Controlled Odor Mimic Permeation Systems for Olfactory Training and Field Testing
Summary
The Controlled Odor Mimic Permeation System is a simple, field-portable, low-cost method of odor delivery for olfactory testing and training. It is constructed of an odorant retained on an adsorbent material and contained inside of a permeable polymer bag allowing controlled release of the odorant vapor over time.
Abstract
The Controlled Odor Mimic Permeation System (COMPS) was developed to provide a convenient field testing method of odor delivery at controlled and reproducible rates. COMPS are composed of an odorant of interest on an absorbent material sealed inside of a permeable polymer bag. The permeable layer allows for a constant release of the odorant over a given amount of time. The permeable bag is further stored in a secondary, impermeable bag. The double-containment procedure allows for equilibration of the odorant from the permeable bag but within the impermeable outer layer, resulting in an instant and reproducible source of odorant vapor upon removal from the outer packaging. COMPS are used in both olfactory testing for experimental scenarios and for olfactory detection training, such as with detection canines. COMPS can be used to contain a wide range of odorants (e.g., narcotics powders) and provide a controlled release of the associated odorants. Odor availability from COMPS is expressed in terms of permeation rate (i.e., the rate of the odorant vapor released from a COMPS per unit time) and is typically measured by gravimetric means. The permeation rate for a given mass or volume of odorant can be adjusted as needed by varying the bag thickness, surface area, and/or polymer type. The available odor concentration from a COMPS can also be measured by headspace analysis techniques such as solid phase microextraction with gas chromatography/mass spectrometry (SPME-GC/MS).
Introduction
Olfaction is a crucial, yet often overlooked, sensing mechanism used by most animals. For many it is the main mechanism for locating food, finding a mate, or sensing danger1. Furthermore, the olfactory capabilities some animals, most notably canines, are regularly exploited by humans for the detection of contraband (e.g., narcotics or explosives), or other objects of interest, such as missing persons, invasive species, or diseases2,3. For canine detection research or other olfaction research topics, investigators often study the process of olfaction and the strengths and limitations of the olfactory system. As such, it is generally desirable to control the release of an odorant vapor into the environment to reproducibly deliver known quantities of odorant during testing. Failure to account for variations in odor availability due to factors such as vapor pressure or environmental effects often complicates data interpretation and applicability4. It is similarly desirable to provide an established quantity of odor during training scenarios for detection canines. For example, studies by Hallowell et al.5 and by Papet6 have indicated the importance of odor intensity in odor perception, and that altering the intensity of an odorant can affect how it is perceived alone or in a mixture.
In laboratory settings, the use of analytical equipment such as permeation tubes with controllable ovens, vapor generators, or olfactometers may be used to control odor delivery. However, this type of equipment is impractical for use during field testing and training scenarios4. The Controlled Odor Mimic Permeation System (COMPS) was developed as a simple, low-cost, and disposable method for controlled odor delivery requiring no external power. Therefore, they can easily be incorporated into a variety of different testing and training scenarios7. COMPS units are simply composed of an odorant of interest on an absorbent material sealed inside of a permeable polymer bag, stored in a secondary containment system. The utilization of COMPS reduces variability between tests and improves consistency during training exercises8.
Odor delivery or availability from COMPS is measured in terms of permeation rate, as determined by gravimetric analysis in terms of mass of vapor released over time. Permeation rates can be controlled by a number of factors, including the thickness of the polymer bag, its available surface area, the type of absorbent material (substrate) used, and the amount of the odorant. Permeation rate is constant for a given period of time (hours or days) depending on the odorant being used. This allows for minimal variability in odor delivery during testing or training. During storage, COMPS come to equilibrium within the impermeable outer container, resulting in an instant source of odorant vapor at a known permeation rate.
COMPS were initially designed to contain odorants associated with explosive materials and to be used as odor mimics7. As defined by Macias et al., an odor mimic simulates a material of interest, such as an explosive, by providing the dominant volatile compounds, or odorants, found in the headspace of that material without the presence of the parent material itself8. To create an odor mimic, the active odorants of the parent material must be determined. An active odorant, in this scenario, is described as a volatile compound that a trained explosive-detection canine detects, believing that there is an actual explosive material present. Having identified dominant volatile compounds in the headspace of several explosive materials, COMPS were prepared to release these individual odorants at a controlled rate for the duration of canine olfactory detection field trials and determine the active odorant associated with several explosive materials. COMPS were successfully used for this purpose7,9 and have since been used as odor mimics for further explosive detection training.
