Generation of Multicellular Human Primary Endometrial Organoids
Summary
A protocol to generate human primary endometrial organoids that consist of epithelial and stromal cells and retain characteristics of the native endometrial tissue is presented. This protocol describes methods from uterine tissue acquisition to the histologic processing of endometrial organoids.
Abstract
The human endometrium is one of the most hormonally responsive tissues in the body and is essential for the establishment of pregnancy. This tissue can also become diseased and cause morbidity and even death. Model systems to study human endometrial biology have been limited to in vitro culture systems of single cell types. In addition, the epithelial cells, one of the major cell types of the endometrium, do not propagate well or retain their physiological traits in culture, and thus our understanding of endometrial biology remains limited. We have generated, for the first time, endometrial organoids that consist of both epithelial and stromal cells of the human endometrium. These organoids do not require any exogenous scaffold materials and specifically organize so that epithelial cells encompass the spheroid-like structure and become polarized with stromal cells in the center that produce and secrete collagen. Estrogen, progesterone and androgen receptors are expressed in the epithelial and stromal cells and treatment with physiological levels of estrogen and testosterone promote the organization of the organoids. This new model system can be used to study normal endometrial biology and disease in ways that were not possible before.
Introduction
The human endometrium lines the uterine cavity and serves as the first contact for the embryo during implantation. The endometrium is comprised of luminal and glandular epithelial cells, supportive stromal fibroblasts, endothelial cells and immune cells. Together, these cell types make up the endometrial tissue which is one of the most responsive tissues to sex steroid hormones1. The changes that occur during each menstrual cycle are striking. Appropriate growth and remodeling of the endometrium is required to allow for embryo implantation to occur. Aberrant response to estrogen and progesterone can result in a refractory endometrium that does not allow for successful establishment of pregnancy and can even result in diseases including endometrial neoplasia.
In order to study the hormone responses and essential changes that occur in the endometrium, cells from endometrial tissues excised from patients during surgery or endometrial biopsy have been propagated in cell culture. Endometrial stromal cells preferentially proliferate and propagate readily, and the process of differentiation induced by progesterone can be recapitulated in vitro. As a result, much has been learned during this differentiation process, termed decidualization2,3. The other major cell type in the endometrium, the luminal and glandular epithelial cells, however, do not grow well as traditional monolayers, losing polarity, becoming senescent, and having limited proliferative potential. As a result, less is known of their biology and their role in the human endometrium. As many neoplasia originate from the epithelial cells, mechanisms associated with hyperplasia or transformation to cancer cells remain to be fully defined. Furthermore, studies have established that hormone response involves the intimate paracrine actions between the epithelial and stromal cells of the endometrium4,5.
Recently, a three-dimensional (3D) organoid culture of endometrial epithelial cells was established by two independent groups6,7, which are the first reports of organoids formed from endometrial tissue. These organoids were comprised of endometrial epithelial cells embedded within a protein matrix (Table of Materials) and did not include an important hormonally responsive compartment of the endometrial endometrium, the stromal fibroblasts. As the matrix proteins can vary from lot to lot and can trigger signaling pathways that do not necessarily occur in the tissue, it would be ideal to replace the matrix proteins with components of the endometrium. In the current study, a protocol to generate scaffold-free human endometrial organoids of epithelial and stromal cells of the human endometrium is presented. The presence of stromal cells not only provides the support for epithelial cells but also provides the necessary paracrine actions that have been established to be important for endometrial hormone response4,8,9.
The new multicellular endometrial organoid offers a model system of the endometrium that is simple to generate and that incorporates both epithelial and stromal cells. These organoids can be used to study long-term hormonal changes and early events of disease such as tumorigenesis due to hormonal imbalance or exogenous insults. The complexity of these organoids could eventually be expanded to include other cell types, including endothelial and immune cells with possibly myometrial cells to truly mimic human tissue physiology.
Protocol
Endometrial samples were collected from premenopausal women undergoing routine hysterectomy for benign uterine conditions at Northwestern University Prentice Women's Hospital, according to an Institutional Review Board-approved protocol. Written consent was obtained from all women included in the study.
1. Preparation of Agarose Molds
- Prior to beginning cell isolation, cast and equilibrate 1.5% agarose micro molds (Table of Materials) to house the organoids according to the manufacturer’s instructions.
