Low-Cost Gait Analysis for Behavioral Phenotyping of Mouse Models of Neuromuscular Disease
Summary
Footprint analysis is a low-cost alternative to digitized gait analysis programs for researchers quantifying movement abnormalities in mice. Because of its speed, simplicity, and longitudinal potential, it is ideal for behavioral phenotyping of mouse models.
Abstract
Measurement of animal locomotion is a common behavioral tool used to describe the phenotype of a given disease, injury, or drug model. The low-cost method of gait analysis demonstrated here is a simple but effective measure of gait abnormalities in murine models. Footprints are analyzed by painting a mouse’s feet with non-toxic washable paint and allowing the subject to walk through a tunnel on a sheet of paper. The design of the testing tunnel takes advantage of natural mouse behavior and their affinity for small dark places. The stride length, stride width, and toe spread of each mouse is easily measured using a ruler and a pencil. This is a well-established and reliable method, and it generates several metrics that are analogous to digital systems. This approach is sensitive enough to detect changes in stride early in phenotype presentation, and due to its non-invasive approach, it allows for testing of groups across life-span or phenotypic presentation.
Introduction
Locomotion requires complex neurological and musculoskeletal coordination, and deficits in a single aspect of motor pathways can produce observable gait abnormalities1,2. Gait analysis is a critical tool for researchers testing mouse models because it provides quantifiable behavioral data on how a given disease, injury, or drug impacts an animal’s movement3. However, digitized gait analysis requires the purchase of a treadmill, a camera, and associated software, which can be prohibitively expensive for researchers. Gait analysis is often used intermittently to track longitudinal changes in motor function, hence it may be difficult to justify the expenditure if sporadically used4. Although digitized analyses may provide more detailed gait metrics than simple footprint analysis, these more complex measures are not always necessary or relevant for the characterization of a behavioral phenotype5.
Here we present a low-cost manual footprint analysis method as a quick and sensitive alternative to digitized gait analysis programs6,7. Manual footprint analysis has been demonstrated to detect significant gait differences in a multitude of murine disease models4,7,8,9,10,11,12,13,14,15,16,17, and in at least one case, this low-cost method identified changes in gait that were not detected by a common digitized gait analysis program12. The total cost of materials is nominal, and it can be easily adapted to other rodent research models.
While there are many different gait metrics from which data can be drawn, the method we describe focuses on three specific metrics: stride length, stride width (a.k.a. “track width”), and toe spread. It is important to note that the parameters to be assessed should be determined on a model-by-model basis. This method of gait analysis is not designed to measure cognitive function, and it is not recommended for studies that require complex biomechanical measurements of gait16.
We present behavioral data from a cohort of pre- and post-symptomatic mice modeling X-linked Spinal and Bulbar Muscular Atrophy (SBMA), a neuromuscular disease characterized by motor neuron degeneration and muscle atrophy. These mice develop progressive deficits in gait that coincide with the onset of other disease-specific phenotypes. This demonstrates the validity and specificity of this method, and confirms that it can reliably discriminate between affected and non-affected animals.
The experimental mice in this study were 2.5 (pre-symptomatic) and 9-month-old (post-symptomatic) BAC fxAR121 transgenic mice on a C57BL/6 background (nexpt=12). This model was generated in our lab and has been fully characterized as a powerful mouse model of SBMA9. Non-transgenic littermates were used as controls (nctrl=8). SBMA is a sex-limited disease which fully manifests in males only, so male mice were used exclusively for this study. During planning stages, researchers must take into account the National Institutes of Health’s considerations of sex as a biological variable to determine group sizes and composition18.
Protocol
All testing conducted with mice was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Duke University. Personnel responsible for testing and scoring must be blinded to animal genotype or experimental condition until gait analysis and scoring of papers has been completed for the entire cohort.
1. Testing material preparation
- Conduct testing with a tunnel built from 3 pre-cut clear acrylic panels that are 0.375 inches thick. Assemble tunnel by gluing panels together with a sealant that specifically bonds acrylic and will not emit odors when dried.
- For standard C57BL/6 mice, use the following tunnel measurements: 2.5 in. wide, 3 in. high, and 13 in. long. Mice must be able to comfortably walk through the tunnel and take enough steps (>4) so that gait can be measured.
