Mouse Footpad Inoculation Model to Study Viral-Induced Neuroinflammatory Responses
Summary
The footpad inoculation model is a valuable tool for characterizing viral-induced neuroinflammatory responses in vivo. In particular, it provides a clear assessment of viral kinetics and associated immunopathological processes initiated in the peripheral nervous system.
Abstract
This protocol describes a footpad inoculation model used to study the initiation and development of neuroinflammatory responses during alphaherpesvirus infection in mice. As alphaherpesviruses are main invaders of the peripheral nervous system (PNS), this model is suitable to characterize the kinetics of viral replication, its spread from the PNS to CNS, and associated neuroinflammatory responses. The footpad inoculation model allows virus particles to spread from a primary infection site in the footpad epidermis to sensory and sympathetic nerve fibers that innervate the epidermis, sweat glands, and dermis. The infection spreads via the sciatic nerve to the dorsal root ganglia (DRG) and ultimately through the spinal cord to the brain. Here, a mouse footpad is inoculated with pseudorabies virus (PRV), an alphaherpesvirus closely related to herpes simplex virus (HSV) and varicella-zoster virus (VZV). This model demonstrates that PRV infection induces severe inflammation, characterized by neutrophil infiltration in the footpad and DRG. High concentrations of inflammatory cytokines are subsequently detected in homogenized tissues by ELISA. In addition, a strong correlation is observed between PRV gene and protein expression (via qPCR and IF staining) in DRG and the production of pro-inflammatory cytokines. Therefore, the footpad inoculation model provides a better understanding of the processes underlying alphaherpesvirus-induced neuropathies and may lead to the development of innovative therapeutic strategies. In addition, the model can guide research on peripheral neuropathies, such as multiple sclerosis and associated viral-induced damage to the PNS. Ultimately, it can serve as a cost-effective in vivo tool for drug development.
Introduction
This study describes a footpad inoculation model to investigate the replication and spread of viruses from the PNS to CNS and associated neuroinflammatory responses. The footpad inoculation model has been intensively used to study alphaherpesvirus infection in neurons1,2,3. The main objective of this model is to allow neurotropic viruses to travel a maximal distance through the PNS before reaching the CNS. Here, this model is used to obtain new insights in the development of a particular neuropathy (neuropathic itch) in mice infected with pseudorabies virus (PRV).
PRV is an alphaherpesvirus related to several well-known pathogens (i.e., herpes simplex type 1 and 2 [HSV1 and HSV2] and varicella-zoster virus [VZV]), which cause cold sores, genital lesions, and chicken pox, respectively4. These viruses are all pantropic and able to infect many different cell types without showing affinity for a specific tissue type. However, they all exhibit a characteristic neurotropism by invading the PNS (and occasionally, the CNS) of host species. The natural host is the pig, but PRV can infect most mammals. In these non-natural hosts, PRV infects the PNS and induces a severe pruritus called the “mad itch”, followed by peracute death5,6. The role of the neuroimmune response in the clinical outcome and pathogenesis of PRV infection has been poorly understood.
The footpad inoculation model allows PRV to initiate infection in the epidermal cells of the footpad. Then, the infection spreads into sensory and sympathetic nerve fibers that innervate the epidermis, sweat glands, and dermis. The infection spreads by virus particles moving via the sciatic nerve to the DRG within approximately 60 h. The infection spreads through the spinal cord, ultimately reaching the hindbrain when animals become moribund (82 h post-infection). During this time window, tissue samples can be collected, processed, and analyzed for virus replication and markers of the immune response. For instance, histological examination and viral load quantification can be performed in different tissues to establish correlations between the initiation and development of clinical, virological, and neuroinflammatory processes in PRV pathogenesis.
