In situ FTIR Spectroscopy as a Tool for Investigation of Gas/Solid Interaction: Water-Enhanced CO2 Adsorption in UiO-66 Metal-Organic Framework
Summary
The use of FTIR spectroscopy for investigation of the surface properties of polycrystalline solids is described. Preparation of sample pellets, activation procedures, characterization with probe molecules and model studies of CO2 adsorption are discussed.
Abstract
In situ infrared spectroscopy is an inexpensive, highly sensitive, and selective valuable tool to investigate the interaction of polycrystalline solids with adsorbates. Vibrational spectra provide information on the chemical nature of adsorbed species and their structure. Thus, they are very useful for obtaining molecular level understanding of surface species. The IR spectrum of the sample itself gives some direct information about the material. General conclusions can be drawn concerning hydroxyl groups, some stable surface species and impurities. However, the spectrum of the sample is "blind" with respect to the presence of coordinatively unsaturated ions and gives rather poor information about the acidity of surface hydroxyls, species decisive for the adsorption and catalytic properties of the materials. Furthermore, no discrimination between bulk and surface species can be made. These problems are solved by the use of probe molecules, substances that interact specifically with the surface; the alteration of some spectral features of these molecules as a result of adsorption provides valuable information about the nature, properties, location, concentration, etc., of the surface sites.
The experimental protocol for in-situ IR studies of gas/sample interaction includes preparation of a sample pellet, activation of the material, initial spectral characterization through the analysis of the background spectra, characterization by probe molecules, and study of the interaction with a particular set of gas mixtures. In this paper we investigate a zirconium terephthalate metal organic framework, Zr6O4(OH)4(BDC)6 (BDC = benzene-1,4-dicarboxylate), namely UiO-66 (UiO refers to University of Oslo). The acid sites of the UiO-66 sample are determined by using CO and CD3CN as molecular probes. Furthermore, we have demonstrated that CO2 is adsorbed on basic sites exposed on dehydroxylated UiO-66. Introduction of water to the system produces hydroxyl groups acting as additional CO2 adsorption sites. As a result, CO2 adsorption capacity of the sample is strongly enhanced.
Introduction
Adsorption and heterogeneous catalysis are processes that are important for a wide range of industrial applications1,2. These processes occur on solid surfaces and therefore detailed characterization of these surfaces is decisive for understanding the processes and for rational design of new effective materials. To ensure high efficiency, the adsorbents and catalysts normally possess high specific surface area and are usually applied in the form of pelletized powders. Characterization of these materials is a primary research goal that can be achieved with the utilization of various analytical techniques.
Without any doubt, in situ infrared spectroscopy is one of the most commonly used methods for studying surface compounds2,3,4,5,6,7,8,9,10,11. The infrared spectral region corresponds to vibrations between atoms, which depend on the symmetry of the molecules, the strength of the bonds, the mass of the atoms, and other molecular constants. Therefore, infrared spectra contain rich information on the structure and symmetry of the adsorbed molecules and on the adsorbent-adsorbate and adsorbate-adsorbate interactions. By studying the adsorption of suitably selected compounds (so-called probe molecules), it is possible to obtain rich information on the structure and chemical composition of the surface, the nature, the acidity or basicity of the active sites, the oxidation and coordination state of the surface-situated cations, the acidity of hydroxyl groups, etc.3,4,5,6,7,8,9,10,11. Via infrared spectroscopy, the pathways for chemical transformation of molecules on the surface and the variety of reaction intermediates can be identified, which is a prerequisite for clarifying the mechanisms of catalytic reactions. The transmission mode of IR is mostly used, but in situ diffuse-reflectance IR spectroscopy is also utilized and, although based on different experimental protocol, gives very similar information12,13,14,15,16. Usually IR spectroscopy is combined with other complementary techniques that allow obtaining more profound information.
In general, there are two reasons for studying surface compounds. First, the adsorption of molecular probes is used to characterize the surface of a given material. Secondly, information is sought about a particular process involving adsorption. The mechanisms of catalytic reactions are most often studied in this way. It should be noted that the two cases are not strictly distinguishable, and in the study of a particular adsorption process, information can be obtained both on the surface of the adsorbent and on the mechanism of a catalytic reaction.
The spectral detection of surface species requires that they have a sufficiently high concentration in the infrared beam path. An optimal concentration of adsorbed compounds can be achieved by using a self-supporting pellet of the sample containing about 2-10 mg cm-2 of the substance. Thicker pellets are practically opaque for the infrared beam, whereas making and using thinner tablets has technical difficulties.
The pellets for IR studies are prepared by compressing sample powder between optically smooth dies of a pre-ground sample. Typically, they are characterized by high transparency in the IR region and have good mechanical properties.
In some cases, it is not possible to prepare a pellet which is thin (transparent) enough; then, a carrier is used: a metal grid, silicon or a KBr wafer. When using KBr, care must be taken because it can easily be oxidized either by the sample (if it possesses oxidizing properties) or by some adsorbates (e.g. NO2)10.
Normally, organic impurities, adsorbed water, carbonates, etc. are present on the surface of the as-prepared adsorbents and catalysts. Therefore, the surface must be cleaned before measurements. This is achieved by activation, which usually consists of two stages: (i) a thermo-oxidation treatment (aimed at the oxidation of organic pollutants) and (ii) thermo-vacuum treatment (mainly directed to the removal of adsorbed water and impurities such as bicarbonates, carbonates, nitrates, etc.). Typically, activation temperatures vary between 573 and 773 K. In some particular cases, the activation can be performed even at room temperature. For some specific materials (supported metals, metal-organic frameworks), the thermo-oxidation treatment is omitted because it can affect the sample.