- Construct the goal chamber with pre-cut gray acrylic panels 0.375 inches thick, glued together with the same sealant as used on the tunnel. The interior measurements of the chamber are 4 in. wide, 4 in. long, and 3 in. tall. Match the opening of this chamber to the opening of the tunnel (2.5 in. wide x 3.0 in. tall). Because the mice naturally prefer darkened spaces to well-lit spaces, use material that is opaque and dark in color.
- Use paper for tracking steps that is thick and smooth (watercolor paper works well). Cut individual papers strips to be slightly wider and longer than the width and length of the tunnel. If using the tunnel dimensions described here, cut papers to 15 in. long by 3.5 in. wide.
- Use two contrasting colors (e.g., green and purple) of non-toxic washable water-based paint. Assign one color for hind-limbs, the second one for forelimbs. Mice will lick the remaining paint from their feet after testing, so the selected paint must be completely non-toxic.
- Use two round barrel paintbrushes, one for each paint color (~0.5 cm in diameter, tapered/pointed brush tip).
- Select a ruler with markings down to millimeters, and a caliper with measurements down to 0.1 mm. Pencil is recommended to write on the scoring papers.
- Optional: For animals with high anxiety or low motivation, provide a behavioral incentive in the goal chamber. This can include small amounts of sterilized sunflower seeds (placed in the home cage 2 days prior to testing to allow habituation). On the day of testing, place sunflower seeds inside the goal chamber to encourage mice to walk through without stopping.
2. Data collection
- If testing is performed in a separate room, acclimate the mice to the new room for 30 minutes and then start the behavioral assays. Additionally, because mice are naturally nocturnal, ensure all mice are fully awake and alert for at least 5 minutes before testing.
- Prepare the testing setup by positioning the tunnel over the paper and marking the paper with mouse ID and testing date. Position the goal chamber at the end of the tunnel, connecting both open ends. Add sunflower seeds at the end of the tunnel (inside the goal chamber) for motivation if needed.
- Remove the mouse to be tested from its cage and grip it firmly by its scruff, making sure to grip the tail to stabilize movement of its hind limbs.
- Paint forepaws so the entire underside of all toes and the center of the foot are fully covered in paint. Repeat this with a contrasting color of paint on its hind paws. Wipe off any paint that the mouse gets on other parts of its body with a clean damp cloth to prevent smudges that may interfere with data collection.
NOTE: Mouse handling must be performed by experienced researchers to minimize animal stress. - Place the mouse at the start of the tunnel and allow it to walk all the way into the goal chamber, and then retrieve the mouse, gently wipe off its feet with a water-dampened cloth, and return it to its home cage.
- Allow paper with footprints to dry fully before scoring. Wipe down the testing area and tunnel with ethanol or an equivalent cleaning solution in between each animal.
3. Scoring criteria
- Use steps that are consistently spaced with clear, non-smudged footprints for scoring. Figure 1B is a good example of a footprint sequence that can be scored. In order to generate sufficient scoring data, there must be at least 2 consecutive steps from each foot, but 4-6 steps per foot is recommended. Do not include the first and last footprints on the paper, as they are unlikely to represent normal gait because the mouse is changing its walking speed.
- Use stride length, stride width, and toe spread as three different measures of gait that can be analyzed using this method.
NOTE: Stride length and width require clear sequential prints where the forefoot region is well defined in paint. Toe spread does not require sequential prints for scoring, only clear prints of the first and last toes on a single foot. However, if a given footprint is not included in measurements of stride length or width, it cannot not be scored for toe spread. All three measures are assessed in centimeters.- Define stride length as the distance between two sequential footprints created by the same foot (i.e., one stride) (Figure 1A, 1B).
- With a pencil, draw a 2-4 mm circle around the fore-foot region of both forelimb footprints (identified by assigned color above) in a single stride and draw a line between them using a ruler.
- Record the distance between two prints from the middle of each circle (i.e. center of each foot pad) as Right-Fore 1 (RF1) or Left-Fore 1 (LF1).
- Repeat for all steps that can be scored (RF2, LF2, RF3, LF3 and so on).
- Repeat for right and left hind-limb footprints.
- Average all individual recorded stride distances for each limb. For statistical analysis, individual cohort members can be averaged together.
- Define stride width as the measure of distance between left and right forelimbs or hind-limbs (Figure 1A, 1B).
- To assess this distance, draw and measure a line from the circled forefoot region of one hind-limb that intersects perpendicularly with the line for stride length on the contralateral hind-limb.