Macias et al. utilized COMPS containing piperonal, a pure chemical solid at room temperature that, in the vapor phase, has been shown to be the active odorant for MDMA (3,4-methylenedioxymethamphetamine), the psychoactive drug known as ecstasy. The researchers used varying thicknesses and surface areas of low-density polyethylene bags to adjust the permeation rate of piperonal vapor. This series of COMPS was then used to estimate piperonal detection threshold for trained narcotics-detection canines8. Conversely, in a separate study, COMPS bag thicknesses were adjusted to minimize the deviation of permeation rates between each compound in a homologous series though they possessed drastically varying vapor pressures. If a single bag thickness had been used in this study, those compounds with higher vapor pressures would have yielded much higher permeation rates. By increasing the bag thickness for the higher volatility compounds, the permeations rates were adjusted so that they were similar for all compounds4. Both studies demonstrate the utility and adaptability of the COMPS to control vapor release. Similar studies optimizing polymer bag thickness as well as absorbent material have been carried out in the creation of odor mimics for synthetic cathinones (i.e., bath salts)10, other narcotics (including heroin and marijuana11), and human odor compounds12,13. In a final example, Simon et al. investigated the active odorants associated with an invasive fungus species14. Whole pieces of infected tree bark, instead of the extracted odorants, were placed directly into the polymer bag to control release during canine olfaction testing14. COMPS can be utilized for a variety of scenarios, and the protocols discussed herein were chosen to demonstrate the diversity of this tool.
Protocol
1. Assembly of COMPS (Figure 1)
- For neat (liquid) compound on a substrate (Figure 1A)
- To impregnate the substrate with odorant, use a calibrated pipette to add 5 µL of neat compound to a 2 x 2 inch cotton gauze pad or other substrate of choice (see Table of Materials).
- Fold the gauze pad in half and place this (or an alternative substrate material) into a 2 x 3 inch low-density polyethylene permeable bag. The suggested bag thicknesses are between 1 MIL, for the fastest permeation rate, to 8 MIL, for a slower permeation rate.
NOTE: Variations in absorbent materials, permeable bag size, polymer chemistry, and thickness can be used, but these changes affect the permeation rate of the odorants (see further discussion in the Results Section). - Immediately seal the polymer bag closed with a heat sealer, eliminating as much air from within the bags as possible.
- Store the bag in an outer nonpermeable bag, or if it will be used immediately, place it in a clean weigh boat in a fume hood (Figure 1B).
- For solid material, no substrate necessary (Figure 1C)
- Weigh the desired amount of solid material, which may be a pure compound or actual target material, and place in a 2 x 3 inch low-density polyethylene (LDPE) permeable bag. Again, the suggested bag thickness ranges from 1 MIL to 8 MIL.
- Immediately heat-seal the polymer bag closed, eliminating as much air from within the bag as possible, and store or set aside in a weigh boat.
2. Gravimetric analysis to determine COMPS permeation rate
NOTE: A constant ambient temperature is important for accurate and reproducible measurements, both gravimetric and headspace. A constant temperature must be maintained during all testing. It is recommended to carry out all analytical measurements at desired temperatures during testing.
- To determine the permeation rate of the odorants through the permeable bag, place a newly made COMPS in a weigh boat inside of a fume hood.
- Place a clean, separate weigh boat on an analytical balance, and zero the balance.
- Remove the COMPS from the fume hood and place on the balance. Record the mass and immediately return to the fume hood.
- Continue recording the mass of the COMPS over regular time increments until the mass of the COMPS no longer changes (±5%). At this point the odor from the COMPS is depleted.
- As a negative control, create an empty COMPS consisting of just the substrate material without odorants sealed into the permeable bag. Treat this negative control in the same manner as the COMPS with odorant to ensure minimal fluctuations in mass over time.
- Calculate the permeation rate from the COMPS.
- Plot the mass of the COMPS versus time on an X-Y plot in appropriate statistical analysis software.
- Fit a linear trendline to just the linear portion of the graph and display an equation on a chart. The trendline should NOT be set to include the origin. The slope of the line (i.e., m in y = m+ b) is the permeation rate in mass per unit time.