As a rule, the sample activation is performed in situ in purpose made vacuum cells. Various laboratories use cells of different designs and made by different materials (metal, glass, quartz), but with a number of common features. An example of a simple glass IR cell is shown on Figure 1. The sample pellet is placed in a mobile holder that has two basic positions. In the first position the holder secures the pellet perpendicular to the infrared beam. In this part, the cell is equipped with windows from material that is transparent to infrared radiation (typically KBr or CaF2). In the second position, the holder secures the sample in a heating zone. In this zone, the cell includes an external furnace. Movement of the pellet from one place to another is accomplished by means of a magnet or a metal chain (for vertical constructions). The cells also provide the possibility of fixing the pellet in an intermediate position both outside the furnace and the infrared beam area, allowing easy registration of the background spectrum while cooling the sample down to room temperature. In our laboratory we use horizontal cells. This design prevents accidental release of the sample holder, which may cause the sample and even the cell to break.
In many cases, it is necessary to perform adsorption at low temperature. For this purpose, low-temperature cells are used in which the volume around the sample, when in the path of the infrared beam, is cooled with liquid nitrogen (Figure 2). In order to protect the cell windows from condensation of water from the air, a thermal buffer (e.g. from constantly circulating water) is applied between them and the cool zone. In some other cases, adsorption should be carried out at high temperatures, using purpose-made IR cells. The IR cells are always directly connected to vacuum/gas manifold system, allowing the adsorption experiments to be conducted in situ.
One of the main shortcomings of transmission infrared spectroscopy in the study of surface species is the existence of spectral regions in which, due to their own absorbance, the samples are opaque. When vibrational modes of adsorbed compounds fall into these regions, they cannot be registered.
The IR spectrum of the sample itself gives some direct information about the material. In the most favorable cases, general conclusions can be drawn concerning the surface hydroxyl groups and some stable surface species, such as sulfates, oxo-groups, foreign phases, etc. However, the IR spectrum of the sample is "blind" with respect to the presence of coordinatively unsaturated ions and gives scarce information about the acidity of surface hydroxyl groups, both species having decisive role for the adsorption and catalytic properties of the materials. Furthermore, no discrimination between bulk and surface species can be made. These problems are solved by the use of probe molecules. These are substances that interact specifically with the surface; the alteration of their spectral features as a result of adsorption provides indirect information about the nature, properties, location, concentration, etc. of the surface sites. Probe molecules are categorized into several groups, e.g., for determination of surface acidity or basicity, establishing the oxidation state of coordinatively unsaturated cations and the number of their coordination vacancies, obtaining information on the accessibility and location of surface sites, etc. There are several basic requirements for the probe molecules7,8: (i) the functional group or atom with which the molecule binds to the surface should be well known, (ii) the molecule should have a pronounced acidic or basic character, (iii) the molecule should bind to the same type of adsorption sites and the formed surface species should have the same structure; (iv) the adsorption complexes should be sufficiently stable, (v) the molecule should possess spectral parameters (frequency, spectral split, spectral shift) sufficiently sensitive to the surface property to be determined; (vi) in case the molecules are adsorbed on more than one type of sites, it is necessary that the different adsorption complexes can be reliably distinguished on the basis of their spectral characteristics; (vii) the informative spectral parameters should fall within the area where the sample is transparent; (viii) the absorption bands of the surface complexes should be characterized by sufficiently high intensity, and (ix) the molecule should not chemically modify the surface. There is virtually no compound that can satisfy all of the above requirements. Therefore, prior to the study, careful selection of a suitable probe molecule is necessary.
Another application of IR spectroscopy is to study the interaction between the substrate and one or more adsorbates of practical interest. In these cases, a variety of tricks are applied, such as co-adsorption with probe molecules (for establishing the nature of the adsorption sites), full or partial isotopic substitutions (for determination of the structure of the surface species), interaction with different reagents (to establish the reactivity of the species), variable-temperature experiments (for calculation of the entropy and enthalpy of adsorption), etc.
Finally, IR spectroscopy is used for mechanistic studies. In this way operando spectroscopy (spectroscopy in real reaction conditions) is applied12,17,18. However, a solid knowledge base must be obtained beforehand through in situ experiments.
In this article we describe the protocol we use for IR characterization of different materials and illustrate the power of the technique by demonstrating the water-enhanced CO2 adsorption on a metal-organic framework (UiO-66) material. For the experiments we used a Nicolet 6700 FTIR spectrometer. The spectra were registered accumulating 64 scans at a spectral resolution of 2 cm-1.
Protocol
1. Synthesis of UiO-66
NOTE: The UiO-66 sample was synthesized via a hydrothermal synthesis following a modified recipe reported elsewhere19.
- Dissolve 0.28 g of zirconium chloride in 4.5 mL of dimethylformamide. Then, prepare another solution consisting of 0.42 g of terephthalic acid and 4.4 g of benzoic acid in 10 mL of dimethylformamide. Heat the solutions to 423 K, combine them in a polytetrafluoroethylene lined autoclave and place in a conventional oven at 453 K for 24 h.
- After the reaction, wash the resultant crystals 3x with CH3OH and then dry them at 313 K.
2. Making pellets
- Making self-supporting pellets
- Spread uniformly, using a grid, about 20 mg of the sample powder on the polished surface of a pressing die. If the powder sticks to the metal surface, use mica or clear packing tape glued to the die. Place another die on top with the polished side facing the powder. Ensure even distribution of the specimen with several gentle rotating movements.