2. Generation of Endometrial Organoids
- Harvest primary stromal and epithelial cells
- In a biosafety cabinet using aseptic technique, prepare enzyme solution (2.5 mg/mL collagenase type I + 0.1 mg/mL DNase I in 10 mL of dispase [500 units]) at 37 °C, and sterile-filter the solution using a 0.2 µm syringe filter into a 15 mL conical tube.
- In a biosafety cabinet using aseptic technique, scrape off endometrium from the uterine biopsies and mince tissue under hood into very small pieces with a scalpel.
NOTE: Endometrial tissue that is at least 20 mm x 10 mm x 1 mm is required to generate sufficient numbers of organoids. - Put freshly minced tissue into the enzyme solution in a 15 mL conical tube. Close the cap and wrap the top with a wax film (Table of Materials) to prevent contamination.
- Put the tube with tissue in a water bath or an incubator at 37 °C for 30 min with gentle shaking (80−100 rpm).
- Stack a 100 µm cell strainer on top of a 20 µm cell strainer on a 50 mL conical tube. Filter the solution through the two strainers, and then rinse the 15 mL conical tube with 10 mL of Hank’s balanced salt solution (HBSS; Table of Materials) and put the wash through the strainer to ensure all cells are collected.
NOTE: The flow-through liquid contains stromal cells and red blood cells (~20 mL total). Epithelial cells are collected on the 20 µm strainer. Chunks of undigested tissue remain on top of the 100 µm strainer. Discard undigested tissue into a biohazard waste container. - Invert the 20 µm cell strainer from step 2.1.5 onto a new 50 mL conical tube and wash the epithelial cells off the strainer with 20 mL of organoid media (Table of Materials) supplemented with 1% pen/strep.
- Centrifuge the conical tubes from steps 2.1.5 and 2.1.6 at 500 x g for 5 min.
- With the collected stromal cells, remove the supernatant and resuspend the pellet with 10 mL of red blood cell lysis buffer. Incubate at 37 °C for 10−15 min. Centrifuge the conical tube at 500 x g for 5 min, remove the supernatant, and resuspend the pellet with 200−300 µL of organoid media (see step 2.2.2 for further instructions).
- With the collected epithelial cells, remove the supernatant and re-suspend with 100 µL of organoid media (see step 2.2.2 for further instructions).
- Seeding cells
- After equilibrating the 1.5% agarose molds in wells (1 mold per well) of a 24-well plate, remove organoid media on the outside of the agarose molds and tilt the tissue culture plate so that the medium from the cell seeding chamber of the agarose dishes can also be carefully removed.
- View epithelial (from step 2.1.9) and stromal (from step 2.1.8) cell suspensions under the microscope. Add more organoid media to either the epithelial or stromal cell suspensions 100 µL at a time, so that the suspensions are roughly equal in density.
NOTE: Epithelial cells will clump together, and it is not advisable to digest/trypsinize epithelial cells into single cell suspensions. - Combine 1 part of stromal cells with 3 parts of epithelial cells by volume.
- Pipette 50 µL (60,000 cells) of the combined cell suspension into the cell seeding chamber of the agarose mold.
- Once the agarose molds are filled with cells, carefully add 400 µL of fresh organoid media into the well of the 24-well plate.
NOTE: The medium reaches just below the surface of the agarose dishes and will not cause the cells to float out of the molds. After 2−3 days, cells will settle and start to form organoids. - Change medium every second day with 500 µL of organoid media.
- After 2-3 days, change medium to one that is supplemented with 0.1 nM estradiol (E) and 0.8 nM testosterone (T) to promote organization of epithelial and stromal cells.
NOTE: Although organization of epithelial and stromal cells can occur with or without hormones, more organoids will organize in the presence of hormones.
3. Harvesting Endometrial Organoids for Experiments
NOTE: After the experiment is done, endometrial organoids can be processed for histology or RNA analysis.The following steps describe how to process the organoids.
- Histology
- Dissolve 1.5% agarose in phosphate-buffered saline (PBS) by boiling. Allow the liquid agarose to cool to approximately 50 °C.
- Under a dissecting microscope, tilt the 24-well plate and carefully pipette out the medium from the outside of agarose mold. Carefully pipette out the medium from the interior of the agarose mold to avoid disrupting the organoids.
- Under a dissecting microscope, carefully pipette 70-75 µL of warm (50 °C) 1.5% agarose into the chamber of the agarose dish. Be careful not to disturb the organoids.
