A Uniaxial Compression Experiment with CO2-Bearing Coal Using a Visualized and Constant-Volume Gas-Solid Coupling Test System
Summary
This protocol demonstrates how to prepare a briquette sample and conduct a uniaxial compression experiment with a briquette in different CO2 pressures using a visualized and constant-volume gas-solid coupling test system. It also aims to investigate changes in terms of coal’s physical and mechanical properties induced by CO2 adsorption.
Abstract
Injecting carbon dioxide (CO2) into a deep coal seam is of great significance for reducing the concentration of greenhouse gases in the atmosphere and increasing the recovery of coalbed methane. A visualized and constant-volume gas-solid coupling system is introduced here to investigate the influence of CO2 sorption on the physical and mechanical properties of coal. Being able to keep a constant volume and monitor the sample using a camera, this system offers the potential to improve instrument accuracy and analyze fracture evolution with a fractal geometry method. This paper provides all steps to perform a uniaxial compression experiment with a briquette sample in different CO2 pressures with the gas-solid coupling test system. A briquette, cold-pressed by raw coal and sodium humate cement, is loaded in high-pressure CO2, and its surface is monitored in real-time using a camera. However, the similarity between the briquette and the raw coal still needs improvement, and a flammable gas such as methane (CH4) cannot be injected for the test. The results show that CO2 sorption leads to peak strength and elastic modulus reduction of the briquette, and the fracture evolution of the briquette in a failure state indicates fractal characteristics. The strength, elastic modulus, and fractal dimension are all correlated with CO2 pressure but not with a linear correlation. The visualized and constant-volume gas-solid coupling test system can serve as a platform for experimental research about rock mechanics considering the multifield coupling effect.
Introduction
The increasing concentration of CO2 in the atmosphere is a direct factor causing the global warming effect. Due to the strong sorption capacity of coal, CO2 sequestration in a coal seam is regarded as a practical and environment-friendly means to reduce the global emission of greenhouse gas1,2,3. At the same time, the injected CO2 can replace CH4 and result in gas production promotion in coalbed methane recovery (ECBM)4,5,6. The ecological and economic prospects of CO2 sequestration have recently attracted worldwide attention among researchers, as well as among different international environmental protection groups and governmental agencies.
Coal is a heterogeneous, structurally anisotropic rock composed of a pore, fracture, and coal matrix. The pore structure has a large specific surface area, which can adsorb a large amount of gas, playing a vital role in gas sequestration, and the fracture is the main path for free gas flow7,8. This unique physical structure leads to a great gas adsorption capacity for CH4 and CO2. Mine gas is deposited in coalbed in a few forms: (1) adsorbed on the surface of micropores and larger pores; (2) absorbed in the coal molecular structure; (3) as free gas in fractures and larger pores; and (4) dissolved in deposit water. The sorption behavior of coal to CH4 and CO2 causes matrix swelling, and further studies demonstrate that it is a heterogeneous process and is related to the coal lithotypes9,10,11. In addition, gas sorption can result in damage in the constitutive relation of coal12,13,14.
The raw coal sample is generally used in coal and CO2 coupling experiments. Specifically, a large piece of raw coal from the working face in a coal mine is cut to prepare a sample. However, the physical and mechanical properties of raw coal inevitably have a high dispersion degree due to the random spatial distribution of natural pores and fractures in a coal seam. Moreover, the gas-bearing coal is soft and difficult to be reshaped. According to the principles of the orthogonal experimental method, the briquette, which is reconstituted with raw coal powder and cement, is regarded as an ideal material used in the coal sorption test15,16. Being cold-pressed with metal dies, its strength can be preset and remains stable by adjusting the quantity of cement, which benefits the comparative analysis of the single-variable effect. Additionally, although the porosity of the briquette sample is ~4-10 times, that of the raw coal sample, similar adsorption and desorption characteristics and stress-strain curve have been found in the experimental research17,18,19,20. In this paper, a scheme of a similar material for gas-bearing coal has been adopted to prepare the briquette21. The raw coal was taken from the 4671B6 working face in the Xinzhuangzi Coal Mine, Huainan, Anhui Province, China. The coal seam is approximately 450 m below ground level and 360 m below sea level, and it dips at about 15° and is approximately 1.6 m in thickness. The height and diameter of the briquette sample are 100 mm and 50 mm, respectively, which is the recommended size suggested by the International Society for Rock Mechanics (ISRM)22.
The previous uniaxial or triaxial loading test instruments for gas-bearing coal experiments under laboratory conditions have some shortages and limitations, presented as fellows23,24,25,26,27,28: (1) during the loading process, the vessel volume decreases with the piston moving, causing fluctuations in gas pressure and disturbances in gas sorption; (2) the real-time image monitoring of samples, as well as circumferential deformation measurements in a high gas pressure environment, is difficult to conduct; (3) they are limited to stimulation of dynamic load disturbances on preloaded samples to analyze their mechanical response characteristics. In order to improve the instrument precision and data acquisition in the gas-solid coupling condition, a visualized and constant-volume test system29 has been developed (Figure 1), including (1) a visualized loading vessel with a constant volume chamber, which is the core component; (2) a gas filling module with a vacuum channel, two filling channels, and a releasing channel; (3) an axial loading module consisting of an electro-hydraulic servo universal testing machine and control computer; (4) a data acquisition module comprised of a circumferential displacement measurement apparatus, a gas pressure sensor, and a camera at the window of the visualized loading vessel.
The core visualized vessel (Figure 2) is specifically designed so that two adjusting cylinders are fixed on the upper plate and their pistons move simultaneously with the loading one through a beam, and the sectional area of the loading piston is equal to the sum of that of the adjusting cylinders. Flowing through an inner hole and soft pipes, the high-pressure gas in the vessel and the two cylinders is connected. Therefore, when the vessel-loading piston moves downward and compresses the gas, this structure can offset the change in volume and eliminate pressure interference. In addition, the enormous gas-induced counterforce exerting on the piston is prevented during the test, significantly improving the safety of the instrument. The windows, which are equipped with tempered borosilicate glass and situated on three sides of the vessel, provide a direct way to take a photograph of the sample. This glass has been successfully tested and proved to resist up to 10 MPa gas with a low expansion rate, high strength, light transmittance, and chemical stability29.
This paper describes the procedure to perform a uniaxial compression experiment of CO2-bearing coal with the new visualized and constant-volume gas-solid coupling test system, which includes the description of all pieces that prepare a briquette sample using raw coal powder and sodium humate, as well as the successive steps to inject high-pressure CO2 and conduct uniaxial compression. The whole sample deformation process is monitored using a camera. This experimental approach offers an alternative way to quantitively analyze the adsorption-induced damage and fracture evolution characteristic of gas-bearing coal.
Protocol
1. Sample preparation
- Collect raw coal blocks from the 4671B6 working face from the Xinzhuangzi coal mine. Note that, due to the low strength and looseness of the structure, the raw coal is broken and probably mixed with impurities. To avoid the influence of these internal and external factors, as well as reduce the inhomogeneity of coal as much as possible, select large coal blocks (about 15 cm long, 10 cm wide, and 10 cm high).
