Transcranial Direct Current Stimulation for Online Gamers
Summary
We present a protocol and a feasibility study for applying transcranial direct current stimulation (tDCS) and neuroimaging assessment in online gamers.
Abstract
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that applies a weak electric current to the scalp to modulate neuronal membrane potentials. Compared to other brain stimulation methods, tDCS is relatively safe, simple, and inexpensive to administer.
Since excessive online gaming can negatively affect mental health and daily functioning, developing treatment options for gamers is necessary. Although tDCS over the dorsolateral prefrontal cortex (DLPFC) has demonstrated promising results for various addictions, it has not been tested in gamers. This paper describes a protocol and a feasibility study for applying repeated tDCS over the DLPFC and neuroimaging to examine the underlying neural correlates in gamers.
At baseline, individuals who play online games report average weekly hours spent on games, complete questionnaires on addiction symptoms and self-control, and undergo brain 18F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET). The tDCS protocol consists of 12 sessions over the DLPFC for 4 weeks (anode F3/cathode F4, 2 mA for 30 min per session). Then, a follow-up is conducted using the same protocol as the baseline. Individuals who do not play online games receive only baseline FDG-PET scans without tDCS. Changes of clinical characteristics and asymmetry of regional cerebral metabolic rate of glucose (rCMRglu) in the DLPFC are examined in gamers. In addition, asymmetry of rCMRglu is compared between gamers and non-gamers at baseline.
In our experiment, 15 gamers received tDCS sessions and completed baseline and follow-up scans. Ten non-gamers underwent FDG-PET scans at the baseline. The tDCS reduced addiction symptoms, time spent on games, and increased self-control. Moreover, abnormal asymmetry of rCMRglu in the DLPFC at baseline was alleviated after tDCS.
The current protocol may be useful for assessing treatment efficacy of tDCS and its underlying brain changes in gamers. Further randomized sham-controlled studies are warranted. Moreover, the protocol can be applied to other neurological and psychiatric disorders.
Introduction
In recent years, increasing attention has been paid to excessive online game use since its associations with negative impact on mental health and daily functioning as well as with internet gaming disorder (IGD) have been reported1,2,3. Although several treatment strategies including pharmacotherapy and cognitive-behavioral therapy have been evaluated, evidence for their effectiveness is limited4.
Previous studies have suggested that IGD may share clinical and neurobiological similarities with other behavioral addictions and substance use disorders5,6. It has been reported that the dorsolateral prefrontal cortex (DLPFC) is closely involved in the pathophysiology of substance and behavioral addiction such as craving7, impulse control8, decision making9, and cognitive flexibility10. Several neuroimaging studies on IGD have reported structural and functional impairments in the DLPFC6. In particular, structural neuroimaging studies revealed a reduction in gray matter density in the DLPFC11,12 and a functional magnetic resonance imaging (fMRI) study found an altered cued-induced activity in the DLPFC of patients with IGD13. In addition, functional asymmetry of the brain may contribute to impulsivity and craving in addictions including IGD. For instance, cue-induced craving for online gaming could be related to right prefrontal activations14. However, alterations of regional cerebral metabolic rate of glucose (rCMRglu) associated with excessive online game use or IGD remain to be further investigated compared to other brain deficits15.
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that applies a weak electric current (1-2 mA) through electrodes attached to the scalp to modulate neuronal membrane potentials. Generally, the cortical excitability is increased under the anode electrode and decreased under the cathode electrode16. tDCS has become a popular method because it is simple, inexpensive, and safe to administer compared to other brain stimulation techniques such as transcranial magnetic stimulation (TMS) that uses a magnetic pulse to generate an electrical current in the brain tissue under the coil. According to a recent review, the use of conventional tDCS protocols has not produced any serious adverse effects or irreversible injury and is associated with only mild and transient itching or tingling sensation under the stimulation area17.
Several studies have demonstrated favorable results of tDCS18,19,20 and repetitive TMS21,22 over the DLPFC for treating behavioral and substance addiction. However, further studies are needed to investigate the effects of brain stimulation techniques on online game use and the underlying brain changes.