Using the footpad inoculation model, the cellular and molecular mechanisms of PRV-induced pruritus in mice can be investigated. Moreover, this model can provide new insight into the initiation and development of virus-induced neuroinflammation during herpesvirus infections. A better understanding of the processes underlying alphaherpesvirus-induced neuropathies may lead to the development of innovative therapeutic strategies. For instance, this model is useful to investigate the mechanisms of neuropathic itch in patients with post-herpetic lesions (e.g., herpes zoster, shingles) and test novel therapeutic targets in mice for the corresponding human diseases.
Protocol
All animal experiments were performed in accordance to a protocol (number 2083-16 and 2083-19) reviewed and approved by the Institution Animal Care and Use Committee (IACUC) of Princeton University. This work was done by strictly following the biosafety level-2 (BSL-2) requirements, to which we have a fully equipped lab approved by the Princeton University biosafety committee. The procedures including mouse footpad abrasion, viral inoculation, mouse dissection, and tissue collection were performed in a biological safety cabinet (BSC) in the Princeton University biocontainment animal facility room. Those performing the procedure wore disposable gowns, head cover, eye protection, sterile gloves, surgical masks, and shoe covers.
1. Mouse footpad abrasion
- Anesthetize a C57BL/6 mouse (5–7 weeks old) with isoflurane gas anesthetic, delivered at a dosage of 3% via a small anesthesia system (chamber).
- Place the mouse in the surgical plane of anesthesia on its back, prior to inoculation, in the hind footpad. Attach a nose cone with a diaphragm (a slit sufficiently sized to fit the muzzle of the animal) to the mouse and deliver isoflurane gas anesthetic at a constant dosage of 1.5%–2.0%.
- Closely monitor the mouse for a response to painful stimulus created by forceful pinching of the toe with forceps.
- Lightly grasp one hind footpad with flat forceps.
- Gently abrade the glabrous skin of the hind footpad approximately 20x, between the heel and walking pads, with an emery board (100–180 grit). Do not induce bleeding by abrading too frequently or applying too much pressure.
- Using fine forceps, slowly peel off the stratum corneum detached by abrasion to expose the stratum basale.
2. PRV footpad inoculation
- Prepare the virus inoculum diluted to the desired titer in DMEM media containing 2% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Table of Materials).
- Quantitate the number of plaque-forming units (PFU) in the virus stock on PK15 cells to calculate and standardize the viral dose and dilute the viral inoculum accordingly.
NOTE: In this study, virulent strain of PRV (PRV-Becker) was used at a dose of 8 x 106 PFU. This dose was optimized in a previous preliminary experiment to ensure that all inoculated animals showed clinical symptoms at 82 h post-inoculation (hpi). - Keep the virus inoculum on ice and gently mix before use.
- Quantitate the number of plaque-forming units (PFU) in the virus stock on PK15 cells to calculate and standardize the viral dose and dilute the viral inoculum accordingly.
- Add a 20 μL droplet of virus inoculum (8 x 106 PFU) onto the abraded footpad (topical administration). Carry out mock inoculations (medium only) in parallel.
- Gently rub 10x with the shaft of a needle to facilitate adsorption of the virus. Avoid scratching with the needle point. Repeat this step every 10 min.
- Keep the mouse under anesthesia for 30 min until the abraded footpad is dry.
- After stopping anesthesia, monitor the mouse until it can maintain sternal recumbency and place it in an individual cage for clinical follow-up and sampling.
3. Mouse dissection and tissue collection
- At appropriate times after infection, euthanize the mouse by the asphyxiation method (CO2).
NOTE: In this study, the humane endpoint (when animals begin to exhibit terminal symptoms) for PRV-Becker infected mice is 82 hpi. Euthanize control animals in parallel. - Place the mouse ventral side facing up on a surgical mat using needles/pins. Secure the limbs of the mouse with pins to a surgical foam board. Wet the ventral side of the mouse with 70% ethanol to minimize fur contamination.
- Pinch the external layer of fur and skin using forceps and make a small initial incision using fine scissors near the urethral opening.
- From this opening, continue the incision on the mid ventral side up to the chin.