- Let cool at 4 °C for 5 min.
- Add 4% paraformaldehyde (PFA) into each well of the 24 well plate and fix the entire sealed agarose mold containing organoids overnight at 4 °C. Then store in 70% ethanol at 4 °C until ready to process for paraffin embedding.
- RNA isolation
- Under a dissecting microscope, tilt the 24-well plate and carefully pipette out the medium from the chamber of the agarose mold.
- Forcefully pipette 1 mL of fresh organoid medium directly into the agarose mold so the organoids are flushed out of the microwells. Be careful not to create too many bubbles.
- Repeat the pipetting again with the same medium from step 3.2.2.
- Collect all the medium containing the organoids. Centrifuge at max speed to collect the organoids and proceed to RNA extraction.
Representative Results
A schematic of the protocol is depicted in Figure 1. Uterine tissue was obtained from surgery after it was examined by pathologists. The endometrial lining was separated from the myometrium by scraping and the endometrial tissue was enzymatically digested to cells as outlined in the protocol. Epithelial and stromal cells were added into microwells in the agarose molds. After 7 days in culture, organoids were treated with E and T for an additional 7−14 days.
For histological processing, the agarose molds containing endometrial organoids were sealed with agarose followed by fixation with 4% PFA overnight. These molds were processed for standard paraffin embedding, sectioned and stained for histology, immunofluorescence or immunohistochemistry. The hematoxylin and eosin (H&E) staining (Figure 2A) revealed a spheroid-like structure with a single layer of cells lining the outside and cells in the center. Cell-specific markers for endometrial epithelial (E-cadherin) and stromal cells (vimentin) revealed epithelial cells surrounding the organoid with stromal cells in the center (Figure 2B).
Markers of endometrial physiology confirmed that the endometrial organoids retained certain characteristics of the native tissue. Trichrome staining, which stains collagen blue and cells red, showed the presence of collagen within the center where stromal cells resided, demonstrating active production and secretion of collagen by stromal cells similar to the native tissue (Figure 3A). In addition, immunohistochemical staining revealed the presence of estrogen receptors (ER), androgen receptors (AR), and progesterone receptors (PR) in both the epithelial and stromal cells (Figure 3B).
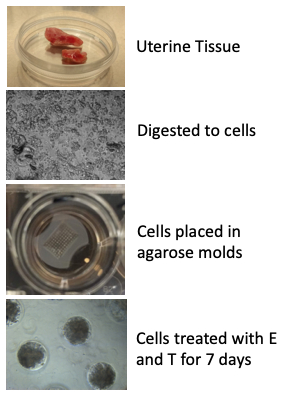
Figure 1: Generation of scaffold-free 3D endometrial organoids from human primary endometrial cells. Uterine tissues were obtained from premenopausal endometrial tissues with benign pathology and the endometrial lining was excised from the tissue piece. Upon enzymatic digestion, endometrial epithelial and stromal cells were seeded into 1.5% agarose molds at a 3:1 ratio by volume and maintained in sex hormone-free medium for up to 7 days. Estradiol (E) and testosterone (T) were added to the 3D cultures to mimic the levels of E and T during the follicular phase of a menstrual cycle10 and to promote organization of the organoids. Adapted from Teerawat et al., JCEM, (2019)11.
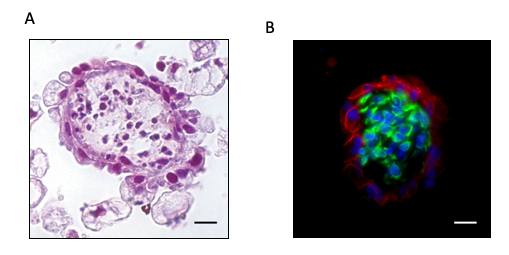
Figure 2: Histology and immunofluorescent staining of cell specific markers on endometrial organoids. Organoids were observed after 14 days of stepwise hormone treatment mimicking the follicular phase of a normal menstrual cycle (0.1 nM E and 0.8 nM T for 7 days, 1 nM E and 0.8 nM T for an additional 6 days, followed by 1 nM E and 1.25 nM T for 1 day). (A) H&E staining was done on paraffin embedded organoids. (B) Cell specific markers were assessed by immunofluorescent staining of E-cadherin (epithelial, red), vimentin (stromal, green) and 4′,6-diamidino-2-phenylindole (DAPI; nucleus, blue) which revealed structural organization of the cells in the organoids. Scale bar = 20 µm. Adapted from Teerawat et al., JCEM, (2019)<11. Please click here to view a larger version of this figure.