The aim of this study is to present a protocol for applying repeated sessions of tDCS over the DLPFC and neuroimaging to examine the underlying neural correlates in gamers using 18F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET), as well as to assess its feasibility. Specifically, we focused on changes in addiction symptoms, average time spent on games, self-control, and asymmetry of rCMRglu in the DLPFC.
Protocol
All experimental procedures presented in this protocol have been approved by the Institutional Review Board and are in accordance with the Declaration of Helsinki.
1. Research Participants
- Recruit individuals who report that they play online games (the gamer group) and those who report that they do not play online games (the non-gamer group).
NOTE: Here, we included individuals with two or more IGD symptoms according to the Diagnostic and Statistical Manual of Mental Disorders-523 or those who play games at least one hour per day on average in the gamer group. The non-gamer group undergoes only baseline brain FDG-PET scans to compare rCMRglu with the gamer group and does not receive tDCS sessions. - For both groups, exclude individuals with (a) major medical, psychiatric, or neurological disorders, (b) history of traumatic brain injury, (c) history of alcohol or other substance abuse or dependence, (d) use of psychotropic medications, or (e) any contraindications for tDCS such as severe headache, metal in the head, history of seizure, epilepsy, or brain surgery, or any lesions or other medical problems on the skin where tDCS electrodes will be attached.
- Explain to each participant the aim of the study, the main experimental procedures, and any potential risks associated with participating in the study. After answering any questions, obtain written consent.
2. Baseline Assessment
- Evaluate clinical characteristics using the following questionnaires: Internet Addiction Test (IAT)24 and Brief Self Control Scale (BSCS)25. In addition, ask participants to report average weekly hours spent playing games.
NOTE: The word "Internet" in the IAT is replaced with "online games" to assess severity of online game addiction. - Perform brain FDG-PET scans.
- Inject participants with 185 – 222 MBq of FDG and have participants rest for 45 min of an uptake period during which they are awake and resting in supine position in a dark and quiet room with their eyes closed.
- Conduct brain FDG-PET scans to acquire transaxial emission images and CT images using a PET-CT scanner in about 15 min. Apply attenuation correction, standard filtering, and standard reconstruction techniques.
3. Application of tDCS
- Within a week after the baseline assessment, apply tDCS to participants. Prepare tDCS sessions with following materials: a tDCS device, wet wipes, saline solution, two sponge electrodes (6 cm in diameter), a cable, a headcap, and a headband.
- Have the participant sit on a chair.
- Set the stimulation parameters for the tDCS device: 2 mA for 30 min (current density = 0.07 mA/cm2). Set the current so it ramps up to 2.0 mA over 30 s, remains at 2.0 mA for 29 min, and ramps down to 0 mA over the last 30 s.
- Put the headcap (the International 10-20 system) on the participant’s head and mark the left dorsolateral prefrontal cortex (F3) and the right dorsolateral prefrontal cortex (F4). Then, remove the headcap from the participant's head.
- Place two sponge electrodes in the rubber holders of the headband and soak them with saline solution.
- Remove any makeup, dirt, or sweat on the scalp where the electrodes will be applied.
- Place the headband over the marking points by putting the anodal electrode over the left DLPFC and the cathodal electrode over the right DLPFC.
- Connect the electrodes to the tDCS device using the cable and turn on the device.
- Ask the participant to report any adverse effects during or after the tDCS session.
- At the end of the 30 min of stimulation, turn off the device and remove the electrodes from the participant.
- Administer a total of 12 tDCS sessions (3 times per week for 4 weeks).
4. Follow-up Assessment
- Perform the follow-up assessment within a week after the last tDCS session using the same protocol as the baseline assessment.
5. Data Analysis
- Use an appropriate software package to preprocess the PET images (e.g., Statistical Parametric Mapping 12).
- Convert DICOM files to NIFTI files.
- Spatially normalize all PET images to the standard PET template.
- Create binary masks for the left and right DLPFC (e.g., WFU PickAtlas toolbox). The DLPFC is defined by the middle frontal gyrus in the Automated Anatomical Labeling atlas.