- Extend two lateral incisions towards the extremities of the forelimbs and hind limbs.
- Separate the skin from the underlying muscular layer and pin down to the side.
- Open the abdominal cavity and incise up to the base of the thorax. Complete the opening of the abdominal cavity by making two transversal incisions.
- Open the thoracic cavity by cutting the diaphragm and ribs on both lateral sides.
NOTE: Cutting the sides of the ribcage prevents the risk of cutting the heart. - Collect exposed organs, including heart, lungs, spleen, pancreas, liver, kidneys, and bladder in 1.5 mL microtubes. Keep the tubes on ice.
- Cut the abraded footpad between the heel and walking pads and place the piece of tissue in a tube as described in step 3.3.6.
- Place the mouse dorsal side up and wet the fur with 70% ethanol. Cut down the skin layer to expose the vertebral column.
- Make a small incision in the region of the pelvis. Strip the skin from the hind limbs towards the head.
- Remove the legs and arms by cutting with scissors parallel with and close to the spinal column on both sides.
- Cut the head off at the base of the skull (C1–C2 level).
- Cut open the skull with scissors from the foramen magnum to the frontal bone.
- Pull open the skull in lateral directions using forceps.
- Gently scoop out the brain using forceps and proceed as described in step 3.3.6.
- Clean the spinal column by cutting muscle, fat and soft tissues using curved scissors. Cut the tail. Place the intact spinal column in a 15 mL tube containing sterile ice-cold phosphate-buffered saline (PBS). Keep tubes on ice until further dissection of the spinal cord and DRG.
4. Spinal cord and DRG extraction
NOTE: Extract spinal cord and DRG from the vertebrae column directly following mouse dissection. The protocol for spinal cord and DRG extraction has been adapted from a previous publication7.
- Remove the vertebrae column from ice-cold PBS tube and remove remaining soft tissues, which becomes easier after refrigeration.
- Make three transverse cuts in the column through the vertebrae (T1, L1, and S2 levels) using a razor blade. Place the three pieces in a 15 mm Petri dish containing sterile ice-cold PBS. Keep track and mark origin of segments for later analysis.
- Take one segment and secure it between forceps, dorsal side facing up. Make a single, longitudinal cut down through the midline using a razor blade, splitting the column in two equal halves.
- Place both halves in a new Petri dish containing new sterile ice-cold PBS. Ensure the correct orientation of the segment to be able to distinguish left and right sides.
- Under a dissecting microscope, gently peel the spinal cord out of the vertebrae column from each half in a rostral to caudal direction using fine forceps.
- Collect both spinal cord halves in 1.5 mL microtubes containing 500 μL of sterile ice-cold PBS. Keep the tubes on ice.
- Gently remove the meninges from one side of the spinal column to the other to expose the DRG.
- Remove each DRG individually by grasping the exposed spinal nerve and pull it gently out of the spinal column. Place the collected DRG in a 15 mm Petri dish containing ice-cold PBS. Harvest all ipsilateral and contralateral DRG separately from the spinal column segment and proceed as described in step 4.5.
NOTE: The DRG is visible as a round transparent structure along the white spinal nerve. - Repeat steps 4.3–4.7 using the two other spinal column segments within 30–45 min.
- Centrifuge all tubes at high speed (17,900 x g) for 3 min at 4 °C.
- Aspirate supernatant and flash-freeze tubes in liquid nitrogen. Store tubes at -80 °C for further tissue homogenization.
- Alternatively, fix and section the cleaned DRG and other mouse tissues for histopathological analyses and/or immunofluorescence staining after step 4.9.
5. Tissue homogenization
- Thaw the tubes containing frozen tissue samples on ice.
- Weigh 100 mg of tissue and place it in a 2 mL microcentrifuge tube containing a sterile steel bead and 500 μL of RIPA buffer containing 0.5 M EDTA (pH = 8.0), 1 M Tris-HCl (pH = 8.0), 5 M NaCl, 10% SDS, and protease cocktail inhibitor tablets.
