granule: human dosage/day x body weight of human x K/body weight of rat = 20 g/70 kg/day x 70 kg x K (K = 0.018) /0.2 kg = 2 g/kg/day.
- Weigh 3-days’ worth of three doses of GSK granules. Weigh 3.6 g, 7.2 g, and 14.4 g of GSK granules and serve as GSKL, GSKM, and GSKH, respectively. Weigh 0.72 tablet for the CAL group.
- Place 7.2 g of GSK granules into a 50 mL tube. Add 36 mL of saline and shake tube to completely dissolve. Repeat this with 3.6 g and 14.4 g of GSK granules.
- Repeat section 1.6 for intragastric administration with 2 mL of GSK working solution.
NOTE: Administer the same volume of saline (2 mL per rat) to prepare serum and serves as a blank control group for in vitro assays. - Preparation of the GSK-containing serum
- Intraperitoneally anesthetize the rats with 300 mL/100 g of 80 mg/kg ketamine 1 h after the last administration of GSK granules. Use a needle pinch of the toes to confirm whether the rat is completely anesthetized. No response indicates successful anesthesia.
- Expose the abdomen to the bottom of the thorax of rats using straight operating scissors after incising the skin and peritoneum.
NOTE: The surgical instrument must be sterilized at high temperatures and high pressures prior to use. The surgical area must be sterilized with 70% ethanol during blood collection. - Remove the connective tissue of the abdominal aorta with tissue paper to expose the vessel clearly.
- Draw blood from the abdominal aorta using a 10 mL, 22 G syringe. Then remove the needle and transfer the blood to a 15 mL sterile tube. Usually, 6−8 mL of blood can be obtained from one rat.
NOTE: Each rat must be kept living when drawing blood. One indicator is that the abdominal aorta pulsates when the rat is alive. The rat is dead after blood draw. - Keep the tube upright at room temperature for 30−60 min until the blood is clotted in the tube. Then centrifuge the tube at 500−600 x g for 20 min. Transfer all the supernatant (serum) from one group (6 rats) to one 50 mL sterile tube and shake to mix.
- Inactivate the serum by incubating in a 56 °C water bath for 30 min. Filter the serum using a 0.22-μm-pore-size hydrophilic polyethersulfone syringe filter. Store at -80 °C for long-term usage (less than 1 year).
NOTE: The filtered serum can be used for in vitro osteoclastogenesis and osteoblastogenesis.
- Application
- In vitro osteoclastogenesis
- Dilute the three dosages of the GSK-containing serum (GSKL, GSKM, GSKH) at the ratio of 1:4 with minimum Eagle’s medium (α-MEM) containing L-glutamine, ribonucleosides, and deoxyribonucleosides.
NOTE: Ensure that the final concentration of GSK-containing serum for in vitro osteoclastogenesis and osteoblastogenesis is 20%. - Add the diluted GSK-containing serum (200 μL/well) from step 3.6.1.1 to bone marrow macrophages (BMMs) from 4−6 week old C57BL/6 mice for osteoclastogenesis and stimulate BMMs with macrophage colony-stimulating factor (M-CSF, 10 ng/mL) and receptor activator for nuclear factor-κB ligand (RANKL, 100 ng/mL) as previously described2.
- Dilute the three dosages of the GSK-containing serum (GSKL, GSKM, GSKH) at the ratio of 1:4 with minimum Eagle’s medium (α-MEM) containing L-glutamine, ribonucleosides, and deoxyribonucleosides.
- In vitro osteoblastogenesis
- Repeat step 3.6.1.1.
- Add the diluted GSK-containing serum (2 mL/well) to bone mesenchymal stem cells (BMSCs) from 4−6 week old C57BL/6 mice to generate osteoblast as previously described16.
- In vitro osteoclastogenesis
Representative Results
Micro-CT scanning results indicated that the OVX mice showed significant bone loss compared to saline control mice (Figure 1A). The intervention (90 days) of GSK granules greatly increased the BMD, particularly in the GSKM group (Figure 1B). The bone structure parameters, such as BMD, BV/TV, Tb.N, and Tb.Th, were quantified. GSK granule treatments led to increased BMD, BV/TV, Tb.N and Tb.Th but decreased Tb.Sp (Figure 1C).