- Repeat this for all hind-limb prints that can be scored, and then average the measurements. The method of calculation for stride width is the same for fore- and hind-limbs.
- Define toe spread as the distance between the first and last toes on a single fore- or hind-limb footprint (Figure 1A, 1B).
- Use calipers to measure the distance between the tip of the first toe print and the tip of the last toe print.
- Repeat for all hind-limb prints that can be scored and average the measurements. The method of calculation for toe spread is the same for fore- and hind-limbs.
- Define stride length as the distance between two sequential footprints created by the same foot (i.e., one stride) (Figure 1A, 1B).
- If the paper cannot be scored, allow the animal to rest for 10 minutes before trying again.
Representative Results
With sufficient numbers of animals, this procedure is capable of detecting gait differences between mouse genotypes, within the same strain over time. Figure 1B shows representative traces of footprint images collected in our lab, using a mouse model of X-linked Spinal and Bulbar Muscular Atrophy (SBMA), a neurodegenerative disorder affecting lower motor neurons and skeletal muscle. We have previously reported that male BAC fxAR121 transgenic mice develop significant weight loss, impairments in grip strength, and shortened stride length at post-symptomatic ages when compared to non-transgenic littermate controls9.
Here we present gait analysis results from a cohort of pre-symptomatic (2.5 months of age) and post-symptomatic (9 months of age) BAC fxAR121 transgenic and littermate control male mice (Figure 2). Prior to disease onset, BAC fxAR121 transgenic mice display similar stride length, stride width, and toe spread compared to their littermate non-transgenic controls. After disease onset, BAC fxAR121 transgenic mice display significantly shorter stride length (pforelimb= 0.001, phind-limb= 0.009) (Figure 2A). Similar longitudinal analysis revealed no differences in stride width at either age tested (p2.5months=0.709, p9 months=0.204) (Figure 2B). Post-symptomatic BAC fxAR121 transgenic mice also have significantly narrower hind toe spread (p=0.01) than age-matched littermate controls (Figure 2C). BAC fxAR121 mice model a neuromuscular disease that primarily affects hind-limbs, so detailed measures of forelimb gait were not collected. We encourage researchers using this gait analysis method to consider the phenotype of their mouse models and choose forelimb or hind-limb gait metrics accordingly.
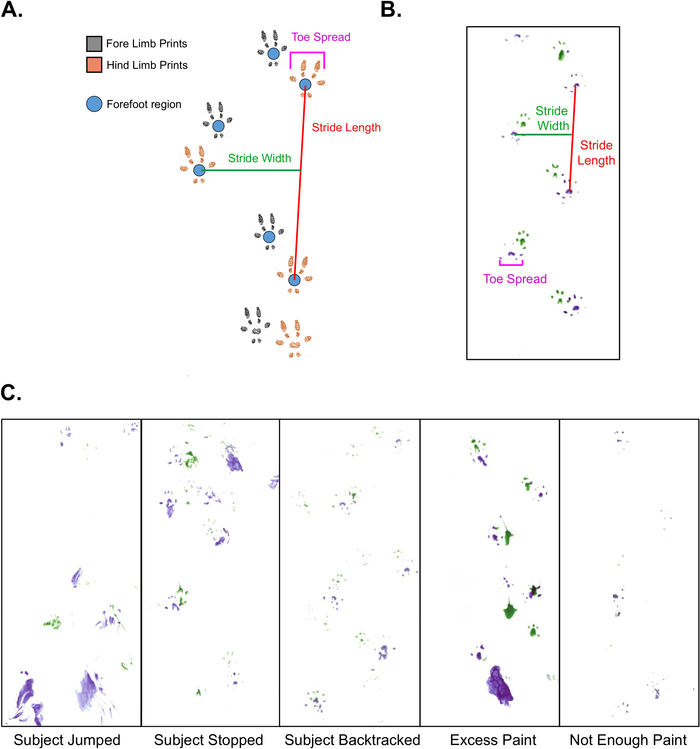
Figure 1: Gait Analysis Measures and Troubleshooting.
A. Schematic representation of gait analysis on mice, depicting stride length, stride width, and toe spread information. B. Representative example of a gait analysis footprint sequence that can be scored, depicting measurement of all three parameters. C. Representative examples of problematic gait analysis footprint sequences that cannot be scored. Please click here to view a larger version of this figure.