- Extract rCMRglu of the left and right DLPFC using the masks (e.g., MarsBaR toolbox). The rCMRglu is normalized to global mean uptake using proportional scaling.
- Calculate asymmetry index (AI) of rCMRglu in the DLPFC as (rCMRglu right – rCMRglu left) / [(rCMRglu right + rCMRglu left) / 2] × 100. Positive AI indicates right-greater-than-left asymmetry of glucose metabolism.
Representative Results
A total of 15 gamers (Table 1) and 10 non-gamers were recruited. The mean age of the gamer group (21.3 ± 1.4) was significantly lower than that of the non-gamer group (28.8 ± 7.5) (t = -3.81, p < 0.001). There were 8 men in the gamer group and 6 men in the non-gamer group (χ2 = 0.11, p = 0.74).
Behavioral results using linear mixed models indicate that the tDCS sessions successfully lowered the IAT score (z = -4.29, p < 0.001), weekly hours spent playing games (z = -2.41, p = 0.02), and improved the BSCS score (z = 2.80, p = 0.01) in the gamer group (Table 1 and Figure 1). No adverse events were reported during the tDCS sessions.
A significant negative correlation was found between changes in the IAT score and those in the BSCS score in gamers (r = -0.77, p < 0.001) (Figure 2). In addition, a decrease of the time spent on games was associated with an increase of the BSCS score in the gamer group at a marginal level (r = -0.50, p = 0.06).
PET analysis revealed that the AI of the DLPFC was significantly different between the gamer group and the non-gamer group (t = 3.53, p = 0.002) at baseline (Figure 3). Despite the significant difference in age between the two groups, rCMRglu may not be affected by aging in young adults26. Following the tDCS sessions, the AI of the DLPFC in the gamer group was significantly decreased (z = -2.11, p = 0.04) (Figure 3).
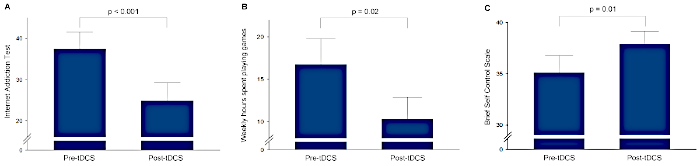
Figure 1: Changes in clinical characteristics of the gamer group. (A) Internet Addiction Test scores, (B) weekly hours spent playing games, and (C) Brief Self Control Scale scores before and after transcranial direct current stimulation (tDCS). Error bars indicate standard errors. Please click here to view a larger version of this figure.
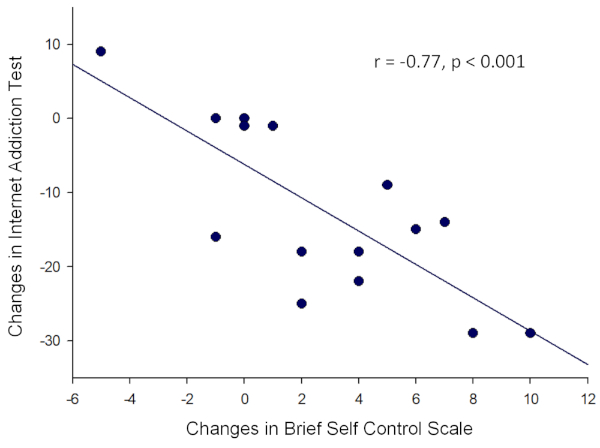
Figure 2: A significant negative correlation between changes in the Brief Self Control Scale and those in the Internet Addiction Test in the gamer group. Please click here to view a larger version of this figure.