Tartrate resistant acid phosphatase (TRAP) staining showed an increase in the number of osteoclasts in OVX mice compared to control mice (Figure 2A). GSK granule treatments decreased TRAP-positive osteoclasts compared to the OVX group. These findings were confirmed by calculating the ratio of TRAP-positive area to trabecular bone surface (OCs/BS%) and the ratio of osteoclast number to bone area (OCs/mm2). These quantitative results showed a significant decrease in the number of osteoclasts in GSK groups compared to the OVX group (Figure 2B,C).
The GSK granule-containing serum was administered to bone marrow macrophages (BMMs) from 4−6 week old C57BL/6 mice to generate osteoclast and the number of osteoclasts was analyzed by TRAP staining. The results showed that GSK granule-containing serum decreased the number of TRAP-positive osteoclasts in GSK groups compared to the control group (Figure 3A,B).
Alkaline phosphatase (ALP) staining showed that GSK granule-medicated serum exerted stimulatory effects on osteoblastogenesis with MSCs from C57BL/6 mice. ALP staining showed that all three groups of GSK granule-medicated serum had increased the activity of ALP (Figure 4A,B) compared to the control group.
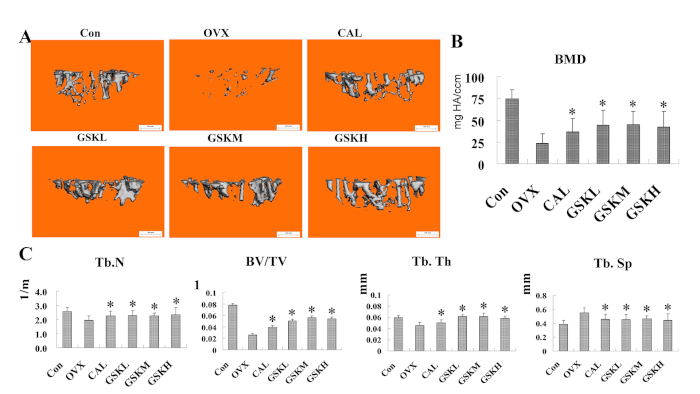
Figure 1: GSK granule prevents bone loss in OVX-induced mice. (A) Mice were treated with GSK granules for 3 months and left tibias were harvested to perform micro-CT analysis. Representative three-dimensional (3D) reconstruction images of the trabecular bone of left tibias were shown. Scale bar = 0.5 mm. (B) Bone mineral density (BMD) was measured and quantified. (C) Bone parameters of left tibias, such as the trabecular bone number (Tb.N), bone volume over total volume (BV/TV), trabecular bone thickness (Tb.Th), and trabecular bone separation (Tb.Sp), related to the trabecular bone structure in all the groups were shown. GSKL, GSKM, and GSKH groups were compared with control (Con; sham+ saline) and the OVX group (n = 6, *P < 0.05, versus control; *P < 0.05, versus OVX). CAL: Calcium carbonate with vitamin D3. Please click here to view a larger version of this figure.
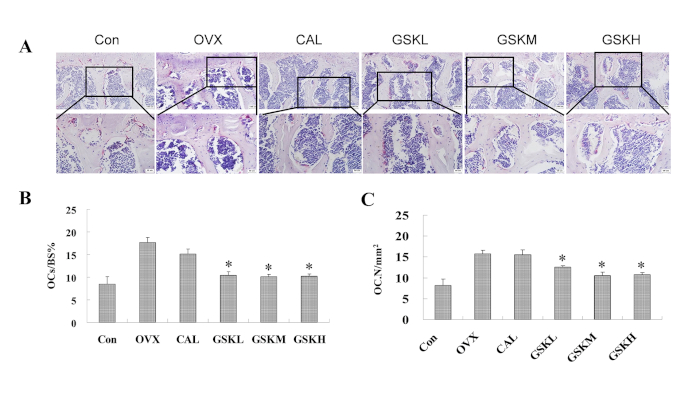
Figure 2: GSK granules suppress the number of osteoclasts in OVX mice. (A) TRAP staining was performed on lumbar vertebra 3 (L3) after the GSK-treated mice were harvested. TRAP results from control (sham + saline), OVX (OVX + saline), CAL (OVX + Caltrate), GSKL (OVX + low dose GSK, 2 g/kg/day), GSKM (OVX + medium dose GSK, 4 g/kg/day), and GSKH (OVX + high dose GSK, 8 g/kg/day) were measured and analyzed. Scale bar = 100 μm (top images) or 50 μm (bottom images). (B) Quantification of osteoclast-covered surface over bone surface. (C) Osteoclast number. Values were expressed as mean ± standard error of the mean (SEM). *P < 0.05, OVX versus control (Con); *P < 0.05, the groups of CAL or GSKL/GSKM/GSKH versus the OVX group. All the assays were repeated with at least 3 mice. Please click here to view a larger version of this figure.