- Then put the two cylinders in a hydraulic press and apply 0.2 tons of pressure. After about two minutes, reduce slowly the pressure and remove the cylinders from the press. If the pellet is not formed, repeat the procedure, applying a higher pressure.
- Using a scalpel or a blade, cut out a piece of the pellet with dimensions of about 10 mm x 10 mm. Measure the geometric surface and the weight of the pellet.
NOTE: Some powders resist tableting. If the pellet is in pieces, a tungsten grid can be used as a carrier20. In other cases, the concentration of the adsorption sites is very high and the IR bands of the adsorbed species are too intense to be measured accurately. The solution is to prepare a pellet containing smaller amount of the substance. To achieve this, the substance is dispersed on preliminary prepared wafer that is transparent in the required IR regions. The wafer material could be silicon21 or KBr22. Below is a description of this technique for making a pellet with a sample spread on a KBr wafer.
- Making KBr-supported pellets
- Prepare a KBr pellet by the conventional technique. After pressing, pull up the piston from die set and spread the desired amount of sample powder uniformly on the KBr pellet, then put the piston back. Press the pellet with a hydraulic press.
NOTE: Initial control of the suitability of the pellet for IR studies is made by analyzing its IR spectrum.
- Prepare a KBr pellet by the conventional technique. After pressing, pull up the piston from die set and spread the desired amount of sample powder uniformly on the KBr pellet, then put the piston back. Press the pellet with a hydraulic press.
3. Pretreatment of the sample
- Positioning of the pellet
- Place the pellet into the sample holder. Put the sample holder into the IR cell and move the sample to the middle of the oven zone.
- Connecting to the vacuum/adsorption apparatus
- Connect the cell to the vacuum/adsorption apparatus, placing between them a reservoir with known volume, in this case about 0.5 mL. Evacuate the system.
- Activation of the sample at 573 K
- Adjust the activation temperature to 573 K, (recommended heating rate of 2-5 K min-1). Then, evacuate the sample at this temperature for 1 h.
NOTE: The sample is heated by an external furnace. The heating temperature has to be calibrated for each IR cell.
- Adjust the activation temperature to 573 K, (recommended heating rate of 2-5 K min-1). Then, evacuate the sample at this temperature for 1 h.
- Activation of the sample at RT
- To obtain sample activated at RT, just evacuate it for 1 h without heating.
NOTE: The activation aims for obtaining a clean surface. Control of the surface state is made through analysis of the IR spectra. If the procedure has not ensured a clean surface, it is necessary to repeat the activation, possibly at higher temperature or for a longer time.
- To obtain sample activated at RT, just evacuate it for 1 h without heating.
4. Registering sample spectrum
- Registering a background spectrum
- Using a magnet, move the pellet outside the oven and wait for 10 min in order to reach room (or ambient) temperature. During that time register a background spectrum.
- Registering a sample spectrum
- Move the pellet to the IR beam path and register the sample spectrum (Figure 3).
NOTE: The sample spectrum is used for background when performing the adsorption studies. Therefore, obtaining good quality sample spectrum is very important for the whole experiments. If the spectrum is of bad quality and noisy, prepare a new, thinner pellet.
- Move the pellet to the IR beam path and register the sample spectrum (Figure 3).
5. Adsorption of CD3CN at room temperature
- Successive adsorption of small doses
- Ensure the sample is situated on the IR beam path. Introduce a small dose, namely 0.5 µmol, of the adsorbate to the cell, in this case deuterated acetonitrile. Record an IR spectrum. Then, introduce a second (next) dose of the adsorbate and repeat the procedure. Do this until no more changes in the spectrum occur.
- Stability of adsorbed species
- Evacuate the sample recording spectra until no more changes occur. Then, move the sample to the oven with a preset temperature of 323 K.
- After 15 min evacuation at this temperature, place the pellet outside the oven and wait for 10 min in order to reach ambient temperature. During that time register a fresh background spectrum. Move the pellet to the IR beam path and register the sample spectrum.
- Repeat the procedure increasing the oven temperature with steps of 50 K until obtaining a spectrum coinciding with the initial sample spectrum.
6. Adsorption of CO at 100 K
- Cooling the sample
- To prevent deep cooling of the cell windows during the low-temperature experiments, first turn on the water circulation system. Then ensure the sample is situated on the IR beam path. Fill in the cell reservoir with liquid nitrogen and keep it full during the whole experiment.
- Recording low-temperature spectra
- After cooling the sample, record a spectrum. Then introduce adsorbate, in this particular case CO, on successive small doses, 0.5 µmol each. Record a spectrum after each dose. Finish this set of experiments with CO equilibrium pressure of 2 mbar.
- Then start to decrease the equilibrium pressure, first by dilution and then by evacuation at low temperature, again recording spectra. Mark the pressure in each spectrum.
- Recording spectra at increasing temperature
- When no more changes occur, stop filling the reservoir with liquid nitrogen and record spectra under dynamic vacuum and at increasing temperature.
NOTE: The spectrum of the sample recorded at low temperature slightly differs from that registered at room temperature. This causes problems with the sample spectrum (used for subtraction) at intermediate temperatures. Usually the temperature variation slightly changes the slope of the spectrum, but if the changes are serious, one should record spectra without adsorbate at different temperatures and use them as appropriate backgrounds. To ensure constant temperature during all low-temperature experiments, add ca. 1 mbar pressure of He into the cell before introduction of the probe molecule.
- When no more changes occur, stop filling the reservoir with liquid nitrogen and record spectra under dynamic vacuum and at increasing temperature.
7. Treatment of the spectra
- Loading source spectra
- Load a spectrum with adsorbed substance (a), the sample spectrum before adsorption (b) and a spectrum of the gas phase adsorbate (c) registered at the same pressure/temperature as spectrum (a).