- Use a tweezer to remove impurities mixed in the coal and scrub the crusher chamber with absorbent cotton and acetaldehyde.
- Smash the coal blocks into small pieces with a jaw crusher, and shelter them in a sieve shaker equipped with standard screens of 6 and 16 mesh. Place the sorted coal powder separately according to diameter.
- Weigh 1,000 g and 300 g of pulverized coal with a particle size distribution of 0–1 mm and 1– 3 mm, respectively. Put them together in a beaker in a mass proportion of 0.76:0.24 and mix them well with a glass rod (with a diameter of 6 mm).
NOTE: According to the Gaudian-Schuman function of continuous packing theory, when the particle size distribution value (m) equals approximately 0.25 (mass of particle size is 1–3 mm: total mass = 0.24), the strength of the briquette is maximal30. - To prepare the cement, put 4 g of sodium humate powder (99.99% purity) into a beaker and add approximately 96 mL of distilled water. Use a glass rod to stir them and make sure that all sodium humate is well dissolved.
NOTE: The concentration of cement directly affects the compressive strength of briquette. Table 1 reveals specific ratios of briquette preparation, of which the No. 2 sample has been used for the representative results. - Put 230 g of mixed coal powder and 20 g of sodium humate solution into a beaker and mix them together.
NOTE: Based on previous experiences of making samples, a briquette produced with 250 g of material, using the cold press method, meets the size requirement of a standard rock sample22, where coal powder accounts for 92% and cement accounts for 8%. - Cold-press the briquette using the shaping tools adapted to the size of the briquette (Figure 3).
- To produce a standard-sized briquette, coat the inner surface of the shaping tools with lubricating oil. Assemble tool components #2, #3, and #4 of Figure 3, and fill the hole with 250 g of mixed material.
- Put component #1 of Figure 3 on top of the material, and place everything under the piston of an electro-hydraulic servo universal testing machine.
- Launch the software WinWdw (or equivalent) to control the electro-hydraulic servo universal testing machine. In the software, click on Force Range to set the maximum force to 50 kN, and click on Reset to clear the displacement value.
- Left-click on the option force loading control. Set the moving ratio at 0.1 kN/s. Set the target force value at 29.4 kN and holding time at 900 s. Then, click on Start.
- Take out the shaping tools and invert them onto a rubber plate. Use a rubber hammer to disassemble tool components #4, #2, #3, and #1 in that order.
- Put the briquette in a 40 °C incubator for 48 h. Then, weigh its mass with electronic scales (with a precision of 0.01 g) and measure its height and diameter with a Vernier caliper (with a precision of 0.02 mm) after drying.
- Measure the moisture content, ash content, and volatile content of the briquette, using a proximate analyzer (see the Table of Materials) at a temperature of 20 °C and a relative humidity of 65% (per standard GB/T 212-2008). Perform a vitrinite reflectance measurement on the polished briquette, using a photometer microscope (per standard GB/T 6948-2008).
- Measure the uniaxial compressive strength, tensile strength, cohesion, and internal friction angle, using a universal testing machine and a strain controlled direct shear apparatus (per standard GB/T 23561-2010). Perform a Poisson ratio measurement using a resistance strain gauge (per standard GB/T 22315-2008).
- Conduct an adsorption test of the raw coal and the briquette, using an isotherm adsorption instrument (per standard GB/T19560-2008).
2. Experimental methods
- Laboratory setup
- Place the test system in a quiet, vibration-free area of a clean laboratory without electromagnetic interference. The room temperature should remain stable during the test.
- Put the visualized vessel on the platform of the electro-hydraulic servo universal testing machine. Connect the piston of the testing machine with that of the visualized vessel with the use of a specific tool (see Figure 4).
- Install a manual pressure-reducing valve in the gas tank nozzle. Connect the valve with the gas filling channel at the bottom plate of the visualized vessel by soft pipe (with an inner diameter of 5 mm and a maximal pressure of 30 MPa). Link the vacuum channel and the vacuum pump with the same pipe.
- Fix the back door of the visualized vessel with high-strength bolts. Connect the computer, data acquisition box (DAQ box), and the embedded gas pressure sensor to the back door.
- Air tightness test and blank measurement
- To acquire the gas pressure data in the visualized vessel, launch the software DAQ Sensor-16 (or equivalent). On the software, click on Start.
- Start the vacuum pump. Open the valve V1 (Figure 2) and close V2, V3, and V4 (Figure 2). Vacuum the visualized vessel chamber. Turn off V1 and vacuum-pump it until it is under vacuum.
- Open V2 and the gas tank (with helium). Use the manual pressure-reducing valve to adjust the outlet pressure of the gas tank to approximate 2 MPa (relative pressure).
- Carefully observe the gas pressure curve displayed on DAQ Sensor-16. When it is about 2 MPa, turn off V2 and the gas tank.
NOTE: After 24 h, if the reduction of the gas pressure is less than 5%, the sealability of the visualized vessel is good. - To measure the friction force of the loading piston moving downward, launch the software WinWdw to control the electro-hydraulic servo universal testing machine.
- In the software, click on Force Range to set the maximum force to 5 kN and click on Reset to clear the displacement value. Left-click on the option Displacement Loading Rate. Set the moving ratio at 1 mm/min; then, click on Start.
- When the displacement displayed on WinWdw is approximately 5 mm, click on Stop. Left-click on Data Save to save the force-displacement curve.
- Open V4 and discharge helium into air. Disassemble the back door of the visualized vessel and close V4.
CAUTION: The door and windows should be open for ventilation during the gas release due to the possible suffocation hazard.
- Uniaxial compression experiment
- Measure the height (h) and diameter (d) of the briquette with a Vernier caliper (with a precision of 0.02 mm). Weigh the mass (m) of the briquette with electronic scales (with a precision of 0.01 g). Calculate its apparent density (
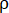 ) with the following equation.
) with the following equation.
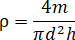
- Install the chain roller of the circumferential deformation test apparatus around the middle position of the briquette (Figure 5, #1) and fix the clamp holder (Figure 5, #2). Connect the sensor (Figure 5, #3) with the DAQ box through the aviation connector in the visualized vessel (Figure 2) and place them under the loading piston.
NOTE: To ensure the accuracy of the data acquisition, adjust the chain roller and the top surface of the sample so that they are parallel to the loading piston. - Launch WinWdw to control the universal testing machine. In the software, left-click on the option Displacement Loading Rate. Set the moving ratio at 10 mm/min. Press the Down button on the remote controller of the universal testing machine until the distance left between the piston and the sample is 1–2 mm. Then, assemble the back door of the visualized vessel.
- Repeat steps 2.2.1–2.2.2. Open V3 and the gas tank (CO2, purity = 99.99%). Use the manual pressure-reducing valve to adjust the outlet pressure of the gas tank to a certain value.
- Carefully observe the gas pressure curve displayed in DAQ Sensor-16. When it gets close enough to the target value, close V3 and the gas tank (CO2).
NOTE: When the gas pressure curve remains stable, the briquette has reached its adsorption and desorption dynamic equilibrium state. Generally, it takes 6–8 h for the briquette to fully adsorb. In this test, the adsorption time is set at 24 h. - After 24 h, place the camera with a tripod beside the window of the visualized vessel. Adjust the height and angle to ensure that the image of the sample is shown in the center of the camera screen.