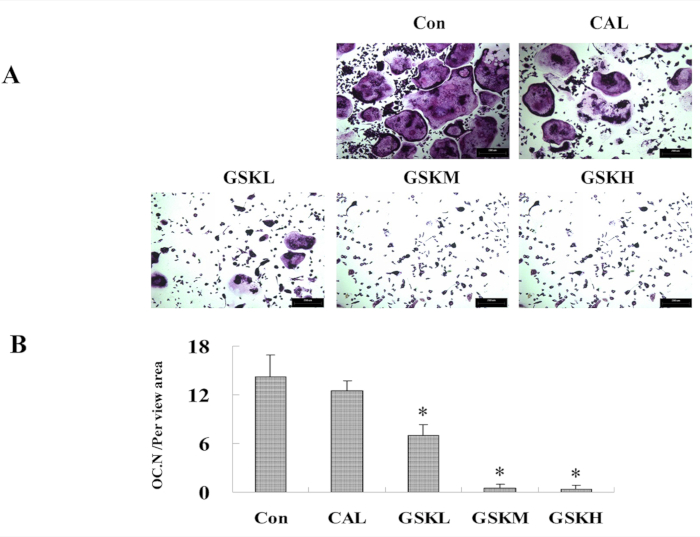
Figure 3: GSK granule medicated-serum decreases osteoclastogenesis from bone marrow macrophages (BMMs). (A) BMMs from C57BL/6 mice (4−6 week old) were harvested, and cultured with M-CSF (10 ng/mL) and RANKL (100 ng/mL) (control), M-CSF and RANKL plus GSK, or CAL medicated serums. Osteoclastogenesis was assessed at day 4−6 by TRAP staining. Scale bar = 100 μm. (B) The number of osteoclasts was quantified. *P < 0.05, the groups of GSKL/GSKM/GSKH versus control. Please click here to view a larger version of this figure.
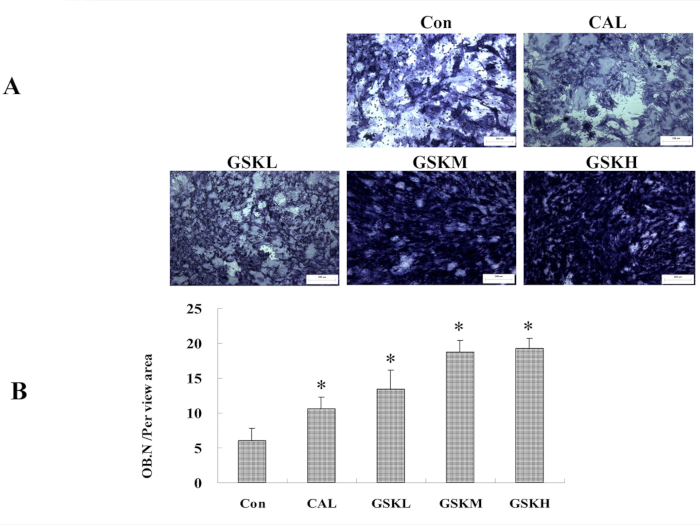
Figure 4: GSK granule-medicated serum promotes osteoblastogenesis. (A) Bone mesenchymal stem cells (MSCs) from C57BL/6 mice (4−6 week old) were isolated and treated with GSK or CAL medicated serum. ALP staining was performed at day 7 to assess osteoblastogenesis. Scale bar = 100 μm. (B) The number of osteoblasts was quantified. *P < 0.05, the groups of CAL or GSKL/GSKM/GSKH versus control. All the assays were repeated with at least 3 mice or 3 times. Please click here to view a larger version of this figure.
Discussion
Granules of TCM agents have become one of the common choices for formulations or prescriptions. GSK granules are composed of several herbal medicines based on clinical experiences or the TCM theory, and they exert better curative effects with fewer side effects4. Compared with water decoction, the granules have these advantages: good taste, convenience of delivery, long-term storage, standard protocol and consistent curative effects, as well as higher productivity. Currently, granules are one of the most commonly used pharmacy formations in TCM. However, the underlying mechanisms of pharmacological effects are still rarely studied. It is necessary to determine the critical steps in the preparation of granules to investigate the underlying pharmacological mechanisms.