- Treatment
- Subtract spectrum (b) from spectrum (a). From the resulting spectrum subtract spectrum (c) using interactive subtraction. The resulting spectrum is a superimposition of the spectrum of the adsorbed substance and the changes in the sample background, as also shown in Figure 4. For instance, the stretching modes of adsorbed CO are observed in the region between 2200 and 2100 cm-1 while the CO-induced shift of the ν(OH) modes can be monitored in the hydroxyl stretching regions.
- Quantifying
- To quantify the adsorbed amount (see Figure 5) calculate the integral absorbance of a chosen band due to adsorbed species. Plot the absorbance vs. the introduced amount of adsorbate or vs. its equilibrium pressure.
Representative Results
Here we report results on the water-induced enhancement of the CO2 adsorption capacity of the UiO-66 metal organic framework. Thorough sample characterization, including confirmation of the structure, is reported elsewhere23.
The activation of UiO-66 was performed by evacuation at desired temperature in order to avoid affecting the organic linkers of the MOF by oxidative treatment. The IR spectrum of UiO-66 registered after evacuation at ambient temperature (Figure 3) contains bands due to the linker, residual dimethylformamide (1667 and 1096 cm-1), terephthalic acid and esters (1732 and 1704 cm-1), and isolated (3673 cm-1) and H-bonded (3500-3000 cm-1) structural OH groups. Evacuation at 573 K leads to almost full disappearance of the residuals and of the structural hydroxyls, i.e., after this pretreatment the sample is practically clean and dehydroxylated.
Samples evacuated at room temperature and at 573 K were characterized by probe molecules (CD3CN and CO). Adsorption of CD3CN – a probe molecule for assessing acidity – on the just evacuated sample reveals existence of Brønsted acid sites (hydroxyl groups) through C-N stretching bands at 2276 and 2270 cm-1. At the same time, the OH band is red shifted by 170 and 250 cm-1, indicating a weak Brønsted acidity. With sample activated at 573 K, the bands indicating Brønsted acidity are practically absent, which is consistent with the observed sample dehydroxylation. However, a band at 2299 cm-1, due to CD3CN on Zr4+ Lewis acid sites, is present (Figure 6). More details are reported elsewhere23.
Low temperature CO adsorption on a sample evacuated at ambient temperature (Figure 4) revealed CO polarized by OH groups through a band at 2153 cm-1 (the unstable bands at 2136 and 2132 cm-1 are associated with physically adsorbed CO). Simultaneously, the original OH band is red shifted from 3676 to 3599 cm-1, i.e. by 77 cm-1, confirming the weak acidity of the hydroxyls. With a sample evacuated at 573 K, a very weak band due to CO polarized by hydroxyl groups was detected at 2154 cm-1, confirming again the low hydroxyl concentration in the sample. Importantly, no CO coordinated to Zr4+ sites was detected. This observation shows that the Lewis acid sites can be monitored only by relatively strong bases, as CD3CN, probably via structural re-arrangement in the Zr4+ environment.
Carbon dioxide (50 mbar) was put in contact with a sample evacuated at 573 K. The adsorbed CO2 is monitored by the antisymmetric stretching modes at 2336 cm-1 (Figure 7). Another weak satellite at 2325 cm-1 was also registered and associated with the so-called "hot" CO2 combination band22. Then, water (ca. 1 mbar partial pressure) was introduced into the system, which led to gradual development of a high-frequency shoulder at 2340 cm-1 which finally dominated the spectrum in the region. In concert, bands due to isolated (3673 cm-1) and H-bonded structural hydroxyls (µ3-OH-OCO adducts at 3647 cm-1 and µ3-OH-OH2 complexes at 3300 cm-1) developed. Note that no erosion of the initial band at 2336 cm-1 was detected, which indicates that, similarly to CO, CO2 is not able to form complexes with Zr4+ sites on the 573 K-activated sample.
In conclusions, the results show that water vapor hydroxylates the sample, creating structural hydroxyl groups that act as CO2 adsorption sites. This observation is important because it (i) evidences that CO2 adsorption could be enhanced in humid atmosphere and (ii) reveals the mechanism of this phenomenon.
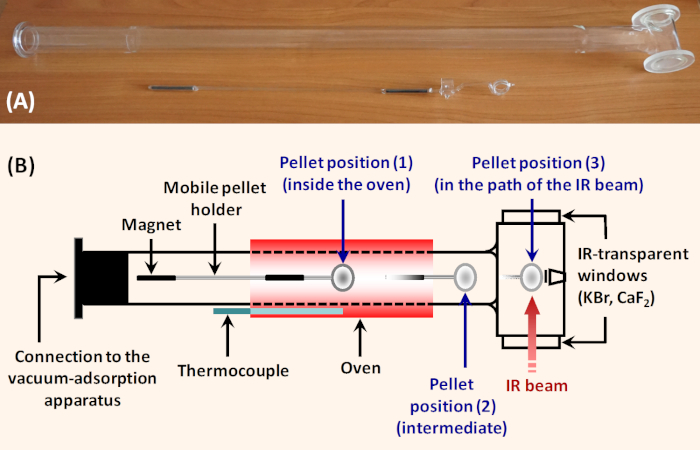
Figure 1: Simple horizontal glass IR cell for adsorption studies. (A) Photograph of the cell. (B) Scheme of the cell. The sample pellet is put into the holder which can be moved along the cell with a ferrite block magnet (60 mm x 30 mm x 10 mm, magnetization Y35). Position (1) is in the sample oven and allows thermal treatment. Position (2) is intermediate and allows tempering the sample and registering background immediately before registering the sample spectrum. In position (3), the sample is fixed perpendicularly to the IR beam for taking spectrum. To ensure transmission of the IR beam, the cell is equipped with IR transparent windows. The cell can be connected to a vacuum/adsorption device. Please click here to view a larger version of this figure.