- Start the software SDU deformation acquisition V2.0 (or equivalent) to monitor the circumferential deformation of the briquette. Click on Start.
- On WinWdw, click on New Sample and type in the height and diameter of the briquette, click on Sectional Area, and then click on Confirm. Click on Force Range to set the maximum force to 5 kN, and click on Reset to clear the displacement value.
- Left-click on the option Displacement Loading Rate and set the moving ratio at 1 mm/min. Click on Start to compress the sample. At the same time, press the Start button on the camera to begin video recording.
- When the sample totally fails, click on Stop and Data Save, in that order, in both WinWdw and SDU deformation acquisition V2.0. Press the Start button again on the camera to stop video recording.
- Repeat step 2.2.8 to release CO2 in the vessel chamber. Disconnect the aviation connectors for the gas pressure sensor and circumferential deformation test apparatus.
- Left-click on the option Displacement Loading Rate on WinWdw. Set the moving ratio at 10 mm/min. Press the Up button on the remote controller of the universal testing machine. When the loading piston of the vessel is around 2–3 mm above the briquette, take the briquette out and remove it from the chain roller.
- Dismantle the connecting tool between the pistons. Clean the visualized vessel with a vacuum cleaner.
- Measure the height (h) and diameter (d) of the briquette with a Vernier caliper (with a precision of 0.02 mm). Weigh the mass (m) of the briquette with electronic scales (with a precision of 0.01 g). Calculate its apparent density (
- Completion
- Based on the stress-axial strain curve and circumferential strain curve obtained from WinWdw and SDU deformation acquisition V2.0, calculate the volume strain of the sample with the following equation.
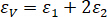
Here,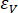 = volume strain;
= volume strain; 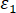 = axial strain;
= axial strain; 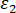 = circumferential strain.
= circumferential strain. - Obtain the peak strength from the stress-axial strain curve. The strength reduction rate is calculated as follows.
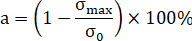
Here, = strength reduction rate;
= strength reduction rate; 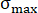 = peak strength of the sample under a different pressure of CO2;
= peak strength of the sample under a different pressure of CO2; 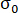 = peak strength of the sample in atmospheric air.
= peak strength of the sample in atmospheric air. - Calculate the elastic modulus using the linear stage in the stress-axial strain curve according to the following equation.
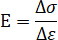
Here,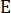 = elastic modulus of the sample;
= elastic modulus of the sample; 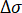 = stress increment of linear the stage (in megapascal);
= stress increment of linear the stage (in megapascal); 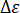 = strain increment of the linear stage. Calculate the elastic modulus reduction rate as follows.
= strain increment of the linear stage. Calculate the elastic modulus reduction rate as follows.
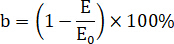
Here,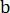 = elastic modulus reduction rate,
= elastic modulus reduction rate, 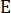 = elastic modulus of the sample under a different pressure of CO2;
= elastic modulus of the sample under a different pressure of CO2; 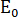 = elastic modulus of the sample in atmospheric air.
= elastic modulus of the sample in atmospheric air. - Select sample photos during the test and statistics fracture covering area using a program (e.g., written in MATLAB) according to the box-counting dimension method.
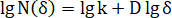
Here,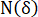 = grid number to cover the fracture area at the square grid side length of
= grid number to cover the fracture area at the square grid side length of 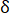 ;
; 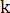 = a constant;
= a constant; 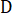 = fractal dimension;
= fractal dimension; 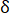 = side length of the square grid. The minimum grid size equals the pixel size in this test.
= side length of the square grid. The minimum grid size equals the pixel size in this test.
- Calculate the correlation coefficient according to the following equation.
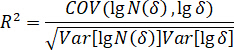
Here,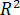 = correlation coefficient;
= correlation coefficient; 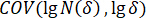 = covariance of
= covariance of 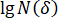 and
and 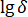 ;
; 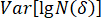 = variance of
= variance of 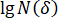 ;
; 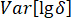 = variance of
= variance of 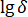 .
.
- Calculate the correlation coefficient according to the following equation.
- Based on the stress-axial strain curve and circumferential strain curve obtained from WinWdw and SDU deformation acquisition V2.0, calculate the volume strain of the sample with the following equation.
Representative Results
The average mass of the briquette sample was 230 g. Depending on the industrial analysis, the briquette exhibited a moisture content of 4.52% and an ash content of 15.52%. Furthermore, the volatile content was approximately 31.24%. As the sodium humate was extracted from the coal, the components of the briquette were similar to raw coal. The physical characteristics are displayed in Table 2.
The comparison of the mechanical properties between raw coal and briquette are shown in Table 3, and the isothermal adsorption test proved their similar capacity for gas adsorption (Figure 6). The strength of the briquette samples used in the test had some fluctuation (Figure 7). However, compared with the strength reduction induced by CO2 adsorption, it was rather slight and had little influence on the analysis of the experimental results.
When under different CO2 pressures, the stress-axial strain curves showed obvious compaction, elastic, and plastic deformation phases (Figure 8a). In the post-peak state, the briquette gradually failed, with a surface crack expanding and connecting. A volume expansion was observed from the stress-volume strain curves, and it increased with the CO2 pressure becoming higher (Figure 8a). The CO2 sorption caused damage to the coal body, which directly reduced its uniaxial compressive strength. The peak strengths of the briquette were 1.011 MPa, 0.841 MPa, 0.737 MPa, 0.659 MPa, 0.611 MPa, and 0.523 MPa under CO2 pressure from 0 MPa, 0.4 MPa, 0.8 MPa, 1.2 MPa, and 1.6 MPa to 2.0 MPa. As the CO2 pressure increased, the peak strength of the coal sample decreased, where it showed a nonlinear relationship (Figure 8b). In addition, the elastic moduli were 66.974 MPa, 48.271 MPa, 42.234 MPa, 36.434 MPa, 32.509 MPa, and 29.643 MPa, in that order, of CO2 pressure from 0 to 2.0 MPa. The results indicate that the elastic modulus decreased under the CO2 saturated condition and that the relationship between the elastic modulus decrease and the gas pressure was nonlinear, which was similar to that of peak strength (Figure 8c).
The images obtained through the camera evince the fractures’ evolution on the sample’s surface under different CO2 pressures. To distinguish different fractures, all photos were transferred into binary images and several colors were used to indicate areas covered by fractures (Figure 9a). The box-counting dimension method was adopted to describe the feature of fractures in failure state (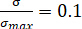 ; here,
; here, 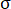 = stress of the sample in post-peak state;
= stress of the sample in post-peak state; 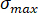 = peak strength of the sample) under different CO2 pressures. The correlation coefficients between the box number (
= peak strength of the sample) under different CO2 pressures. The correlation coefficients between the box number (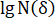 ) and the side length (
) and the side length (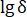 ) were all more than 0.95 (Figure 9b), which verifies the obvious fractal characteristics of fractures. The fractal dimensions (
) were all more than 0.95 (Figure 9b), which verifies the obvious fractal characteristics of fractures. The fractal dimensions (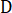 ) were 1.3495, 1.3711, 1.4336, 1.4637, 1.5175, and 1.5191 for the briquette under 0 MPa, 0.4 MPa, 0.8 MPa, 1.2 MPa, 1.6 MPa, and 2.0 MPa CO2, respectively. The values of the fractal dimension were proportional to those of CO2 pressure, and their trend indicated similarity to that of the degree of damage to the coal body.