In the past decades, one or more representative effective components from herbal medicine have usually been used to perform molecular assays and pharmacological outcomes due to their structural clarity. Many investigations have been performed to understand the curative effects with effective components from TCM herbs5,6,7. However, it is still difficult to mimic what will happen in a patient due to the complex environment, with many effective components working together. To resolve this problem, investigations with granules can explore pharmacological processes and are one choice in performing molecular studies compared to investigations with effective components.
Preparation of working solutions for granules contains four basic steps. The first step is dissolution. Granules are commonly mixed in saline after stirring to complete dissolve before further investigations. The quantity and property of granules affects the time and stability of granules during the dissolution process. The variation in dissolution time and stability depends on the herbs, due to their physical, chemical, and pharmacological characteristics17. Proper shaking and higher temperature usually promote and ensure complete dissolution of granules. The next step is concentration. The proper volume of gavage administration for animals is carefully considered and is determined by the volume of the working solution. Oral gavages at high concentrations, such as 10 mL/kg or more, can lead to several absorption-related problems. Rapid shunting of the working solution of granules into the duodenum is one common problem. Other problems, such as aspiration pneumonia, due to the passive reflux of the working solution of granules into the esophagus, are also observed18. Filtration is the third step, which helps the gavage needle to decrease in volume and prevents it from being clogged with herbal granules, as well as aids the digestion of granules. The fourth step is storage. The storage of working solutions of granules at low temperature (-20 °C) guarantees better outcomes.
The approach to calculate the animal bioequivalent dose is important to determine the effects of granules in the practice of TCM. The body weight (mg/kg) and species are commonly considered. The body surface area (mg/m2) is frequently used to perform the calculation19 because the metabolic rate is related to the size of the individual animal. It is common sense to consider both body surface area and body weight, and therefore, the Meeh-Rubner equation was used, which is common in in vivo investigations in pharmacological studies19,20.
Several kinds of animals are chosen for drug-containing serum preparation, such as rabbits, guinea pigs, rats, and mice. For in vivo investigations, the same species is preferred. Rats were selected because they not only provide more serum than mice but are also closer to mice in terms of evolution than other animals. The dose equivalent in vivo (rat: 7-fold of the equivalent dose) and clinical usage for patients are also recommended. Ten times the equivalent dose of the serum-provided animals is not commonly applied for in vivo investigations because treated cells or organs can lead to potential toxic reactions21. Methods such as injection, skin administration, and inhalation are the commonly used administration procedures in accordance with in vivo administrations. Oral administration by gavage needles was chosen in the present study. The granule administration frequency varies from once to twice per day, and the intervention period is 3−14 days. The final collection of blood is usually performed within 2 h after the last administration22,23, when the concentration of granules in blood is relatively stable and at the peak level according to a previous study24.
Drug-containing serum for in vitro assays before use is still controversial. Some researchers hold that it may result in unexpected reactions or side effects, which affect the results because of the presence of numerous active components in serum, including enzymes, hormones, antibodies, and complements25. However, some researchers hold the opposite opinion that active components might also be removed by the inactivation process26. To reach a middle ground, the serum in this study was inactivated before incubation in a water bath at 56 °C for 30 min. Moreover, a blank serum group was included, in which the serum from saline-treated animals is used, to rule out potential side effects. Therefore, drug-containing serum may serve as a potential method to investigate the pharmacological mechanisms or therapeutic outcomes.
Compared to similar methods, the protocol here has the following advantages: (1) Comprehensiveness. Both in vitro and in vivo methods are used simultaneously and can mutually support each other in pharmacological effects. (2) Suitability. Only mice and rats are included because they are closely related. (3) Repeatability. Both mice and rats are easily purchased at low cost, and the methods can be easily repeated. (4) Low cost. The OVX-induced osteoporotic mouse model is commonly used and reliable27,28 and can be easily made or purchased. Therefore, the protocols here are more suitable compared to other methods for studying the pharmacological effects of herbal medicine, such as granules.