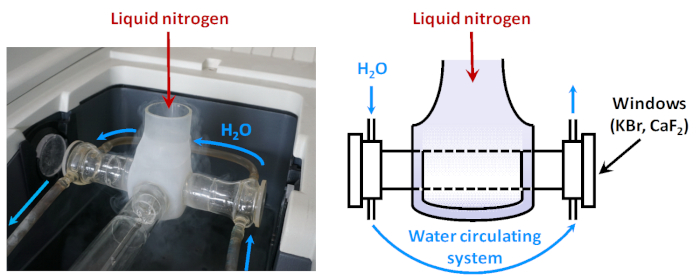
Figure 2: Scheme of low-temperature IR cell. When the sample is fixed in a position on the path of the IR beam, it is surrounded by a Dewar which can be filled with liquid nitrogen. Between the Dewar and the cell windows there is a water circulating system aimed at keeping the temperature of the window enough high (to prevent condensation of water vapors). Please click here to view a larger version of this figure.
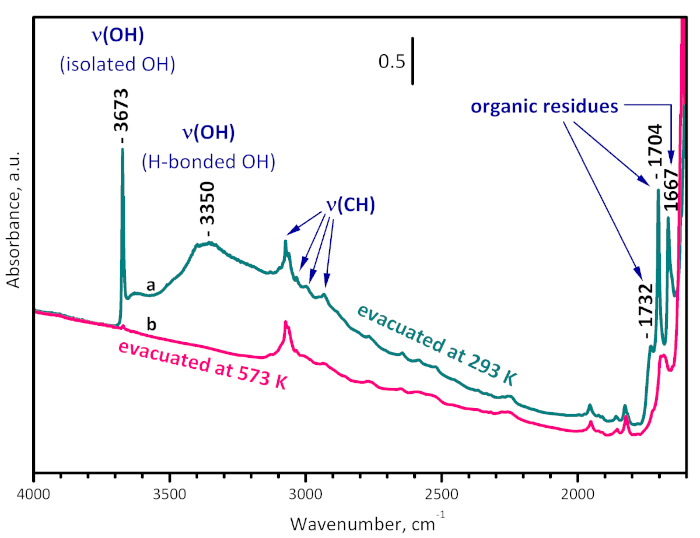
Figure 3: FTIR spectra of UiO-66. Sample evacuated at room temperature (a) and at 573 K (b). The bands at 1732, 1704 and 1667 cm-1 are due to organic residues and are removed by evacuation at 573 K. The band at 3350 cm-1 characterizes OH groups H-bonded to the organic residues. The band at 3673 cm-1 arises from the structural µ3-OH groups and practically disappears after evacuation at 573 K, indicating sample dehydroxylation. Please click here to view a larger version of this figure.
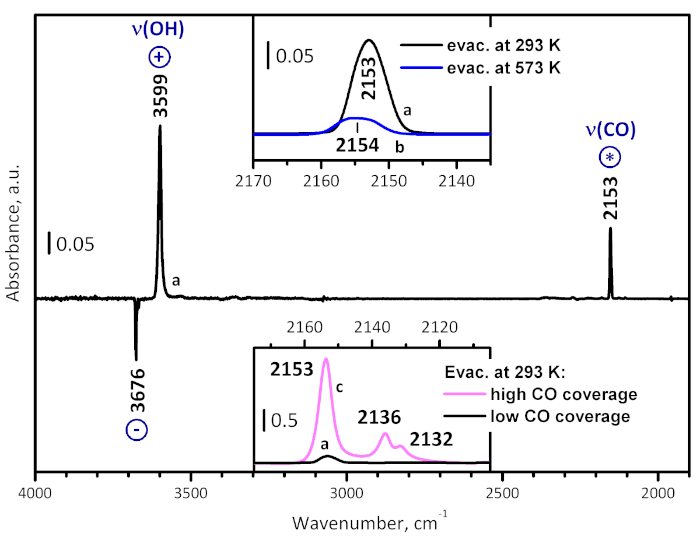
Figure 4: Typical difference spectrum. This spectrum (a) is obtained after low-temperature CO adsorption on UiO-66 activated at room temperature, followed by evacuation. The spectrum consists of bands due to adsorbed CO (marked with *), as well as positive (marked with +) and negative (marked with -) bands due to alteration of the sample own spectrum. In particular, the negative bands at 3676 and 3669 cm-1 and the positive band at 3599 cm-1 show the CO-induced shift of ν(OH) of the μ3-hydroxyls, and the shift value is a measure for the acidity of the OH groups. The top inset compares spectrum (a) in the carbonyl stretching region with a spectrum (b) registered at analogous conditions with a sample pre-evacuated at 573 K for 1 h. The bottom inset compares spectrum (a) in the carbonyl stretching region with a spectrum (c), registered in the presence of 1 mbar equilibrium pressure of CO. Please click here to view a larger version of this figure.