) were 1.3495, 1.3711, 1.4336, 1.4637, 1.5175, and 1.5191 for the briquette under 0 MPa, 0.4 MPa, 0.8 MPa, 1.2 MPa, 1.6 MPa, and 2.0 MPa CO2, respectively. The values of the fractal dimension were proportional to those of CO2 pressure, and their trend indicated similarity to that of the degree of damage to the coal body.
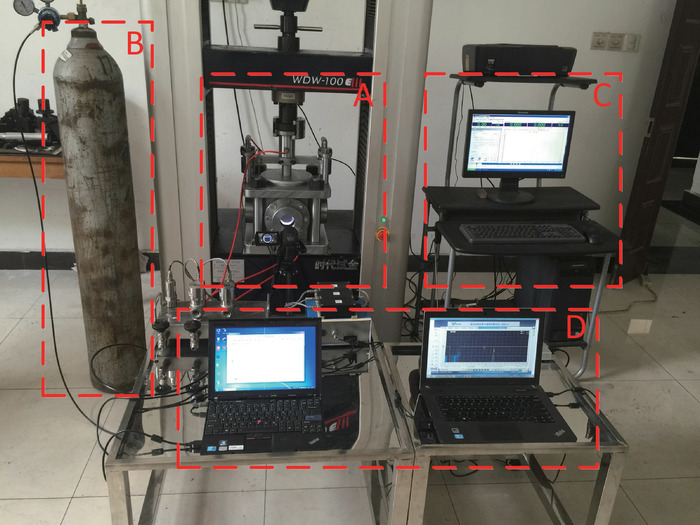
Figure 1: Experimental setup of the visualized and constant-volume gas-solid coupling test system. The figure demonstrates the setup of a uniaxial compression experiment of CO2-bearing coal. (A) Visualized loading vessel. (B) Gas filling module. (C) Axial loading module. (D) Data acquisition module. Please click here to view a larger version of this figure.
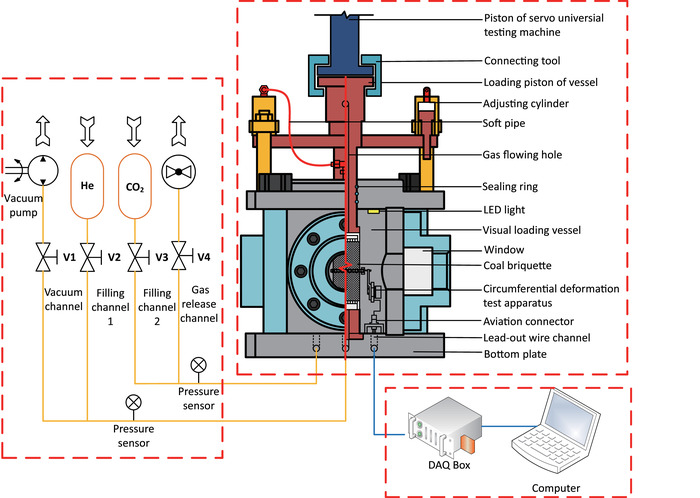
Figure 2: The visualized loading vessel. Schematic drawings of the vessel are shown above. While the sample (height = 100 mm, diameter = 50 mm) lay within the vessel, axial pressure was applied by the independent universal testing machine through the loading piston, and high-pressure gas was injected from the gas tank through the soft pipe and the filling channel. When the sample was warped by the thermal contractible plastic sleeve, the confining pressure was also provided by high-pressure helium. The two adjusting cylinder pistons and the loading one of the visualized vessel moved simultaneously, where the movement-induced volume change was offset because of their same sectional area. This structure kept the vessel volume constant and eliminated the antiforce applied on the loading piston from gas. The sample could be monitored with a camera through the windows on three sides. The aviation connector was set in the vessel for a lead-out wire connection. Please click here to view a larger version of this figure.
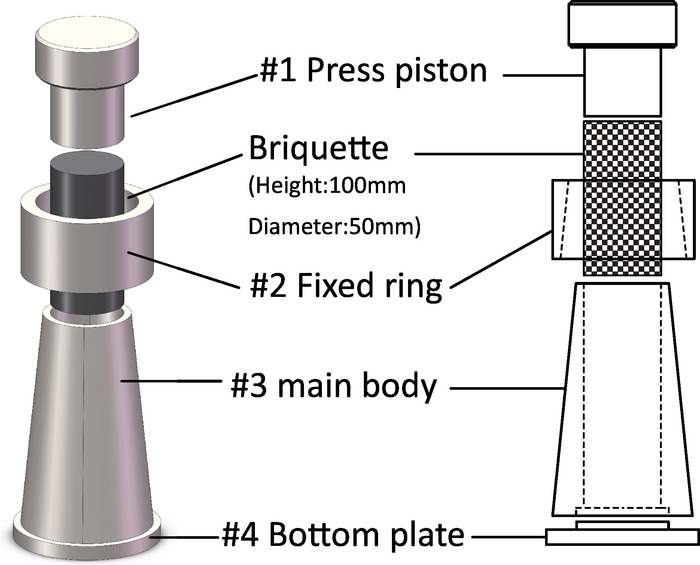
Figure 3: Shaping tools required to cold-press the standard briquette. 3D schematic views of how the briquette was pressed (29.4 KN for 15 min). The sample lay in the inner hole of the tool components, and its height and diameter were 100 mm and 50 mm, respectively. Please click here to view a larger version of this figure.
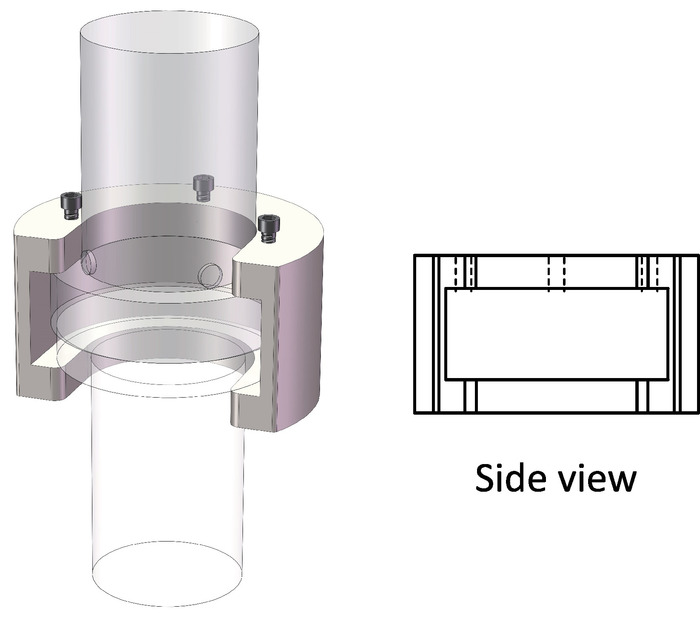
Figure 4: Tool required to connect the loading pistons. 3D schematic views of the fixing tool between the piston of the electro-hydraulic servo tester and that of the visualized vessel. Please click here to view a larger version of this figure.