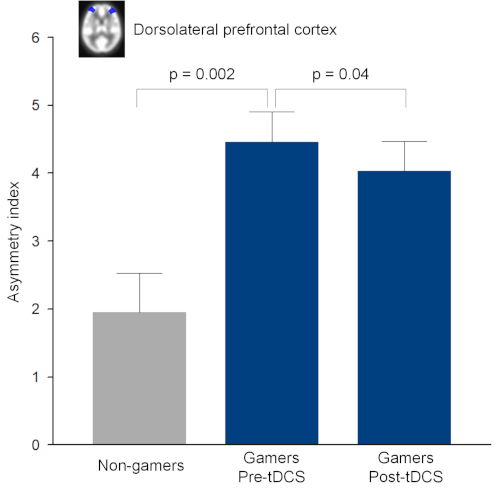
Figure 3: Asymmetry index of regional cerebral metabolic rate of glucose (rCMRglu) in the dorsolateral prefrontal cortex. Asymmetry index was defined as (rCMRglu right – rCMRglu left) / [(rCMRglu right + rCMRglu left) / 2] × 100. Error bars indicate standard errors. This figure has been modified from Lee et al.27. tDCS, transcranial direct current stimulation. Please click here to view a larger version of this figure.
| Characteristics | Pre-tDCS | Post-tDCS | Test statistics |
| (mean ± SD or n) | (mean ± SD) | ||
| Age | 21.3 ± 1.4 | ||
| Sex (male/female) | 8/7 | ||
| Internet Addiction Test | 37.5 ± 15.7 | 24.9 ± 16.7 | z = -4.29, p < 0.001 |
| Weekly hours spent playing games | 16.8 ± 11.7 | 10.3 ± 9.9 | z = -2.41, p = 0.02 |
| Brief Self Control Scale | 35.1 ± 6.4 | 37.9 ± 4.7 | z = 2.80, p = 0.01 |
| Note: SD = standard deviation; tDCS = transcranial direct current stimulation. | |||
| The gamers received a total of 12 tDCS sessions over the dorsolateral prefrontal cortex (2 mA for 30 min per session, 3 times per week for 4 weeks). | |||
Table 1: Demographic and clinical characteristics of gamers. The gamers received a total of 12 tDCS sessions over the dorsolateral prefrontal cortex (2 mA for 30 min per session, 3 times per week for 4 weeks).
Discussion
We have presented a tDCS and neuroimaging protocol for online gamers and assessed its feasibility. The results demonstrated that repeated sessions of tDCS over the DLPFC reduced online game addiction symptoms and average time spent on games and increased self-control. An increase in self-control was correlated with a decrease in addiction symptoms. Moreover, the abnormal asymmetry of rCMRglu in the DLPFC where the right side was greater than the left side was improved after the tDCS sessions in the gamer group. These results may suggest the feasibility of tDCS for reducing online game use. However, since our experiment did not have a sham control group and the participants were aware of the aim of the study at the time of recruitment, further randomized sham-controlled studies are warranted to evaluate the efficacy of tDCS in online gamers. In addition, the long-term effects of tDCS should also be investigated.
Although we defined our inclusion criteria broadly to include both normal gamers and individuals with IGD, it may also be informative to only include IGD patients as study participants in future studies. Otherwise, the effects of tDCS can be compared between normal gamers and IGD patients in larger samples. In addition, any contraindications for tDCS such as severe headache, metal in the head, history of seizure or epilepsy, and lesions on the scalp should be carefully screened for safety.
Using appropriate tDCS parameters is also a critical step for the current protocol. In general, higher current intensity (or current density) and longer stimulation duration are associated with stronger and longer-lasting effects. In most studies, a current intensity and a stimulation duration range from 1 to 2 mA and from 10 to 30 min, respectively28. Although a single session of tDCS with current up to 4 mA was safe and tolerable in stroke patients29, 2 mA is recommended as a safety threshold for human studies30. In addition, some studies reported that an increase in stimulation duration alters the effects of polarity, suggesting that the effects of current intensity and stimulation duration may not be necessarily linear30.
Electrode size influences the current density and the spatial focality. Since smaller electrodes may be associated with not only larger current density but also shunting effect31, electrode sizes between 25 and 35 cm2 are commonly used30. With regard to the stimulation polarity, a previous tDCS study in alcohol dependence reported that both anodal F3/cathodal F4 and anodal F4/cathodal F3 montages significantly reduced alcohol craving18. Thus, the effects of these two montages may also be compared in future tDCS studies in gamers.