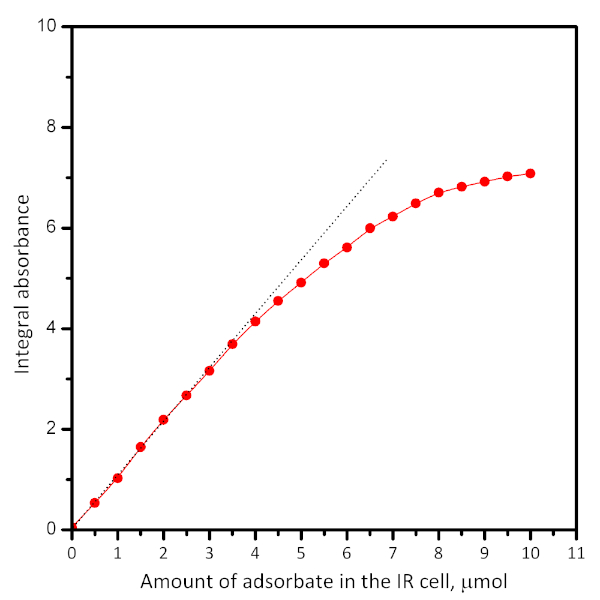
Figure 5: Typical plot showing the dependence of the absorbance of a particular band vs. the amount of introduced adsorbate. Extrapolation (see dotted lines) shows the adsorbate uptake. The plots can be used for calculation of the extinction coefficient of the IR band. Please click here to view a larger version of this figure.
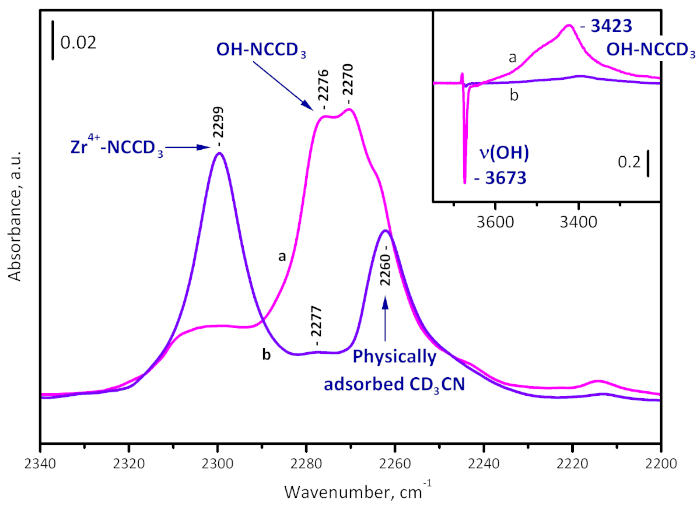
Figure 6: Difference spectra of CD3CN adsorbed on UiO-66. Sample activated at room temperature (a) and at 573 K (b). The band at 2299 cm-1 is due to Zr4+-NCCD3 complexes, those at 2276 and 2270 cm-1 to OH-NCCD3 species, and at 2260 cm-1 to physically adsorbed CD3CN. The formation of OH-NCCD3 species is visible also by the shift of the µ3-OH band from 3673 to 3423 cm-1. Please click here to view a larger version of this figure.
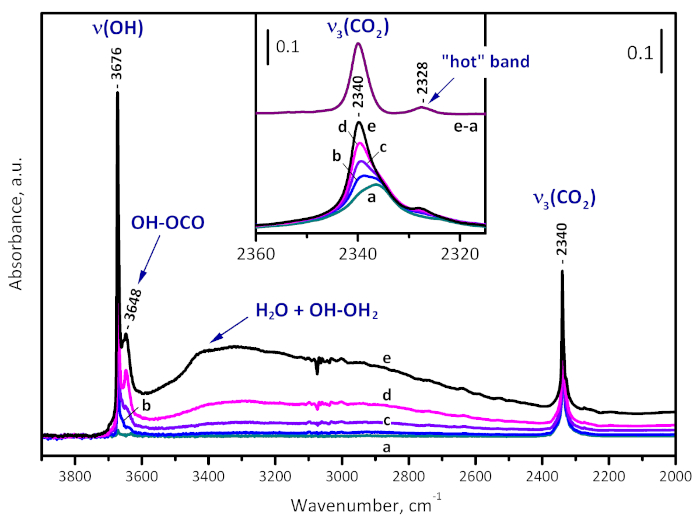
Figure 7: Water enhanced CO2 adsorption on UiO-66. FTIR spectra of CO2 (50 mbar equilibrium pressure) adsorbed on UiO-66 activated at 573 K (a) and time development of the spectra after introduction of water vapor (1 mbar equilibrium pressure) into the system (b-e). The new band developed at 2340 cm-1 is due to OH-OCO adducts and is consistent with sample hydroxylation. Please click here to view a larger version of this figure.
Discussion
The initial step, preparation of the sample pellet, is critical for the whole experiments. If the pellet is thick, the spectra are noisy, which hinders their analysis. Attention should be paid when using a pellet that is not self-supporting. In this case, special care should be taken in order to ensure that no interaction occurs between the supporting wafer and the sample or the adsorbates. Another critical step of the procedure is appropriate sample activation. The activation conditions depend on the sample nature and on the aims of the experiments. For instance, oxidative pretreatment could destroy some samples, as metal-organic and covalent-organic frameworks can oxidize supported metals. High activation temperature could lead to sample sintering or structure collapse. In this point of view, removal of foreign species by chemical treatment before making pellet is applied in some cases.