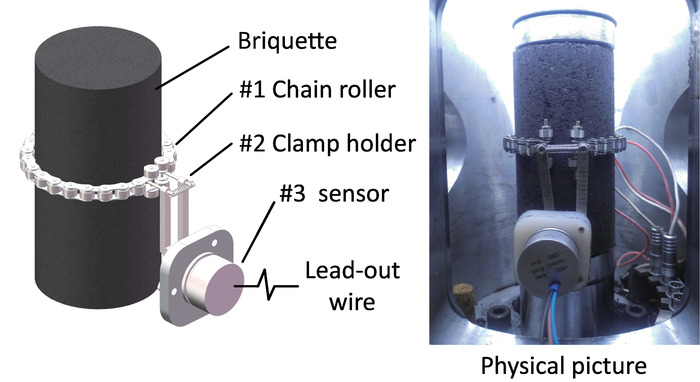
Figure 5: Standard test apparatus for the circumferential deformation of rock samples. Schematic and physical representation of the circumferential deformation acquisition used in the protocol. By measuring the angular displacement induced by sample circumferential deformation, the circumferential strain was obtained. This apparatus can stably operate in high-pressure gas and hydraulic oil. Please click here to view a larger version of this figure.
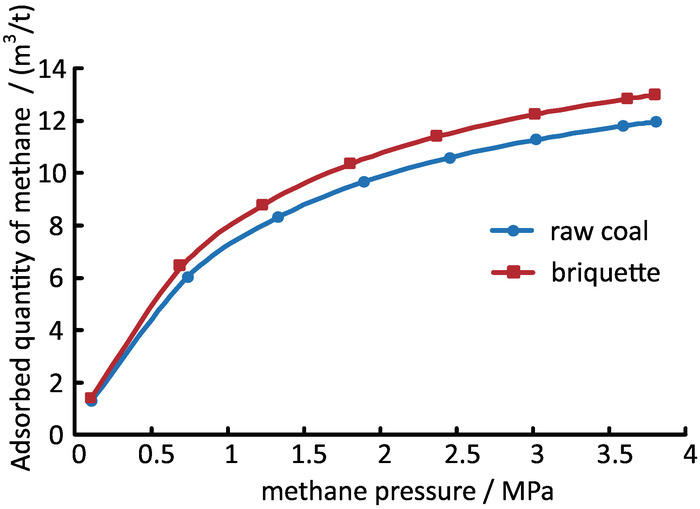
Figure 6: Comparison of the adsorption capacity between raw coal and briquette. The panel shows the methane isothermal adsorption data using raw coal and briquette according to per standard GB/T19560-2008. Please click here to view a larger version of this figure.
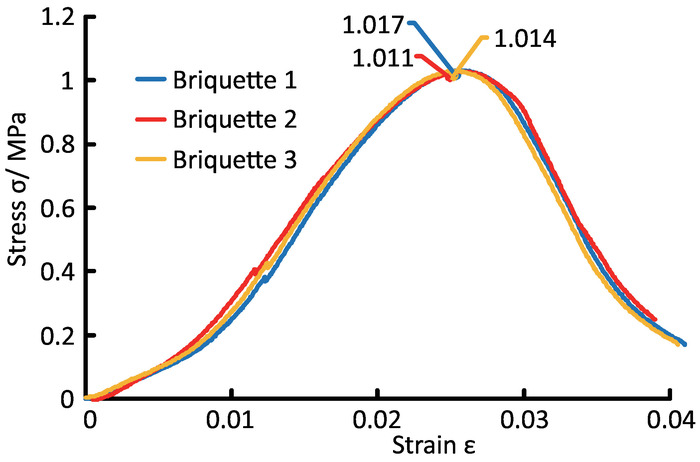
Figure 7: The full stress-strain curves generated from the test system using briquette. A uniaxial compression test was conducted using three briquette samples without CO2 filling, and results show that briquette has a stable uniaxial compression strength (1.0 MPa). Please click here to view a larger version of this figure.
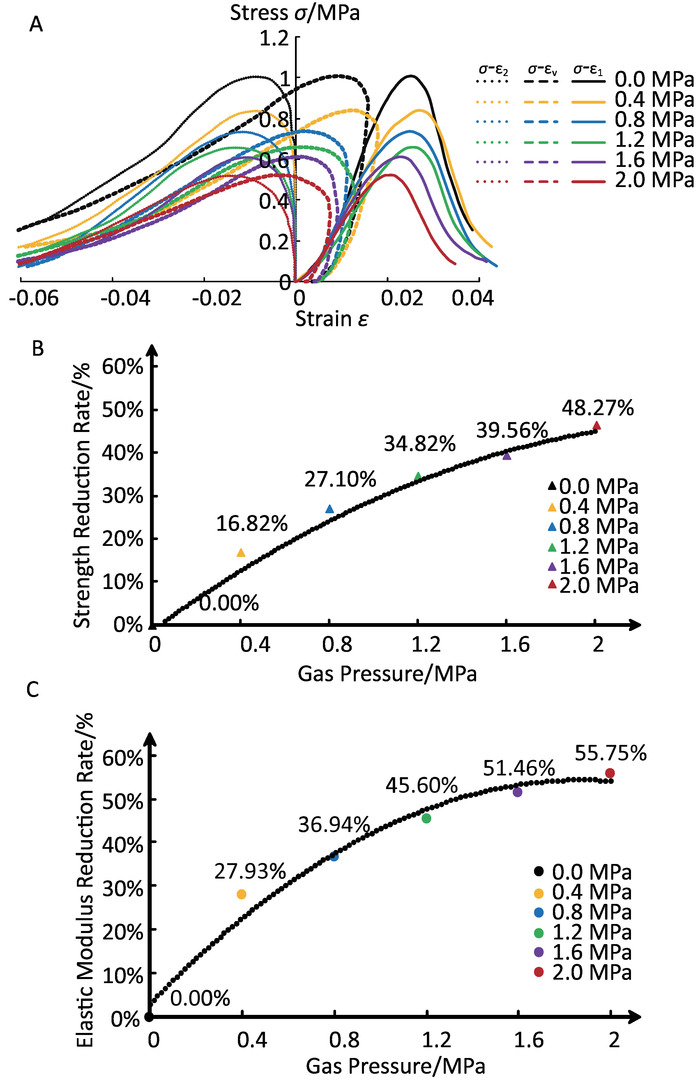
Figure 8: Uniaxial compression experiment of CO2-bearing coal. (A) Stress-strain curves under different CO2 pressures. (B) Trend of change in peak strength. (C) Trend of change in elastic modulus. The stress- axial strain curves ( ), stress-circumferential strain curves (
), stress-circumferential strain curves ( ), and stress-volume strain curves (
), and stress-volume strain curves ( ) are shown in panel A. After filling with CO2, the briquette experienced peak strength and elastic modulus reduction, and the curves in panels B and C indicate a nonlinear relationship between the reduction rate and the gas pressure. Please click here to view a larger version of this figure.