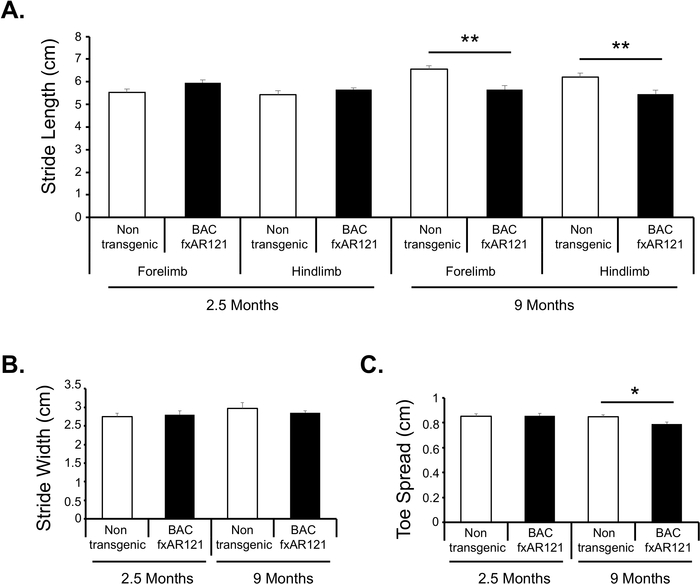
Figure 2: SBMA BAC fxAR121 transgenic mice exhibit a progressive, neurodegenerative gait phenotype that can be detected via gait analysis.
A. Despite no differences at pre-symptomatic ages (2.5 months, nctl=11, nexpt=12), BAC fxAR121 mice develop significantly reduced stride length compared to their non-transgenic littermate controls at post-symptomatic stages (9 months, nctl=8, nexpt=12). B. No changes were detected in stride width at either age. C. Symptomatic SBMA BAC fxAR121 transgenic mice display significantly reduced hind limb toe spread compared to non-transgenic littermate controls. N= 8-12/group. ANOVA with post-hoc Tukey test * p < 0.05, ** p < 0.01. Error bars represent SEM. Please click here to view a larger version of this figure.
Discussion
Using the low-cost gait analysis method described above, we show successful identification of several parameters of gait dysfunction at post-symptomatic ages in the BAC fxAR121 mouse model of SBMA. Decreases in stride length are consistent with prior SBMA studies of mouse models and human patients9. We also show for the first time that there are significant differences in hind-limb toe spread in symptomatic SBMA mice compared to non-transgenic littermate controls. Interestingly, decreases in hind toe spreading can be caused by weakness in paw extensor muscles, tightness in paw flexor muscles, or poor nerve innervation2,19, which is also consistent with the etiology of SBMA.
The mice should readily run to the goal chamber due to their natural behavioral preference for small dark spaces, but some mice may not continuously move through the tunnel. If a mouse jumps, stops, or turns around within the tunnel (see examples in Figure 1C), repeat the assay after a rest period on a new scoring paper. The results may be salvageable if a mouse stops at the very beginning of the tunnel since it can often be gently prodded into running to the goal box.
Applying too much or too little paint to a mouse’s feet can produce unusable results. Excess paint can lead to smudged or distorted prints, while insufficient paint can produce faint or unidentifiable prints (Figure 1C). In either case, repeat the assay on a clean scoring paper to prevent inaccurate measurements.
Very young mice (<3 months old) are more likely to jump forward in the tunnel, whereas older (>8 months old) or very phenotypic mice are more likely to stop or resist forward movement entirely. Adding a behavioral incentive (sunflower seeds) in the goal chamber can help decrease the frequency of problematic behaviors by encouraging unmotivated mice to traverse the tunnel without stopping.
Tunnel dimensions should reflect the dimensions of the subject; if using mice that are significantly larger or smaller than an average lab mouse (due to age, diet, or genetic mutations), we recommend changing the tunnel and goal chamber dimensions to match the animal’s size. In the tunnel, the mice should be able to walk comfortably in a straight line, but should have some difficulty turning around to discourage this behavior. The goal chamber should match the height of the tunnel and mice should fit comfortably inside the chamber.
Researchers who use the toe-clipping method of identification for their mice may not be able to collect data on toe spread, but other measures of gait like stride length and stride width can still be collected. Toe-clipping does not significantly impact gait in mice as long as no more than two toes are clipped per mouse20.