However, there are several limitations to the protocols with GSK granules. First, three dosages were administered, although the granules showed no significant dose-dependent tendency for in vivo investigations. The reason may be that dosages for animal studies are not sensitive and the intervention time is not sufficiently long, which requires further testing. Next, a longer period of intervention is needed for in vitro parallel investigations. The drug-containing serum, although inactivated, may cause side effects after prolonged intervention. Third, only one volume of working solution is used for animal administration, which can be modified in future studies. Finally, animal species chosen for the preparation of drug-containing serum and the administration routines can be changed and will be tested in further studies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81804116, 81673991, 81770107, 81603643, and 81330085), the program for Innovative Team, Ministry of Science and Technology of China (2015RA4002 to WYJ), the program for Innovative Team, Ministry of Education of China (IRT1270 to WYJ), Shanghai TCM Medical Center of Chronic Disease (2017ZZ01010 to WYJ), Three Years Action to Accelerate the Development of Traditional Chinese Medicine Plan (ZY(2018-2020)-CCCX-3003 to WYJ), and national key research development projects (2018YFC1704302).
Materials
| α-MEM | Hyclone laboratories |
SH30265.018 | For cell culture |
| β-Glycerophosphate | Sigma | G5422 | Osteoblastogenesis |
| Caltrate (CAL) | Wyeth | L96625 | Animal interventation |
| C57BL/6 mice | SLAC Laboratory Animal Co. Ltd. |
Random | Ainimal preparation |
| Dexamethsome | Sigma | D4902 | |
| Dimethyl sulfoxide | Sigma | D2438 | Cell frozen |
| Ethylene Diamine Tetraacetic Acid (EDTA) | Sangon Biotech | 60-00-4 | Samples treatmnet |
| Fetal bovine serum | Gibco | FL-24562 | For cell culture |
| Gushukang granules | kangcheng companyin china | Z20003255 | Herbal prescription |
| Light microscope | Olympus BX50 | Olympus BX50 | Images for osteoclastogenesis |
| L-Ascorbic acid 2-phosphate sequinagneium slat hyclrate | Sigma | A8960-5G | Osteoblastogenesis |
| Microscope | Leica | DMI300B | Osteocast and osteoblast imagine |
| M-CSF | Peprotech | AF-300-25-10 | Osteoclastogenesis |
| Μicro-CT | Scanco Medical AG |
μCT80 radiograph microtomograph | Bone Structural analsysis |
| RANKL | Peprotech | 11682-HNCHF | Osteoclastogenesis |
| Sprague Dawley | SLAC Laboratory Animal Co. Ltd. |
Random | Blood serum collection |
| Tartrate-Resistant Acid Phosphate (TRAP) Kit | Sigma-Aldrich | 387A-1KT | TRAP staining |
References
- Shu, B., Shi, Q., Wang, Y. J. Shen (Kidney)-tonifying principle for primary osteoporosis: to treat both the disease and the Chinese medicine syndrome. Chinese Journal of Integrative Medicine. 21 (9), 656-661 (2015).
- Zhao, D., et al. The naturally derived small compound Osthole inhibits osteoclastogenesis to prevent ovariectomy-induced bone loss in mice. Menopause. 25 (12), 1459-1469 (2018).
- Liu, S. F., Sun, Y. L., Li, J., Dong, J. C., Bian, Q. Preparation of Herbal Medicine: Er-Xian Decoction and Er-Xian-containing Serum for In vivo and In vitro Experiments. Journal of Visualized Experiments. (123), e55654 (2017).
- Wang, Q., et al. The systemic bone protective effects of Gushukang granules in ovariectomized mice by inhibiting osteoclastogenesis and stimulating osteoblastogenesis. Journal of Pharmacological Sciences. 136 (3), 155-164 (2018).
- Bian, Q., et al. Oleanolic acid exerts an osteoprotective effect in ovariectomy-induced osteoporotic rats and stimulates the osteoblastic differentiation of bone mesenchymal stem cells in vitro. Menopause. 19 (2), 225-233 (2012).
- Zhao, D., et al. Oleanolic acid exerts bone protective effects in ovariectomized mice by inhibiting osteoclastogenesis. Journal of Pharmacological Sciences. 137 (1), 76-85 (2018).
- Tang, D. Z., et al. Osthole Stimulates Osteoblast Differentiation and Bone Formation by Activation of β-Catenin-BMP Signaling. Journal of Bone and Mineral Research. 25 (6), 1234-1245 (2010).
- Tashino, S. “Serum pharmacology” and “serum pharmaceutical chemistry”: from pharmacology of Chinese traditional medicines to start a new measurement of drug concentration in blood. Therapeutic Drug Monitoring Research. 5, 54-64 (1988).