) are shown in panel A. After filling with CO2, the briquette experienced peak strength and elastic modulus reduction, and the curves in panels B and C indicate a nonlinear relationship between the reduction rate and the gas pressure. Please click here to view a larger version of this figure.
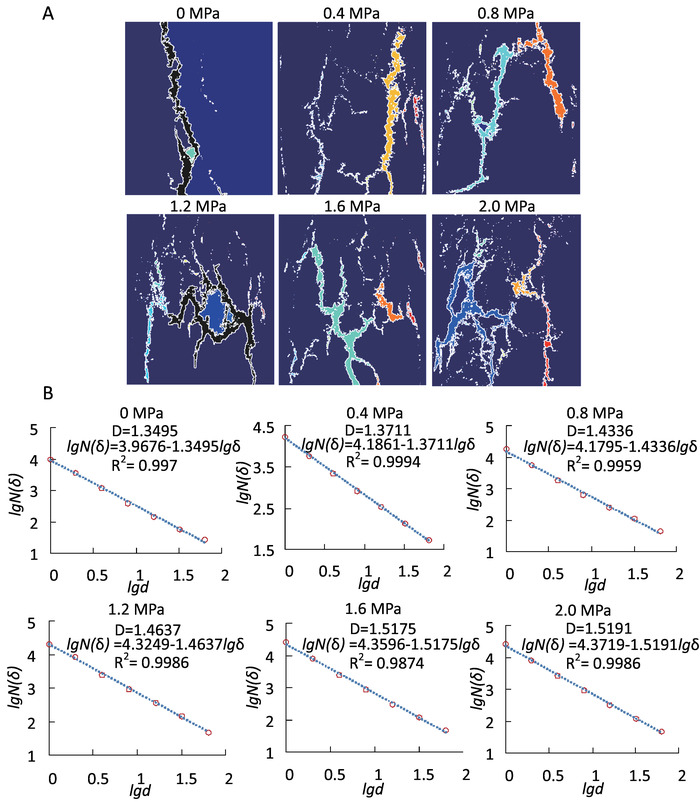
Figure 9: The images of fractures and fractal calculation in failure state (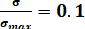 ). (A) Fracture evolution on briquettes’ surfaces, with different colors representing varied fractures. (B) Fractal dimension curves using the box-counting dimension method. Fractures were extracted and the covering area was calculated based on fractal geometry. All correlation coefficients (R2) under different CO2 pressures were more than 0.95, which proves the fractal characteristics. Please click here to view a larger version of this figure.
). (A) Fracture evolution on briquettes’ surfaces, with different colors representing varied fractures. (B) Fractal dimension curves using the box-counting dimension method. Fractures were extracted and the covering area was calculated based on fractal geometry. All correlation coefficients (R2) under different CO2 pressures were more than 0.95, which proves the fractal characteristics. Please click here to view a larger version of this figure.
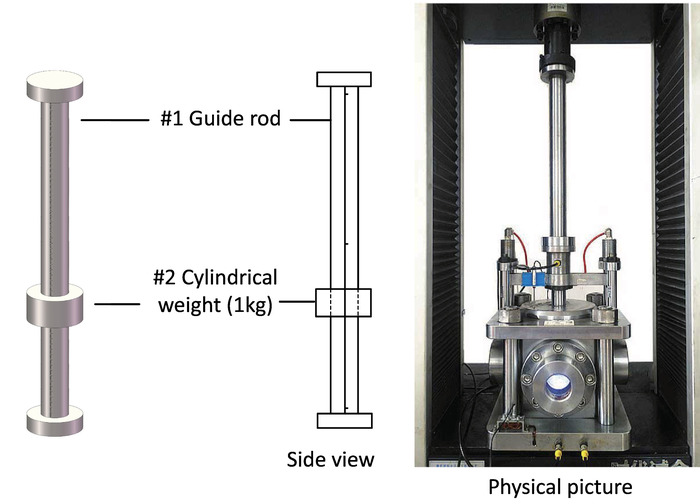
Figure 10: Tools required to apply dynamic load and photo of the test system. 3D view and physical picture of the guide rod and the cylindrical weight for dynamic load applying. Please click here to view a larger version of this figure.
| NO. | Coal grain Composition (0~1 mm:1~3 mm) |
Concentration of solidum humate solution/ % |
Raito (coal powder: cement) |
Mass/ g | Molding Pressure / MPa |
Time / min |
Peak Strength / MPa |
| 1 | 0.76:0.24 | 1 | 0.92:0.08 | 250 | 15 | 15 | 0.5 |
| 2 | 4 | 1 | |||||
| 3 | 7 | 1.5 | |||||
| 4 | 12 | 2 |
Table 1: Scheme of briquette preparation.
| sample | apparent density (g/cm3) |
Porosity (%) |
Moisture content (%) |
Ash content (%) |
Volatile content (%) |
Maximum vitrinite reflectance (%) |
| Briquette | 1.17 | 15 | 4.52 | 15.52 | 31.24 | 0.82 |
| Raw coal | 1.4 | 3.45 | 4.09 | 15.36 | 31.17 | 0.85 |
Table 2: The comparison of industrial analysis parameters for briquette and raw coal.
| Sample | Uniaxial compressive strength (MPa) |
Elastic modulus (Gpa) |
Tensile strength (MPa) |
Internal friction angle (°) |
Cohesion (MPa) |
Pission ratio |
| raw coal | 25.23 | 4.529 | 2.30 | 30 | 0.800 | 0.25 |
| briquette | 1.011 | 0.067 | 0.11 | 29 | 0.117 | 0.25 |
Table 3: The mechanical characteristics of raw coal and briquette.
Discussion
Considering the danger of high-pressure gas, some critical steps are important during the test. The valves and O rings should be inspected and replaced regularly, and any source of ignition should not be allowed in the laboratory. When using the manual pressure-regulating valve, the experimenter should twist the valve slowly to make the pressure in the visualized vessel increase gradually. Do not disassemble the vessel during the test. When the experiment is finished, the back door of the vessel should be opened after the total release of the high-pressure gas; otherwise, there is a danger of injury. Use a vacuum cleaner to remove all pieces of briquette from the vessel, so as not to affect the quantity of gas adsorption during the next test.
The CO2-coal coupling experimental method was designed to promote test precision and provide photograph monitoring for gas-bearing coal experiments. The briquette sample possesses several advantages, such as cost-effectiveness, nontoxicity, easy manufacture, stable performance, and adjustable strength, and its isothermal adsorption curve agrees well with that of raw coal. The model test of coal and gas outburst also proves that briquette can simulate the adsorptive and desorptive behavior of gas-bearing coal29,31. In addition, after five generations of improvement, the experimental apparatus now has high accuracy, precision, stability, and safety, which complies with the standards for the safety of high-pressure experiments. There is no particular requirement for the species of the sample, as long as it is a porous rock, including raw coal and shale rock.