This gait analysis method does not reflect cognitive function, so it should not be used as a measure of cognition. Others intending to use this method should consider the neuromuscular groups affected in their mouse model, and then choose fore- or hind limb metrics accordingly. This method of gait analysis is not recommended for researchers who study pain responses requiring footpad injections, or for studies requiring biomechanical measures of locomotion that cannot be described by footprints alone, like temporal measurements of limb motion or joint rotation21.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank A.M. for animal identification assistance. This work was supported by grants from the US National Institutes of Health (R01 7 RF1 AG057264 to A.R.L.S. and C.J.C. and R01 NS100023 to A.R.L.S) and the Muscular Dystrophy Association (Basic Research Grant to A.R.L.S., Development Grant to C.J.C.).
Materials
| Caliper | n/a | n/a | must have markings down to 0.1 mm |
| Craft Glue | E6000 | n/a | |
| Footprint Paint (Tempera Paint) | Artmind | n/a | must be non-toxic |
| Round Barrel Paintbrushes | Symply Simmons | n/a | 0.5 cm diameter |
| Ruler | n/a | n/a | must have markings down to millimeters |
| Scoring Paper (Watercolor Pads) | Canson | n/a | cut to size |
| Tunnel and Goal Chamber | Interstate Plastics | n/a | cut to size |
References
- Clarke, K. A., Still, J. Development and consistency of gait in the mouse. Physiology & Behavior. 73 (1-2), 159-164 (2001).
- Mendes, C. S., et al. Quantification of gait parameters in freely walking rodents. BMC Biology. 13, 50 (2015).
- Carter, R. J., Morton, J., Dunnett, S. B. Motor coordination and balance in rodents. Current Protocols in Neuroscience. , (2001).
- Tillerson, J. L., Caudle, W. M., Reveron, M. E., Miller, G. W. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 119 (3), 899-911 (2003).
- Pallier, P. N., Drew, C. J., Morton, A. J. The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington’s disease are task- and protocol-dependent: influence of non-motor factors on locomotor function. Brain Research Bulletin. 78 (6), 347-355 (2009).
- Sugimoto, H., Kawakami, K. Low-cost Protocol of Footprint Analysis and Hanging Box Test for Mice Applied the Chronic Restraint Stress. Journal of Visualized Experiments. (143), (2019).
- Carter, R. J., et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. Journal of Neuroscience. 19 (8), 3248-3257 (1999).
- Barlow, C., et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 86 (1), 159-171 (1996).
- Cortes, C. J., et al. Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron. 82 (2), 295-307 (2014).
- D’Hooge, R., et al. Neuromotor alterations and cerebellar deficits in aged arylsulfatase A-deficient transgenic mice. Neuroscience Letters. 273 (2), 93-96 (1999).
- Fernagut, P. O., Diguet, E., Labattu, B., Tison, F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. Journal of Neuroscience Methods. 113 (2), 123-130 (2002).
- Guillot, T. S., Asress, S. A., Richardson, J. R., Glass, J. D., Miller, G. W. Treadmill gait analysis does not detect motor deficits in animal models of Parkinson’s disease or amyotrophic lateral sclerosis. Journal of Motor Behavior. 40 (6), 568-577 (2008).
- Harper, S. Q., et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 102 (16), 5820-5825 (2005).
- Lin, C. H., et al. Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Human Molecular Genetics. 10 (2), 137-144 (2001).
- Sopher, B. L., et al. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 41 (5), 687-699 (2004).
- Tillerson, J. L., Caudle, W. M., Reveron, M. E., Miller, G. W. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Experimental Neurology. 178 (1), 80-90 (2002).
- Wheeler, V. C., et al. Early phenotypes that presage late-onset neurodegenerative disease allow testing of modifiers in Hdh CAG knock-in mice. Human Molecular Genetics. 11 (6), 633-640 (2002).
- Clayton, J. A., Collins, F. S. Policy: NIH to balance sex in cell and animal studies. Nature. 509 (7500), 282-283 (2014).
- Maricelli, J. W., Lu, Q. L., Lin, D. C., Rodgers, B. D. Trendelenburg-Like Gait, Instability and Altered Step Patterns in a Mouse Model for Limb Girdle Muscular Dystrophy 2i. PLoS One. 11 (9), e0161984 (2016).
- Castelhano-Carlos, M. J., Sousa, N., Ohl, F., Baumans, V. Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab Animals. 44 (2), 88-103 (2010).
- Lakes, E. H., Allen, K. D. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage. 24 (11), 1837-1849 (2016).