For cumulative and long-lasting effects, we applied a total of 12 tDCS sessions over 4 weeks. This schedule consists of a relatively large number of sessions over long period compared to previous tDCS studies32. Recently, remotely supervised portable tDCS has been developed for repeated self-administration at home and would be convenient and time-saving for participants33,34. Since anatomical variability including the head size, skull thickness, and morphologies of cortical gyri and sulci may influence the current distribution, computational models of tDCS can be applied to predict the current flow and to optimize and individualize the electrode montages35.
For the sham tDCS protocol, the current may be set to ramp up to 2 mA over 30 s and ramp down to 0 mA over next 30 s. With this sham protocol, participants have difficulties distinguishing between active and sham stimulation because they feel the same sensations under the electrodes as in active tDCS sessions in the beginning. This initial and short stimulation has been proven to be a reliable technique for sham tDCS36 and to be one of the advantages of tDCS over other noninvasive neuromodulation techniques. Further research is warranted to optimize and standardize various tDCS parameters for gamers.
Regarding the protocol for assessing addiction severity for games, other scales have been developed and validated37, and therefore can be used instead of IAT. In the imaging analysis, although we focused on asymmetry of rCMRglu in the target site, analyzing whole-brain voxel-wise changes in rCMRglu may also be informative. Furthermore, other imaging modalities such as fMRI can be used to investigate changes of the brain induced by tDCS. For instance, an fMRI study reported that bupropion treatment decreased cue-induced activity in the DLPFC in patients with Internet video game addiction38.
Our protocol showed the feasibility and safety for reducing addiction severity and online game use using tDCS and for evaluating the underlying neural correlates. With appropriate modifications, it could be applicable to other neurological and psychiatric disorders.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2015M3C7A1064832, 2015M3C7A1028373, 2018M3A6A3058651) and by the National Institutes of Health (NIHNIMH 1R01MH111896, NIH-NINDS 1R01NS101362).
Materials
| Discovery STE PET/CT Imaging System | GE Healthcare | ||
| MarsBaR region of interest toolbox for SPM | Matthew Brett | Neuroimaging analysis software; http://marsbar.sourceforge.net/ | |
| Statistical Parametric Mapping 12 | Wellcome Centre for Human Neuroimaging | Neuroimaging analysis software; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ | |
| Transcranial direct current stimulation device | Ybrain | YDS-301N | |
| WFU_PickAtlas | ANSIR Laboratory, Wake Forest University School of Medicine | Neuroimaging analysis software; https://www.nitrc.org/projects/wfu_pickatlas/ | |
References
- Chen, Y. F., Peng, S. S. University students’ Internet use and its relationships with academic performance, interpersonal relationships, psychosocial adjustment, and self-evaluation. CyberPsychology & Behavior. 11 (4), 467-469 (2008).
- Ho, R. C., et al. The association between internet addiction and psychiatric co-morbidity: a meta-analysis. BMC Psychiatry. 14, 183 (2014).
- Pawlikowski, M., Brand, M. Excessive Internet gaming and decision making: do excessive World of Warcraft players have problems in decision making under risky conditions. Psychiatry Research. 188 (3), 428-433 (2011).
- Zajac, K., Ginley, M. K., Chang, R., Petry, N. M. Treatments for Internet gaming disorder and Internet addiction: A systematic review. Psychology of Addictive Behaviors. 31 (8), 979-994 (2017).
- Weinstein, A. M. An Update Overview on Brain Imaging Studies of Internet Gaming Disorder. Frontiers in Psychiatry. 8, 185 (2017).
- Park, B., Han, D. H., Roh, S. Neurobiological findings related to Internet use disorders. Psychiatry and Clinical Neurosciences. 71 (7), 467-478 (2017).
- Kober, H., et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 107 (33), 14811-14816 (2010).
- Li, C. S., Luo, X., Yan, P., Bergquist, K., Sinha, R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcoholism: Clinical and Experimental Research. 33 (4), 740-750 (2009).
- Fecteau, S., Fregni, F., Boggio, P. S., Camprodon, J. A., Pascual-Leone, A. Neuromodulation of decision-making in the addictive brain. Substance Use & Misuse. 45 (11), 1766-1786 (2010).
- Fujimoto, A., et al. Deficit of state-dependent risk attitude modulation in gambling disorder. Translational Psychiatry. 7 (4), 1085 (2017).