The main limits of the CO2-coal coupling experimental method are, first, that briquette has a lower strength compared with raw coal, due to its way of formation. The similarity of mechanical properties between the raw coal and the briquette still needs improvement, and related experimental results should be evaluated and validated by raw coal and an in situ test. Second, since the LED lights and aviation connector were set in a visualized vessel, it should not be filled with any flammable gas, such as CH4. Otherwise, an explosive accident is likely to occur during the gas filling. Fortunately, a noncombustible gas similar to methane can simulate the CH4-coal interaction and it has been proven as a safe and effective material to apply in coal and gas outburst physical simulation experiments32.
Additionally, the briquette is wrapped by a thermal contractible plastic sleeve for confining the pressure applied during the triaxial compression test, which will evidently degrade the quality of the sample image. When the sample is loaded under a different gas, temperature, and gas pressure, the dynamic index of the refraction needs to be taken into consideration during image capturing. As the pressure difference in the test is relatively low, the index of refraction can be seen as a constant33.
Apart from the uniaxial and triaxial compression, dynamic load disturbance can be applied during the test to investigate the interaction between the sample and the gas. The guide rod and a 1 kg cylindrical weight are added between the pistons of the universal testing machine and the visualized vessel (Figure 10). The pressure sensor is installed on the bottom of the loading piston to acquire the dynamic pressure applied to the sample. During the test, the cylindrical weight, at a certain height, is released in different stress states to study the sample’s dynamic failure characteristics.
The sorption-induced damage to the coal body is macroscopically revealed as a reduction of the uniaxial compressive strength and elastic modulus. The higher the sorption pressure is, the greater the coal damage causes, which is a nonlinear relationship. The adsorption process can be described by the Langmuir model34. According to the model equation, 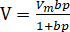 (V = equivalent adsorption volume; Vm, b = constant; p = gas pressure), the adsorption quantity increases as the gas pressure increases. This difference results in the different reduction rates of peak strength of briquette. The coal strength or elastic modulus reduction by CO2 saturation observed from experimental results have good conformity with previous research35,36,37. In conclusion, there must be a certain relationship between mechanical damage caused by sorption and gas adsorption quantity.
(V = equivalent adsorption volume; Vm, b = constant; p = gas pressure), the adsorption quantity increases as the gas pressure increases. This difference results in the different reduction rates of peak strength of briquette. The coal strength or elastic modulus reduction by CO2 saturation observed from experimental results have good conformity with previous research35,36,37. In conclusion, there must be a certain relationship between mechanical damage caused by sorption and gas adsorption quantity.
The deformation characteristics of briquette are summarized as the compression/expansion connection of microcracks and the final formation of macroscopic fractures. It is suggested that the fracture evolution of CO2-bearing coal showed fractal characteristics. The maximum fractal dimension was 1.5191 (2 MPa CO2) in the test. Considering that raw coal is more heterogeneous than briquette, the value of the fractal dimension may be different for the raw coal test.
Rock is a solid medium, and various external effects will cause damage to it. Due to the uncertainty of crack propagation during the failure process, especially considering the coupling effect of sorption and loading, some traditional rock mechanics research methods manifest obvious limitations. However, the fractal theory provides a new way to describe and study the complex mechanical processes and mechanisms of rock fracture development. Previous studies have made it clear that the fracture evolution of rock materials has fractal features38,39,40,41. However, test research on the fracture evolution of gas-bearing coal is lacking, mainly because of a limitation of the experimental apparatus. The CO2-coal coupling experimental method provides scientists with a way to capture and extract the surface fracture network of the sample through windows and obtains the fractal dimension in different coupling conditions. The fractal dimension can be used to quantitatively describe the damage degree, fracture development, and section complexity of coal body under the loading status. It can become an evaluation index for structural characteristics and mechanical properties of coal. Therefore, it is of great significance to the evaluation of gas storage capacity and injection influence parameters in the practice of CO2 geological sequestration.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the China National Major Scientific Instruments Development Project (Grant No. 51427804) and the Shandong Province National Natural Science Foundation (Grant No. ZR2017MEE023).
Materials
| 3Y-Leica MPV-SP photometer microphotometric system | Leica,Germany | M090063016 | Used for vitrinite reflectance measurement |
| Automatic isotherm adsorption instrument | BeiShiDe Instrument Technology (Beijing)CO.,Ltd. | 3H-2000PH | Isothermal adsorption test |
| Electro hydraulic servo universal testing machine | Jinan Shidaishijin testing machine CO.,Ltd | WDW-100EIII | Used to provide axial pressure |
| Gas pressure sensor | Beijing Star Sensor Technology CO.,LTD | CYYZ11 | Gas pressure monitoring |
| Gas tank(carbon dioxide/helium) | Heifei Henglong Gas.,Ltd | Gas resource | |
| high-speed camera | Sony corporation | FDR-AX30 | Image monitoring |
| Incubator | Yuyao YuanDong Digital Instrument Factory | XGQ-2000 | Briquette drying |
| jaw crusher | Hebi Tianke Instrument CO.,Ltd | EP-2 | Coal grinding |
| Manual pressure reducing valve | Shanghai Saergen Instrument CO.,Ltd | R41 | Outlet gas pressure adjustment |
| Proximate Analyzer | Changsha Kaiyuan Instrument CO.,Ltd | 5E-MAG6700 | Coal industrial analysis |
| Resistance strain gauge | Jinan Sigmar Technology CO.,LTD | ASMB3-16/8 | Poisson ratio measurement |
| Sieve shaker (6,16mesh) | Hebi Tianguan Instrument CO.,Ltd | GZS-300 | Coal powder shelter |
| Soft pipe | Jinan Quanxing High pressure pipe CO.,Ltd | Inner diameter=5 mm maximal pressure=30 MPa |
|
| Standard rock sample circumferential deformation test apparatus | Huainan Qingda Machinery CO.,Ltd | Circumferential deformation acquisition |
|
| Strain controlled direct shear apparatus |
Beijing Aerospace Huayu Test Instrument CO.,LTD | ZJ-4A | Tensile strength, cohesion, internal friction angle measurement |
| Vaccum pump | Fujiwara,Japan | 750D | Used to vaccumize the vessel |
| Valve | Jiangsu Subei Valve Co.,Ltd | S4 NS-MG16-MF1 | Gas seal |
| Visual loading vessel | Huainan Qingda Machinery CO.,Ltd | Instrument for sample loading and real-time monitoring |
References
- Mazzotti, M., Pini, R., Storti, G. Enhanced coalbed methane recovery. Journal of Supercritical Fluids. 47 (3), 619-627 (2009).
- Litynski, J., et al. U.S. Department of Energy’s Regional Carbon Sequestration Partnership Program: Overview. Energy Procedia. 1 (1), 3959-3967 (2009).
- Lackner, K. S. A Guide to CO2 Sequestration. Science. 300 (5626), 1677-1678 (2015).
- Zhou, F. D., et al. A feasibility study of ECBM recovery and CO2, storage for a producing CBM field in Southeast Qinshui Basin, China. International Journal of Greenhouse Gas Control. 19 (19), 26-40 (2013).
- Zhou, F., Hussain, F., Cinar, Y. Injecting pure N2 and CO2 to coal for enhanced coalbed methane: Experimental observations and numerical simulation. International Journal of Coal Geology. 116 (5), 53-62 (2013).