Deuterated acetonitrile (CD3CN) and CO are probe molecules widely used for measuring surface acidity8,9. CD3CN is bound to acid sites (both Lewis and Brønsted) via its nitrogen atom8. Upon coordination to metal cation, the C-N modes (2263 cm-1 in gas phase) shift to higher frequencies (up to 2335 cm-1) and the shift increases with the acidity of the Lewis sites. CD3CN is bonded to the hydroxyl groups through an H-bond and the C-N modes are typically observed in the region of 2300-2270 cm-1: the higher the frequency, the stronger the H-bond. In this case the ν(OH) modes are red shifted and the value of the shift is a quantitative measure of the acidity of the hydroxyls. Carbon monoxide is coordinated to surface metal or cationic sites and the ν(CO) frequency is highly sensitive to the oxidation and coordination state of the center9. With d0 metal cations, the ν(CO) frequency is blue shifted with respect to the gas-phase frequency (2143 cm-1) and the shift value is proportional to the cation acidity. When bound to hydroxyl groups via an H-bond, CO causes a red shift of the ν(OH) modes and the Δν(OH) value is used as a measure of the acidity of the hydroxyl.
A very important issue is the proper functionalization of the vacuum/gas manifold system. Entering of air to the system could lead to accumulation of water on the sample and partial or even full blocking of the adsorption sites. With reduced samples, re-oxidation could occur. The purity of the adsorbates is also very important. Sometimes, traces of impurities could affect the results. For instance, hydrogen adsorption is normally weak and high H2 equilibrium pressures are applied even at low temperature. Even ppm levels of N2 impurities could strongly affect the spectra because normally N2 is more strongly bound to the same sites where hydrogen is adsorbed. When performing low temperature experiments, some water could condense on the outer surface of the optical windows. This could hinder or distort the analysis in the OH stretching region, giving information on the acidity of the hydroxyl groups. If, for some reason, the technical problem could not be resolved, one could continue with the experiment using deuterated samples based on the fact that the OD region lies far away from the OH region. Deuteration can also be applied in cases where the sample is opaque in the OH region. In order to obtain the energetic characteristics of the adsorption (entropy, enthalpy), one should perform variable-temperature experiments where the exact measurement of the sample temperature is essential24.
The amount of gas adsorbate introduced by one dose can be adjusted by its pressure and by knowing the volume of the reservoir. To calculate the adsorbate density, one needs to know the mass of the pellet and the specific surface area of the material. Successive adsorption of adsorbate known doses allows the quantification of adsorption. A typical plot of the absorption vs. adsorbed amount is shown in Figure 4. It allows calculation of the extinction coefficient and the number of adsorption sites with knowledge of the sample weight. However, performing dosed adsorption is often accompanied by the so-called wall-effect. Briefly, the adsorbate is not uniformly distributed on the sample surface but first saturates the particles from the geometric surface of the pellet. Therefore, the spectra from the desorption experiments are more representative for equilibrium states.
A sister technique of the in situ transmission IR spectroscopy is diffuse reflectance spectroscopy (DRIFTS). Although it provides essentially the same information, DRIFTS is not so convenient for quantitative studies. In addition, DRIFTS is usually performed in gas flow. This can be an advantage, because the experiments are performed at conditions similar to the real ones, but also brings the risk of accumulation of impurities on the sample surface. Transmission IR spectroscopy can also be performed in real conditions (e.g. operando spectroscopy).
In conclusion, in situ IR spectroscopy provides valuable information on different surfaces and on the nature and properties of the adsorption sites. It can also reveal the method of interaction between the solid and particular gases. However, the technique is often not able to give unambiguous information on some important characteristics, such as the structure of the solid, the location of some sites, etc. This is why combination with other techniques is recommended.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Bulgarian Ministry of Education and Science (contract DO1-214/28.11.2018) under the National Research Programme "Low-carbon Energy for the Transport and Domestic Use – EPLUS" approved by DCM #577/17.08.2018. IS is grateful to the Hannover School for Nanotechnology (HSN), organized by R. Haug and F. Schulze-Wischeler.
Materials
| Acetonitrile-D3 | Uvasol, Merck | 1.13753.0009 | 99.69% deuteration degree (for NMR spectroscopy) |
| Benzoic acid | Sigma Aldrich | 242381-500G | C7H6O ≥99.5% |
| Carbon dioxide | Linde Gaz Magyarorszad | GA 473 | 99.9993% purity |
| Carbon monoxide | Merck-Schuchardt | 823271 | 99.5% purity |
| Ethanol | Carl Roth | 9065.1 | 99.8% |
| Glass sample holder | Self-made | ||
| HiCube80 Eco Turbo Pumping Station including HiPace 80 Turbo Pump, MVP 015 Diaphragm Vacuum Pump and DCU 002 Control Unit | Pfeiffer Vacuum | PM S74 150 00 | |
| Horizontal glass IR cells for adsorption studies | Self-made | ||
| Methanol | Carl Roth | 4627.5 | ≥99.9% |
| N,N-Dimethylformamide | Sigma Aldrich | 33120-2.5L-M | 99.8% |
| Nicolet 6700 FTIR spectrometer | Thermo Scientific | USA | |
| Specac Atlas Manual 15T Hydraulic Press | Specac | GS 15011 | |
| Terephthalic acid | Sigma Aldrich | 185361-100G | 98% |
| UIO-66 | Synthesized at Institute of Physical Chemistry and Electrochemistry, Leibniz Universität Hannover, Germany | ||
| Vacuum valve | Ellipse Labo | 248.904 | 90° branches, Ø 0-4 mm |
| Vacuum valve | Ellipse Labo | 248.910 | 90° branches, Ø 0-10 mm |
| Zirconium(IV) chloride | Sigma Aldrich | 357405-10G | Anhydrous, 98% |
References
- Ross, J. R. H. . Heterogeneous Catalysis: Fundamentals and Applications. , (2012).
- Busca, G. . Heterogeneous Catalytic Materials: Solid State Chemistry, Surface Chemistry, Surface Chemistry and Catalytic Behaviour. , (2014).