- Choi, J., et al. Structural alterations in the prefrontal cortex mediate the relationship between Internet gaming disorder and depressed mood. Scientific Reports. 7 (1), 1245 (2017).
- Yuan, K., et al. Microstructure abnormalities in adolescents with internet addiction disorder. PLoS One. 6 (6), 20708 (2011).
- Ko, C. H., et al. Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatric Research. 43 (7), 739-747 (2009).
- Gordon, H. W. Laterality of Brain Activation for Risk Factors of Addiction. Current Drug Abuse Reviews. 9 (1), 1-18 (2016).
- Tian, M., et al. PET imaging reveals brain functional changes in internet gaming disorder. European Journal of Nuclear Medicine and Molecular Imaging. 41 (7), 1388-1397 (2014).
- Nitsche, M. A., Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 527, 633-639 (2000).
- Bikson, M., et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulation. 9 (5), 641-661 (2016).
- Boggio, P. S., et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug and Alcohol Dependence. 92 (1-3), 55-60 (2008).
- Martinotti, G., et al. Gambling disorder and bilateral transcranial direct current stimulation: A case report. Journal of Behavioral Addictions. 7 (3), 834-837 (2018).
- Martinotti, G., et al. Transcranial Direct Current Stimulation Reduces Craving in Substance Use Disorders: A Double-blind, Placebo-Controlled Study. Journal of ECT. , (2019).
- Gay, A., et al. A single session of repetitive transcranial magnetic stimulation of the prefrontal cortex reduces cue-induced craving in patients with gambling disorder. European Psychiatry. 41, 68-74 (2017).
- Pettorruso, M., et al. Dopaminergic and clinical correlates of high-frequency repetitive transcranial magnetic stimulation in gambling addiction: a SPECT case study. Addictive Behaviors. 93, 246-249 (2019).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Association. , (2013).
- Young, K. S. Internet addiction: the emergence of a new clinical disorder. CyberPsychology & Behavior. 1 (3), 237-244 (1998).
- Tangney, J. P., Baumeister, R. F., Boone, A. L. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality. 72 (2), 271-324 (2004).
- Bentourkia, M., et al. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. Journal of the Neurological Sciences. 181 (1-2), 19-28 (2000).
- Lee, S. H., et al. Transcranial direct current stimulation for online gamers: A prospective single-arm feasibility study. Journal of Behavioral Addictions. 7 (4), 1166-1170 (2018).
- Bikson, M., et al. Response to letter to the editor: Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimulation. 10 (5), 986-987 (2017).
- Chhatbar, P. Y., et al. Safety and tolerability of transcranial direct current stimulation to stroke patients – A phase I current escalation study. Brain Stimulation. 10 (3), 553-559 (2017).
- Thair, H., Holloway, A. L., Newport, R., Smith, A. D. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Frontiers in Neuroscience. 11, 641 (2017).
- Wagner, T., et al. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 35 (3), 1113-1124 (2007).
- Lefaucheur, J. P., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 128 (1), 56-92 (2017).
- Carvalho, F., et al. Home-Based Transcranial Direct Current Stimulation Device Development: An Updated Protocol Used at Home in Healthy Subjects and Fibromyalgia Patients. Journal of Visualized Experiments. (137), (2018).
- Shaw, M. T., et al. Remotely Supervised Transcranial Direct Current Stimulation: An Update on Safety and Tolerability. Journal of Visualized Experiments. (128), (2017).
- Bikson, M., Rahman, A., Datta, A. Computational models of transcranial direct current stimulation. Clinical EEG and Neuroscience. 43 (3), 176-183 (2012).
- Gandiga, P. C., Hummel, F. C., Cohen, L. G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 117 (4), 845-850 (2006).
- Cho, H., et al. Development of the Internet addiction scale based on the Internet Gaming Disorder criteria suggested in DSM-5. Addictive Behaviors. 39 (9), 1361-1366 (2014).
- Han, D. H., Hwang, J. W., Renshaw, P. F. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. Experimental and Clinical Psychopharmacology. 18 (4), 297-304 (2010).