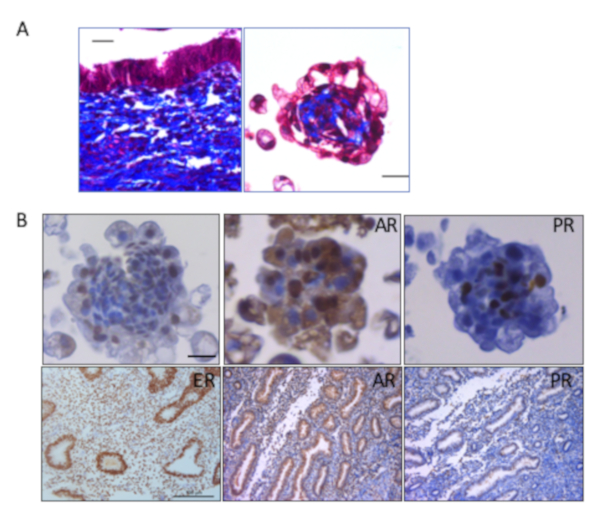
Figure 3: Endometrial organoids exhibit characteristics of native tissue after 14 days of follicular phase hormone treatment. (A) Trichrome staining was done to visualize collagen (blue) and cells (red). Trichrome stain was performed on endometrial tissue (left panel) and endometrial organoids (Scale bars = 20 µm, right panel). (B) Immunohistochemical staining for ER, AR and PR was done in endometrial tissue (Scale bar = 100 µm bottom panels) and endometrial organoids (Scale bar = 20 µm, top panels). Positive stain is shown in brown and hematoxylin stain solution was added as the counter stain shown in blue. Adapted from Teerawat et al., JCEM, (2019)11. Please click here to view a larger version of this figure.
Discussion
We have generated human endometrial organoids comprised of epithelial and stromal cells of the endometrium without the use of exogenous scaffold materials. While it has already been shown that primary endometrial epithelial cells can form organoids6,7, these cells were embedded in a gelatinous matrix of proteins secreted by mouse sarcoma cells (see Table of Materials) to help form spheroid-like structures. In addition, endometrial stromal cells were absent. We speculate that the stromal cells in our system, which is an essential supportive component of the endometrial tissue, provided the scaffold for epithelial cells to adhere to and paracrine signaling occurred between cell types. The organization of the epithelial cells around the stromal cells indicated an active interaction between the two cell types which provided the physiologic cues. As hormonal response of the endometrium relies on paracrine interactions between the epithelial and stromal cells4,8,9, our multicellular organoids provide an alternate, more complex mimic of the native tissue.
The organoids that were generated here were mostly somatic cells, although a small percent of cells was stem-like. We have not yet passaged these organoids for multiple generations and do not know if they would survive and propagate. In addition, endometrial organoids are heterogeneous in nature in that not all organoids contained the same number of epithelial, stromal or stem cells, even those originating from the same tissue. Sizes of the organoids also differed and while most cells formed spheroid like structures, loose cells within each microwell were observed. The presence of E and T appeared to promote organoid formation with the specific organization of epithelial cells lining the outside and stromal cells in the center. We have not tested whether E or T alone would promote organoid formation and what the optimal length of treatment would be. Additional testing of parameters and conditions would be beneficial to generate the ideal endometrial organoids suited for the experiment planned.
Medium is an important component of culturing organoids for promoting growth and survival. From our experience in working with endometrial cells, growth of epithelial cells in culture is the most challenging in contrast to stromal cells which propagate well. Different media were tested, including the organoid media of choice (Table of Materials), which is used to generate mammospheres and tumorspheres of breast cancer, which are of epithelial cell origin. Our tests revealed that this medium permitted organoids to maintain their structural integrity and viability over the course of 14−28 day cultures. The effect of this medium on stromal cells, however, needs additional investigation. Despite their survival and production of collagen in the organoid media, the proliferation rate observed in 3D culture was much less than in monolayers. The decreased proliferation may however be due to the 3D structure as well. Given that the organoid media used is commercially available, medium components remain unclear due to its proprietary nature.