3. Headspace analysis by solid phase microextraction with gas chromatography/mass spectrometry (SPME-GC/MS) (optional)
- Prepare a fresh COMPS following the instructions above and allow it to equilibrate in an open weigh boat inside of a fume hood for 30 min.
- Remove the COMPS from the weigh boat, place it into a 1 pint epoxy-lined metal sample container without a lid, and put it into a 1 gallon epoxy-lined metal container. The containers should be kept in a fume hood for the duration of the experiment.
- Allow at least 30 min for equilibration in the container prior to sampling.
- For sampling after equilibration, place a lid with a previously drilled 1 cm hole atop the outer container. Insert an appropriate SPME fiber through the hole on the lid to extract the analyte of interest. When the SPME fiber is not used, cover the hole with paraffin film or the like. The extraction time and fiber coating will be dependent on the type and amount of analyte vapor present, as well as the size of the sampling vessel and environmental conditions15.
- Remove the SPME fiber after the allotted extraction time and place in the heated inlet of a GC/MS for thermal desorption and analysis.
- Run the GC/MS method appropriate for the compound used in the COMPS16.
- For quantitation, compare the resulting peak area to an external calibration curve16 and/or internal standard17 as appropriate for the method and experimental design.
NOTE: 1) In this example, epoxy-lined metal sample containers were used, but other types of containers would also be suitable. To directly compare the odor availability to field olfactory evaluations, it would be best to use the same container, cleaned between each test, for both experiments; 2) For reproducible results, all aspects of the sampling procedure should be maintained in all replicate experiments, including but not limited to equilibration time, SPME extraction time, container type and size, and environmental conditions (i.e., temperature and humidity).
4. COMPS storage
- Place a single COMPS in a metalized barrier bag (3.5 x 4.5 inch) and heat-seal to close, removing as much air as possible from the bag prior to sealing (Figure 1B).
- Store in cool ambient or refrigerated conditions but not below or close to freezing to avoid the formation of condensation as the COMPS thaws.
- If testing multiple odorants or odorant delivery rates in a single experiment, secondary containment is recommended to eliminate any possible cross contamination during transportation and storage.
- Place replicate multiple barrier bags each containing individual COMPS of the same analyte and permeation rate in an outer, larger metalized bag or glass jar for storage and transport.
5. Field olfactory testing
NOTE: Olfactory testing can be carried out in many different ways depending on the animal being tested, the goal of the experiment, and the environmental conditions. The protocol below describes one such manner of testing. All animal testing should first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC).
- First, create blank or negative control COMPS as described above. Make enough so every container in the testing scenario will contain spare COMPS (2–3 depending on the number of animals involved in the experiment). Package all blank COMPS together in secondary containment (i.e., a larger metalized bag or glass jar with sealing lid).
- Create fresh COMPS as needed for the intended field test protocol. Eliminate all possible sources of contamination between the COMPS and the metalized bag. This can be accomplished by regular changing of gloves and cleaning of the laboratory work surface.
- Store the COMPS for at least 1 day prior to use to allow for equilibration. Store any replicates in the same secondary container. However, different COMPS should be in separate secondary containers.
- To set up a basic canine olfactory test, lay out multiple lines of at least five identical containers (e.g., metal cans, boxes), with the number of lines dependent on the number of variables being tested.
- Set up the trial so that each line contains one container with the target COMPS and four with blank COMPS. Positive control lines, prepared in the same manner but with a known target odor, may be used as appropriate for the experiment, training, or testing scenario. An additional negative control or blank line should contain five blank COMPS and no targets. Order this negative control line, positive control line (if using), and testing lines at random, and change order using a random number generator for each canine olfactory test as practical for the testing scenario.
- Include one distractor odorant/material per line as well.
- Randomize the order and location of the target and distractor odorants in each line for each canine being tested using a random number generator.
- Set up the trial so that each line contains one container with the target COMPS and four with blank COMPS. Positive control lines, prepared in the same manner but with a known target odor, may be used as appropriate for the experiment, training, or testing scenario. An additional negative control or blank line should contain five blank COMPS and no targets. Order this negative control line, positive control line (if using), and testing lines at random, and change order using a random number generator for each canine olfactory test as practical for the testing scenario.