- Davydov, A. A. . Molecular Spectroscopy of Oxide Catalyst Surfaces. , (2003).
- Hadjiivanov, K., Knözinger, H. Characterization of Vacant Coordination Sites of Cations on the Surfaces of Oxides and Zeolites using Infrared Spectroscopy of Adsorbed Probe Molecules. Surface Science. 603 (10-12), 1629-1636 (2009).
- Knözinger, H., Huber, S. IR Spectroscopy of Small and Weakly Interacting Molecular Probes for Acidic and Basic Zeolites. Journal of the Chemical Society, Faraday Transaction. 94 (15), 2047-2059 (1998).
- Paukshtis, E. A., Yurchenko, E. N. Study of the Acid-Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russian Chemical Reviews. 52 (3), 242-258 (1983).
- Kustov, L. M. New Trends in IR-Spectroscopic Characterization of Acid and Basic Sites in Zeolites and Oxide Catalysts. Topics in Catalysis. 4 (1-2), 131-144 (1997).
- Hadjiivanov, K. I. Identification and Characterization of Surface Hydroxyl Groups by Infrared Spectroscopy. Advances in Catalysis. 57, 99 (2014).
- Hadjiivanov, K. I., Vayssilov, G. N. Characterization of Oxide Surfaces and Zeolites by Carbon Monoxide as an IR Probe Molecule. Advances in Catalysis. 47, 305 (2002).
- Hadjiivanov, K. Identification of Neutral and Charged NxOy Surface Species by IR Spectroscopy. Catalysis Reviews – Science and Engineering. 42 (1-2), 71-144 (2000).
- Lamberti, C., Zecchina, A., Groppo, E., Bordiga, S. Probing the Surfaces of Heterogeneous Catalysts by In Situ IR Spectroscopy. Chemical Society Reviews. 39 (12), 4951-5001 (2010).
- Meunier, F. C. Pitfalls and Benefits of In situ and Operando Diffuse Reflectance FT-IR Spectroscopy (DRIFTS) Applied to Catalytic Reactions. Reaction Chemistry & Engineering. 1 (2), 134-141 (2016).
- Ruggeri, M. P., Nova, I., Tronconi, E., Pihl, J. A., Toops, T. J., Partridge, W. P. In-Situ DRIFTS Measurements for the Mechanistic Study of NO Oxidation Over a Commercial Cu-CHA Catalyst. Applied Catalysis B: Environmental. 166, 181-192 (2015).
- Lentz, C., Jand, S. P., Melke, J., Roth, C., Kaghazchi, P. DRIFTS Study of CO Adsorption on Pt Nanoparticles Supported by DFT Calculations. Journal of Molecular Catalysis A: Chemical. 426, 1-9 (2017).
- Zhang, W., Liu, X., Dong, X., Dong, F., Zhang, Y. Facile Synthesis of Bi12O17Br2 and Bi4O5Br2 Nanosheets: In Situ DRIFTS Investigation of Photocatalytic NO Oxidation Conversion Pathway. Chinese Journal of Catalysis. 38 (12), 2030-2038 (2017).
- Hill, I. M., Hanspal, S., Young, Z. D., Davis, R. J. DRIFTS of Probe Molecules Adsorbed on Magnesia, Zirconia, and Hydroxyapatite Catalysts. Journal of Physical Chemistry C. 119 (17), 9186-9197 (2015).
- Aldana, P. A. U., Ocampo, F., Kobl, K., Louis, B., Thibault-Starzyk, F., Daturi, M., Bazin, P., Thomas, S., Roger, A. C. Catalytic CO2 Valorization into CH4 on Ni-Based Ceria-Zirconia. Reaction Mechanism by Operando IR Spectroscopy. Catalysis Today. 215, 201-207 (2013).
- Bañares, M. A. Operando Methodology: Combination of In Situ Spectroscopy and Simultaneous Activity Measurements Under Catalytic Reaction Conditions. Catalysis Today. 100 (1-2), 71-77 (2005).
- Friebe, S., Mundstock, A., Volgmann, K., Caro, J. On the Better Understanding of the Surprisingly High Performance of Metal-Organic Framework-Based Mixed-Matrix Membranes Using the Example of UiO-66 and Matrimid. ACS Applied Materials Interfaces. 9 (47), 41553-41558 (2017).
- Ballinger, Y. H., Wong, J. C. S., Yates, J. T. Transmission Infrared Spectroscopy of High Area Solid Surfaces. A Useful Method for Sample Preparation. Langmuir. 8 (6), 1676-1678 (1992).
- Vimont, A., et al. Evidence of CO2 Molecule Acting as an Electron Acceptor on a Nanoporous Metal-Organic-Framework MIL-53 or Cr3+(OH)(O2C-C6H4-CO2). Chemical Communications. 43 (31), 3291-3293 (2007).
- Mihaylov, M., et al. Adsorption Forms of CO2 on MIL-53(Al) and NH2-MIL-53(Al) as Revealed by FTIR Spectroscopy. Journal of Physical Chemistry C. 120 (41), 23584-23595 (2016).
- Chakarova, K., Strauss, I., Mihaylov, M., Drenchev, N., Hadjiivanov, K. Evolution of Acid and Basic Sites in UiO-66 and UiO-66-NH2 Metal-Organic Frameworks: FTIR Study by Probe Molecules. Microporous and Mesoporous Materials. 281, 110-122 (2019).
- Delgado, M. R., Bulánek, R., Chlubná, P., Arean, C. O. Brønsted Acidity of H-MCM-22 as Probed by Variable-Temperature Infrared Spectroscopy of Adsorbed CO and N2. Catalysis Today. 227, 45-49 (2014).