In future studies, we envision increasing the complexity of these endometrial organoids to incorporate other cell types found in the endometrium, including endothelial and immune cells. This is just the beginning of engineering a complete endometrial mimic that can be used to study biology, function, disease, and to test drugs.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was funded by NIEHS/NIH/NCATS UG3 grant (ES029073) and Northwestern Feinberg School of Medicine Bridge fund (JJK). We would like to acknowledge the Northwestern Pathology Core Facility for processing the fixed organoids for paraffin embedding. We would like to acknowledge the entire UG3 team including the Woodruff, Burdette, and Urbanek labs for the insightful discussions and collaborations.
Materials
| Agarose HS, molecular biology grade | Denville Scientific | CA3510-6 | |
| Agarose molds | Sigma-Aldrich | Z764043 | (https://www.microtissues.com/) |
| Ammonium chloride (NH4Cl) | Amresco | 0621 | |
| β-Estradiol | Sigma-Aldrich | E2257 | |
| Collagenase, Type II, powder | Thermo Fisher Scientific | 17101015 | |
| Dispase | Corning | 354235 | |
| DNase I | Sigma-Aldrich | D4513 | |
| EDTA | Fisher Scientific | BP120-1 | |
| Eosin Stain | VWR | 95057-848 | |
| Estrogen Receptor (SP1), rabbit monoclonal antibody | Thermo Fisher Scientific | RM-9101-S | |
| Fluoroshield with DAPI, histology mounting medium | Sigma-Aldrich | F6057 | |
| Hank's Balanced Salt Solution (HBSS) | Corning | 21-022-CV | 1x without calcium, magnesium, and phenol red |
| Hematoxylin Stain Solution | Thermo Fisher Scientific | 3530-32 | Modified Harris formulation, mercury free |
| Heparin solution | STEMCELL Technologies | 07980 | added to MammoCult media |
| Hydrocortisone stock solution | STEMCELL Technologies | 07925 | added to MammoCult media |
| Organoid media – Mammocult | STEMCELL Technologies | 05620 | supplemented with 2 µL/mL heparin and 5 µL/mL hydrocortisone |
| Paraformaldehyde, 16% solution | Electron Microscopy Sciences | 15710 | |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | |
| Phosphate buffered saline, pH 7.4 | Sigma-Aldrich | P3813 | |
| Progesterone Receptor, PgR 1294, unconjugated, culture supernatant | Agilent Technologies | M356801-2 | |
| protein matrix – Matrigel | BD Biosciences | 356231 | |
| Purified mouse anti-E-cadherin antibody | BD Biociences | 610181 | Clone 36 |
| Recombinant anti-vimentin antibody [EPR3776] | Abcam | ab92547 | |
| RNA lysis and isolation kit | Zymo Research | R2060 | |
| Sodium bicarbonate (NaHCO3) | Sigma-Aldrich | S6014 | |
| Testosterone | Sigma-Aldrich | 86500 | |
| Trichrome Stain | Abcam | ab150686 | |
| Wax film – Parafilm | VWR | 52858-000 |
References
- Henriet, P., Gaide Chevronnay, H. P., Marbaix, E. The endocrine and paracrine control of menstruation. Molecular and Cellular Endocrinology. 358 (2), 197-207 (2012).
- Gellersen, B., Brosens, I. A., Brosens, J. J. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Seminars in Reproductive Medicine. 25 (6), 445-453 (2007).
- Gellersen, B., Brosens, J. J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocrine Reviews. 35 (6), 851-905 (2014).
- Li, Q., et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 331 (6019), 912-916 (2011).
- Wetendorf, M., DeMayo, F. J. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Molecular and Cellular Endocrinology. 357 (1-2), 108-118 (2012).
- Boretto, M., et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 144 (10), 1775-1786 (2017).
- Turco, M. Y., et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nature Cell Biology. 19 (5), 568-577 (2017).
- Kurita, T., et al. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 139 (11), 4708-4713 (1998).
- Kim, J. J., Kurita, T., Bulun, S. E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine Reviews. 34 (1), 130-162 (2013).
- Bui, H. N., et al. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 78 (1), 96-101 (2013).
- Wiwatpanit, T., Murphy, A. R., Lu, Z., Urbanek, M., Burdette, J. E., Woodruff, T. K., Kim, J. J. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. The Journal of Clinical Endocrinology & Metabolism. , (2019).