- To prepare the containers, remove COMPS from the secondary and outer containers, placing only the permeable bag in the trial container.
- Allow the COMPS to equilibrate in the container for a minimum of 30 min prior to testing.
- Repeat steps for each COMPS being used in the test, starting with blanks, followed by positive controls (if using), and then testing odorants, changing gloves each time.
NOTE: Detailed examples of canine testing scenarios can be found in Simon et al.4 or Macias et al.8.
Representative Results
The primary objective of using COMPS in olfactory testing/training is to control the release of the chosen odorants and deliver a controlled amount of the odorant over the duration of the test or training session. Odorant release is measured by gravimetric analysis in terms of mass loss per unit time. Figure 2 gives an example of gravimetric results from the permeation of three identical COMPS prepared from 5 µL of pentanoic acid on cotton gauze through a 3 MIL LDPE bag. A line of regression was added to the graph, and the slope of the line represents the permeation rate of 37 µg/min for this set of COMPS.
It is often desirable to be able to adjust the amount of odorant released for a given test. This can be done in several ways, including adjusting the amount of material in the bag, the surface area of the permeable bag material, or the bag thickness. Figure 3 shows how all three of these factors were used to control the release of piperonal. Figure 3A indicates a logarithmic relationship between the mass in piperonal in the permeable bag (3 x 3 inch, 2 MIL LDPE), where the permeation rate increased quickly at the lower masses, then slowed after 500 mg due to physical restriction in the amount of odorant that could be released from the given bag at a time. The data in Figure 3B depict a linear relationship between permeation rate and surface area of the permeable bag for 2 g of piperonal in a 2 MIL LDPE bag. Finally, permeation rate decreased linearly with increased bag thickness (2 g of piperonal in a 3 x 3 inch bag), as shown in Figure 3C, because the thicker bag restricts and slows emission.
In another example of the utility of controlling permeation rates, Simon et al.4 used bag thickness to standardize permeation rates for compounds of varying vapor pressures in order to present canines with similar odorant availability for each analyte during field testing. A volume of 5 μLs of each neat analyte was pipetted onto separate cotton gauze pads and placed into 2 x 3 inch LDPE permeable bags. The permeation rates were measured by gravimetric analysis. Figure 4 shows the variation in vapor pressures (Figure 4A) across the groups of analytes (RSD = 138%) compared to the variation in permeation rate after adjusting the bag thickness (Figure 4B) to control the rate and make them as similar as possible (RSD = 31.8%). Furthermore, adjusting the bag thickness allowed permeation rates to vary by three orders of magnitude (Table 1).
Headspace measurements can be used to better measure the amount of odorant available during a given testing or training scenario. Macias18 measured the amount of piperonal in the headspace of three COMPS with permeation rates of 1,000, 100, and 10 ng/min (Figure 5). The COMPS were placed in a 1 quart sampling can, and the headspace was extracted for 30 min using SPME. The resulting chromatograph in Figure 5 shows the piperonal peak areas increasing with increasing permeation rate18.
Macias then used these three sets of piperonal COMPS in canine trials. Trained narcotics detection canines were tested on the 0 (blank), 10, 100, and 1,000 ng/s piperonal COMPS in a scent cage (Table 2). The results showed that as the permeation rate, and thus odor availability, increased the number of canines alerting to the appropriate COMPS increased18.

Figure 1: Examples of COMPS. (A) A COMPS constructed from a cotton gauze pad in permeable polymer bag. Reproduced from Simon et al.4 (B) A COMPS inserted into an outer impermeable bag. (C) A COMPS containing infected wood as an odor source in a polymer bag. Figures B and C were reproduced with permission from Simon et al.19. Please click here to view a larger version of this figure.
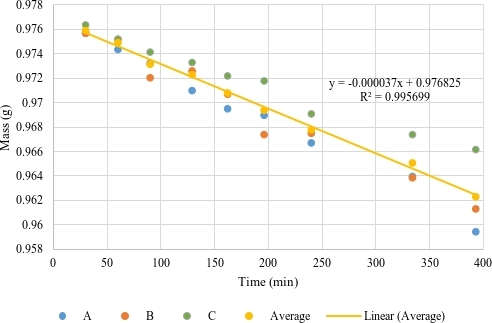
Figure 2: Example measurement of permeation rate by gravimetric analysis. The mass loss of the analyte (i.e., pentanoic acid) on gauze through a 3 MIL LDPE bag measured over time. A, B, and C indicate replicates of the same material, while "Average" is the averaged value of the three at each time point. The given equation describes the linear fit to the average data. Please click here to view a larger version of this figure.