- Pini, R., Ottiger, S., Storti, G., Mazzotti, M. Pure and competitive adsorption of CO2, CH4 and N2 on coal for ECBM. Energy Procedia. 1 (1), 1705-1710 (2009).
- Nie, B. S., Li, X. C., Cui, Y. J., Lu, H. Q. . Theory and application of gas migration in coal seam. , (2014).
- Scott, A. R., Mastalerz, M., Glikson, M., Golding, S. D. Improving coal gas recovery with microbially enhanced coalbed methane. Coalbed Methane: Scientific, Environmental and Economic Evaluation. , 89-110 (1999).
- Gorucu, F., et al. Effects of matrix shrinkage and swelling on the economics of enhanced-coalbed-methane production and CO2 sequestration in coal. Spe Reservoir Evaluation Engineering. 10 (4), 382-392 (2007).
- Liu, S. M., Wang, Y., Harpalani, S. Anisotropy characteristics of coal shrinkage/swelling and its impact on coal permeability evolution with CO2 injection. Greenhouse Gases Science & Technology. 6 (5), 615-632 (2016).
- Larsen, J. W. The effects of dissolved CO2, on coal structure and properties. International Journal of Coal Geology. 57 (1), 63-70 (2004).
- Mastalerz, M., Gluskoter, H., Rupp, J. Carbon dioxide and methane sorption in high volatile bituminous coals from Indiana, USA. International Journal of Coal Geology. 60 (1), 43-55 (2004).
- Li, X. C., Nie, B. S., He, X. Q., Zhang, X., Yang, T. Influence of gas adsorption on coal body. Journal of China Coal Society. 36 (12), 2035-2038 (2011).
- Du, Q. H., Liu, X. L., Wang, E. Z., Wang, S. J. Strength Reduction of Coal Pillar after CO2 Sequestration in Abandoned Coal Mines. Minerals. 7 (2), 26-41 (2017).
- Zhao, B., et al. Similarity criteria and coal-like material in coal and gas outburst physical simulation. International Journal of Coal Science and Technology. 5 (2), 167-178 (2018).
- Xu, J., Ye, G. -. b., Li, B. -. b., Cao, J., Zhang, M. Experimental study of mechanical and permeability characteristics of moulded coals with different binder ratios. Rock and Soil Mechanics. 36 (1), 104-110 (2015).
- Barbara, D., et al. Balance of CO2/CH4 exchange sorption in a coal briquette. Fuel Processing Technology. 106 (2), 95-101 (2013).
- Benk, A., Coban, A. Molasses and air blown coal tar pitch binders for the production of metallurgical quality formed coke from anthracite fines or coke breeze. Fuel Processing Technology. 92 (5), 1078-1086 (2011).
- Zhao, H. B., Yin, G. Z. Study of acoustic emission characteristics and damage equation of coal containing gas. Rock and Soil Mechanics. 32 (3), 667-671 (2011).
- Cao, S. G., Li, Y., Guo, P., Bai, Y. J., Liu, Y. B. Comparative research on permeability characteristics in complete stress-strain process of briquette and coal samples. Chinese Journal of Rock Mechanics and Engineering. 29 (5), 899-906 (2010).
- Wang, H. P., et al. Development of a similar material for methane-bearing coal and its application to outburst experiment. Rock and Soil Mechanics. 36 (6), 1676-1682 (2015).
- Ulusay, R. . The ISRM Suggested Methods for Rock Characterization, Testing and Monitoring: 2007-2014. , (2015).
- Ranathunga, A. S., Perera, M. S. A., Ranjith, P. G. Influence of CO2 adsorption on the strength and elastic modulus of low rank Australian coal under confining pressure. International Journal of Coal Geology. 167, 148-156 (2016).
- Ranjith, P. G., Perera, M. S. A. Effects of cleat performance on strength reduction of coal in CO2, sequestration. Energy. 45 (1), 1069-1075 (2012).
- Masoudian, M. S., Airey, D. W., El-Zein, A. Experimental investigations on the effect of CO2, on mechanics of coal. International Journal of Coal Geology. 128 (3), 12-23 (2014).
- Wang, S. G., Elsworth, D., Liu, J. S. Rapid decompression and desorption induced energetic failure in coal. Journal of Rock Mechanics and Geotechnical Engineering. 7 (3), 345-350 (2015).
- Hadi Mosleh, M., Turner, M., Sedighi, M., Vardon, P. J. Carbon dioxide flow and interactions in a high rank coal: Permeability evolution and reversibility of reactive processes. International Journal of Greenhouse Gas Control. 70, 57-67 (2018).
- Abhijit, M., Harpalani, S., Liu, S. M. Laboratory measurement and modeling of coal permeability with continued methane production: Part 1 – Laboratory results. Fuel. 94 (1), 110-116 (2012).
- Li, Q. C., et al. Development and application of a gas-solid coupling test system in the visualized and constant volume loading state. Journal of China University of Mining & Technology. 47 (1), 104-112 (2018).
- Allen, T. . Particle Size Measure. , (1984).
- Wang, H. P., et al. Coal and gas outburst simulation system based on CRISO model. Chinese Journal of Rock Mechanics and Engineering. 34 (11), 2301-2308 (2015).
- Zhang, Q. H., et al. Exploration of similar gas like methane in physical simulation test of coal and gas outburst. Rock and Soil Mechanics. 38 (2), 479-486 (2017).
- Xia, G. Z. . Study on density and refractive index of near-critical fluid. , (2009).
- Ruppel, T. C., Grein, C. T., Bienstock, D. Adsorption of methane on dry coal at elevated pressure. Fuel. 53 (3), 152-162 (1974).
- Ranjith, P. G., Jasinge, D., Choi, S. K., Mehic, M., Shannon, B. The effect of CO2 saturation on mechanical properties of Australian black coal using acoustic emission. Fuel. 89 (8), 2110-2117 (2010).
- Viete, D. R., Ranjith, P. G. The effect of CO2, on the geomechanical and permeability behaviour of brown coal: Implications for coal seam CO2 sequestration. International Journal of Coal Geology. 66 (3), 204-216 (2006).
- Jiang, Y. D., Zhu, J., Zhao, Y. X., Liu, J. H., Wang, H. W. Constitutive equations of coal containing methane based on mixture theory. Journal of China Coal Society. 32 (11), 1132-1137 (2007).
- Xie, H. P., Gao, F., Zhou, H. W., Zuo, J. P. Fractal fracture and fragmentation in rocks. Journal of Seismology. 23 (4), 1-9 (2003).
- Miao, T. J., Yu, B. M., Duan, Y. G., Fang, Q. T. A fractal analysis of permeability for fractured rocks. International Journal of Heat & Mass Transfer. 81 (81), 75-80 (2015).
- Liu, R. C., Jiang, Y. J., Li, B., Wang, X. S. A fractal model for characterizing fluid flow in fractured rock masses based on randomly distributed rock fracture networks. Computers & Geotechnics. 65, 45-55 (2015).
- Pan, J. N., et al. Micro-pores and fractures of coals analysed by field emission scanning electron microscopy and fractal theory. Fuel. 164, 277-285 (2016).