- Fu, L., et al. Ex vivo Stromal Cell-Derived Factor 1-Mediated Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells into Hepatocytes Is Enhanced by Chinese Medicine Yiguanjian Drug-Containing Serum. Evidence Based Complement Alternative Medicine. , 7380439 (2016).
- Cao, Y., Liu, F., Huang, Z., Zhang, Y. Protective effects of Guanxin Shutong capsule drug-containing serum on tumor necrosis factor-alpha induced endothelial dysfunction through nicotinamide adenine dinucleotide phosphate oxidase and the nitric oxide pathway. Experimental and Therapeutic. 8 (3), 998-1004 (2014).
- Chen, X., et al. Application of serum pharmacology in evaluating the antitumor effect of Fuzheng Yiliu Decoction from Chinese Medicine. Chinese Journal of Integrative Medicine. 20 (6), 450-455 (2014).
- Li, X. L., Wang, L., Bi, X. L., Chen, B. B., Zhang, Y. Gushukang exerts osteopreserve effects by regulating Vitamin D and Calcium metabolism in ovariectomized mice. Journal of Bone Mineral Metabolism. , 1-11 (2018).
- Cui, S. Q., et al. Mechanistic study of Shen (Kidney)tonifying prescription Gushukang in Preventing and Treating Primary Osteoporosis. Journal of Chinese Medical University. 30 (16), 351-354 (2001).
- Wang, Y., Shang, K., Li, Y. K., Tao, X. L. Effect of gushukang on osteoclast cultured from type I diabetic rat in vitro-a preliminary study. Chinese Journal of Bone Tumor and Bone Disease. 3 (12), 22-24 (2004).
- Zhang, Y. P. . Pharmacology Experiment. , (1996).
- Zhao, D. F., et al. Cyclophosphamide causes osteoporosis in C57BL/6 male mice: suppressive effects of cyclophosphamide on osteoblastogenesis and osteoclastogenesis. Oncotarget. 8 (58), 98163-98183 (2017).
- Zhong, L. L., et al. A randomized, double-blind, controlled trial of a Chinese herbal formula (Er-Xian decoction) for menopausal symptoms in Hong Kong perimenopausal women. Menopause. 20 (7), 767-776 (2013).
- Zhang, D. Issues and strategies for study of serum pharmcology in oncology. Zhong Yi Yan Jiu. 17 (5), 13-14 (2004).
- Nair, A. B., Jacob, S. A simple practice guide for dose conversion between animals and human). Journal of Basic and Clinical Pharmacy. 7 (2), 27-31 (2016).
- Xu, X., et al. Protective effect of the traditional Chinese medicine xuesaitong on intestinal ischemia-reperfusion injury in rats. International Journal of Clinical and Experiments Medicine. 8 (2), 1768-1779 (2015).
- Jiang, Y. R., et al. Effect of Chinese herbal drug-containing serum for activating-blood and dispelling-toxin on ox-LDL-induced inflammatory factors’ expression in endothelial cells. Chinese Journal of Integrative Medicine. 18 (1), 30-33 (2012).
- Li, Y., Xia, J. Y., Chen, W., Deng, C. L. Effects of Ling Qi Juan Gan capsule drug-containing serum on PDGF-induced proliferation and JAK/STAT signaling of HSC-T6 cells. Zhonghua Gan Zang Bing Za Zhi. 21 (9), 663-667 (2013).
- Guo, C. Y., Ma, X. J., Liu, Q., Yin, H. J., Shi, D. Z. Effect of Chinese herbal drug-containing serum for activating blood, activating blood and dispelling toxin on TNF-alpha-induced adherence between endothelial cells and neutrophils and the expression of MAPK pathway. Zhongguo Zhong Xi Yi Jie He Za Zhi. 35 (2), 204-209 (2015).
- Li, Y. K. Some issues in methology of Chinese herbs serum pharmcology. Zhong Yao Xin Yao Yu Lin Chuang Yao Li. 10 (5), 263 (1999).
- Zhang, L., et al. A review of Chinese herbs serum pharmcology methodological study. Nan Jing Zhong Yi Yao Da Xue Xue Bao. 18 (4), 254 (2002).
- Pacifici, R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. Journal. Bone Mineral Research. 11, 1043-1051 (1996).
- Ammann, P., et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. Journal Clinical Investigation. 99, 1699-1703 (1997).
- Kimble, R. B., et al. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 136, 3054-3061 (1995).