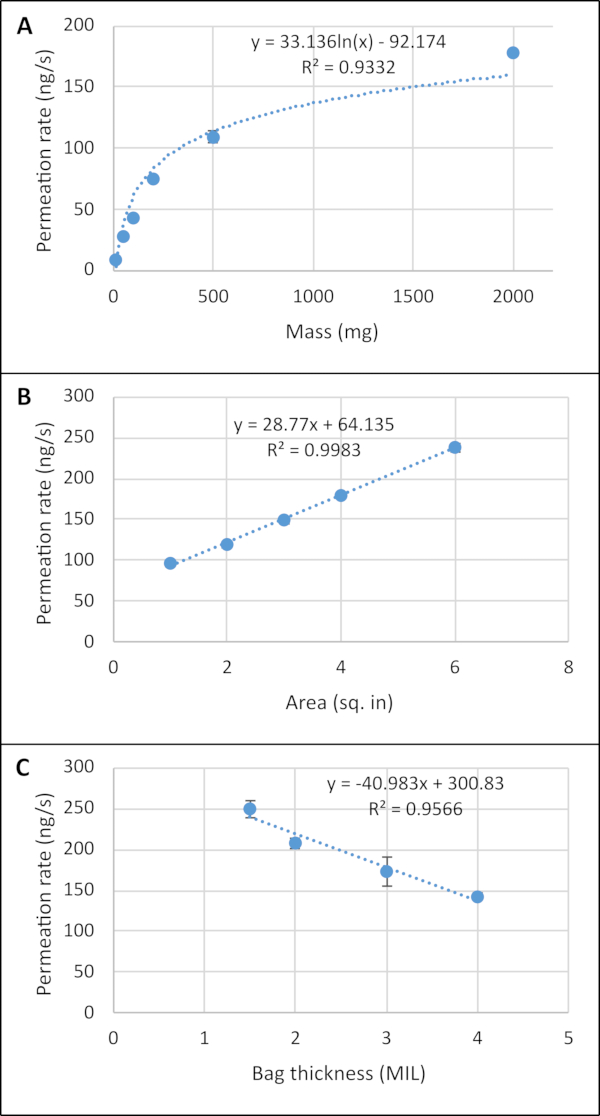
Figure 3: Examples of factors adjusting the permeation rate. Graphs of piperonal dissipation indicate experimentally measured permeation rates when changing (A) the mass of piperonal (3 x 3 inch, 2 MIL LDPE bag), (B) the surface area of the permeable bag (2 g of piperonal, 2 MIL LDPE), and (C) the bag thickness (2 g of piperonal, 3 x 3 inch bag). All error bars represent one standard deviation of the mean (some bars are within the size of the marker). These figures have been reproduced with permission from Macias et al.18. Please click here to view a larger version of this figure.
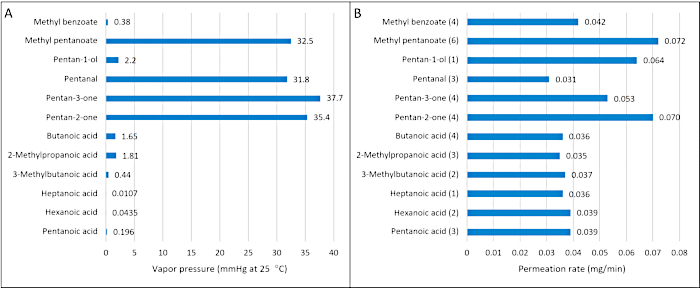
Figure 4: Comparison of vapor pressure variation across a group of compounds versus permeation rate variation. (A) Vapor pressures for a selection of 12 compounds (RSD = 138%) compared to (B) permeation rates for the same compounds with selected COMPS thicknesses (RSD = 31.8%). Numbers in parentheses represent LDPE bag thickness in MIL. These figures have been reproduced with permission from Simon et al.4. Please click here to view a larger version of this figure.
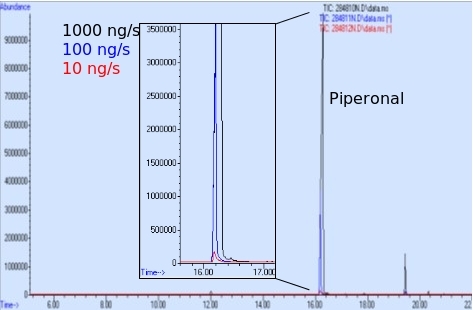
Figure 5: Headspace analysis of piperonal COMPS at three permeation rates. Overlaid chromatograms of the headspace components of piperonal COMPS adjusted to permeation rates of 1,000, 100, and 10 ng/s. Reproduced with permission from Macias et al.18. Please click here to view a larger version of this figure.
| Bag thickness | Permeation rate (mg/min) | R-squared |
| 1 MIL | 0.108 | 0.974 |
| 4 MIL | 0.042 | 0.991 |
| 8 MIL | 0.00499 | 0.99 |
| 4 MIL in metal bag w/ 1/8" hole | 0.000179 | 0.972 |
Table 1: Permeation rate versus bag thickness. Permeation rate of methyl benzoate on cotton gauze in COMPS of varying bag thicknesses. Note that the lowest permeation rate was achieved by placing a 4 MIL COMPS inside of a metalized bag with a 1/8 in hole. The R2 value indicates the fit of the line to the gravimetric plot.
| Piperonal COMPS permeation rate | Number of alerts | % alert |
| 0 ng/s (blank) | 0 | 0% |
| 10 ng/s | 4 | 25% |
| 100 ng/s | 7 | 44% |
| 1000 ng/s | 12 | 75% |
Table 2: Example of canine field trial results. Canine responses to piperonal COMPS with permeation rates ranging from 0–1,000 ng/s. Reproduced with permission from Macias et al.18.
Discussion
Controlled Odor Mimic Permeation Systems (COMPS) are easily created by sealing an odorant of interest into a permeable bag. This may be done by pipetting a neat liquid compound onto an absorbent material and then placing the absorbent material into the bag; by placing a pure, solid compound directly into the bag4, as was done in the case of piperonal8; or by placing the target material containing multiple or unknown odorants into a permeable bag, as was done with fungus-infected wood14. The permeable bag controls the release of the odorant so a known and reproducible amount can be delivered over a given training or testing period. Permeation rate is typically measured by gravimetric analysis, plotting mass loss over time, and can be adjusted by altering a number of parameters, including the absorbent material, the mass/volume of odorant, or parameters of the permeation bag (i.e. thickness, surface area, or polymer type). COMPS are stored in an outer nonpermeable envelope, which allows the COMPS to equilibrate prior to use, thus providing a known amount of odorant immediately upon use.
The greater the permeation rate of a COMPS, the greater concentration of odorant available during a training or testing scenario. To quantitate or compare the odor concentration emitted from a COMPS, headspace analysis of the COMPS in the testing/training container should be completed. This is most often done by extracting the odor using SPME with analysis by GC/MS. For quantitation or comparison purposes, it is recommended to use an internal standard and/or an external calibration curve.
COMPS serve as a low-cost, field-amenable devices for controlling the release of an odorant for olfactory training or testing, such as with canine detectors. COMPS can be used repeatedly until depletion, each time delivering the same odorant emission rate, although the length of time the emission rate is constant will change for each analyte and should be tested in the laboratory prior to use. This overcomes a widely recognized limitation of controlling odor delivery for field use and advances olfaction research and detection animal training.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded in part by the Office of Naval Research and the National Institute of Justice (2006-DN-BX-K027). The authors wish to thank the many “Furton Group” students that have participated in this project, as well as collaborators from the U.S. Naval Research Laboratory and the Naval Surface Warfare Center (Indian Head EOD Technology Division). Finally, the authors thank Peter Nunez of U.S. K-9 Academy, Tony Guzman of Metro-Dade K9 Services, and Miami-Dade area law enforcement canine teams.
Materials
| 16 oz economy jars (70-450 finish) | Fillmore container | A16-08C-Case 12 | |
| 7890A gas chromatograph / 5975 mass selective detector | Agilent | ||
| Analytical balance | Mettler Toledo | 01-911-005 | |
| Ball regualr bands and dome lids | Fillmore container | J30000 | |
| Cotton gauze (2" x 2") | Dukal | ||
| Disposable weighing boats | VWR | 10803-148 | |
| Epoxy-lined sample containers, 1 gallon | TriTech Forensics | CANG-E | |
| Epoxy-lined sample containers, 1 pint | TriTech Forensics | CANPT-E | |
| Low density polyetheylene bag | Uline | S-5373 | |
| Rtx-Volatiles (30 m x 0.32 mmID) column | Restek | 10901 | |
| Silver metalized mylar barrier bag (3.5" x 4.5") | ESP Packaging | 95509993779 | |
| Silver metalized mylar barrier bag (5" x 8.5" x 3") | ESP Packaging | 95509993793 | |
| Solid phase microextration fiber assembly (PDMS/DVB/CAR) | Sigma-Aldrich | 57328-U | |
| Solid phase microextration holder | Sigma-Aldrich | 57330-U | |
| Tabletop Impulse Sealer | Uline | H-190 | Heat sealer |
References
- Buck, L., Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 65, 175-187 (1991).
- Furton, K. G., Myers, L. J. Scientific foundation and efficacy of the use of canines as chemical detectors for explosives. Talanta. 54, 487-500 (2001).
- Leitch, O., Anderson, A., Kirkbride, K., Lennard, C. Biological organisms as volatile compound detectors: A review. Forensic Science International. 232, 92-103 (2013).
- Simon, A. G., et al. Method for controlled odor delivery in canine olfactory testing. Chemical Senses. 44 (6), 399-408 (2019).
- Hallowell, L. R., et al. Detection of hidden explosives: New challenges and progress (1998-2009). Forensic Investigation of Explosives. 2nd Ed. , 53-77 (2012).
- Papet, L. Narcotic and explosive odors: Volatile organic compounds as training aids for olfactory detection. Canine Olfaction Science and Law. , 265-278 (2016).
- Furton, K., Harper, R. Controlled Odor Mimic Permeation System. US Patent. , (2017).
- Macias, M. S., Guerra-Diaz, P., Almirall, J. R., Furton, K. G. Detection of piperonal emitted from polymer controlled odor mimic permeation systems utilizing canis familiaris and solid phase microextract-ion mobility spectrometry. Forensic Science International. 195, 132-138 (2010).
- Harper, R., Almirall, J., Furton, K. Identification of dominant odor chemicals emanating from explosives for use in developing optimal training aid combinations and mimics for canine detection. Talanta. 67, 313-327 (2005).
- Francis, V. S. The identification of volatile organic compounds from synthetic cathinone derivatives for the development of odor mimic training aids. Florida International University. , (2017).
- Huertas-Rivera, A. M. Identification of the active odors from illicit substances for the development of optimal canine training aids. Florida International University. , (2016).
- DeGreeff, L. E., Furton, K. G. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Analytical and Bioanalytical Chemistry. 401, 1295-1307 (2011).
- DeGreeff, L. E., Curran, A. M., Furton, K. G. Evaluation of selected sorbent materials for the collection of volatile organic compounds related to human scent using non-contact sampling mode. Forensic Science International. 209 (1-3), 133-142 (2011).
- Simon, A. G., Mills, D. K., Furton, K. G. Chemical and canine analysis as complimentary techniques for the identification of active odors of the invasive fungs, Raffaelea lauicola. Talanta. 168, 320-328 (2017).
- Penton, Z. Method development with solid phase microextraction. Solid Phase Microextraction: A Practical Guide. , 27-58 (1999).
- Robards, K., Haddad, P. R., Jackson, P. E. . Principles and Practice of Modern Chromatographic Methods. , (2004).
- MacCrehan, W., Moore, S., Schantz, M. Evaluating headspace component vapor-time profiles by solid-phase microextraction with external sampling of an internal standard. Analytical Chemistry. 83, 8560-8565 (2011).
- Macias, M. S. . The Development of an Optimized System of Narcotic and Explosive Contraband Mimics for Calibration and Training of Biological Detectors. , (2009).
- Simon, A. G. . The Detection of an Invasive Pathogen through Chemical and Biological Means for the Protection of Commercial Crops. , (2017).