- Disrupt tissues at room temperature (RT) using a homogenizer at 20 cycles/s for 2 min, followed by a 1 min waiting period, then 20 cycles/s for 2 min.
- Centrifuge the tube at high speed (17,900 x g) for 10 min at RT.
- Collect supernatant (500 μL) in a new tube and store it at -20 °C until performing ELISA and qPCR to quantify inflammatory markers and viral loads, respectively.
NOTE: For qPCR, collect all samples with RNase-free material (i.e., beads, tubes, etc.) and disrupt tissues with RNA lysis buffer containing 1% betamercaptoethanol (Table of Materials).
6. Preparation and immunofluorescence staining of frozen DRG sections
- Tissue processing
- Fix the dissected and cleaned DRG in 1% paraformaldehyde (PFA) for 2 h at RT.
- Transfer the tissue into a 15 mL tube with 10% sucrose in PBS. Incubate overnight at 4 °C.
- Transfer the tissue into a 15 mL tube with 20% sucrose in PBS. Incubate overnight at 4 °C.
- Transfer the tissue into a 15 mL tube with 30% sucrose in PBS. Incubate overnight at 4 °C.
- Embed tissues in OCT using a cryomold and place blocks in dry ice for rapid freezing.
- Store the samples at -80 °C until use.
- Cryosectioning preparation
- Remove the frozen block from -80 °C and allow it to equilibrate in the cryostat chamber temperature for 30 min.
- Cryosection the DRG at a thickness of 15 µm and mount the sections onto slides.
- Use a pen to draw a hydrophobic circle around the slide-mounted tissue.
- Keep the slides at 4 °C until staining.
- Immunofluorescence staining
- Wash the DRG sections 3x in PBS for 10 min at RT.
- Incubate the sections with 100 µL of desired primary antibody (e.g., mouse antibody against PRV glycoprotein B) for 1 h at 37 °C. Dilute the primary antibody in PBS containing 10% negative goat serum.
- Repeat step 6.3.1.
- Incubate the sections with 100 µL of desired secondary antibody (goat anti-mouse Alexa Fluor 488) for 50 min at 37 °C. Dilute secondary antibody in PBS only.
- Add 100 µL of DAPI (4′,6-diamidino-2-phenylindole) dye diluted in PBS onto the section. Further incubate samples 10 min at 37 °C.
- Repeat step 6.3.1.
- Mount the samples using a fluorescence mounting medium onto glass slides and secure and cover with coverslip.
7. Preparation and H&E staining of paraffin-embedded tissue sections
- Fix the tissue in 10% formalin for 24 h at 4 °C.
- Perform standard processing schedule for paraffin embedding and sectioning of fixed tissues. Transfer fixed tissues to 70% ethanol and process through upgraded alcohols to xylene followed by paraffin, as previously described8.
NOTE: Use polyester sponges to keep small tissue samples such as DRG inside cassettes. - Perform standard deparaffinization followed by H&E staining of tissue sections, as previously described9.
Representative Results
The mouse footpad inoculation model allows for characterization of the immunopathogenesis of alphaherpesvirus infection in vivo, including replication and spread of the infection from the inoculated footpad to the nervous system and the induction of specific neuroinflammatory responses.
In this study, we first abraded the mouse hind footpad and either mock-inoculated or inoculated the abraded region with a virulent strain of PRV (PRV-Becker). The site of abrasion was visible in the control footpad. A crust was formed at the abrasion site as part of the healing process (Figure 1, black arrow). In contrast, mice inoculated with PRV showed severe inflammation at the humane endpoint (82 hpi), characterized by swelling of the footpad and redness.
Following footpad inoculation with the virulent PRV-Becker strain, mice began showing clinical signs at 72 hpi, characterized by swelling of the inoculated footpad and increasingly frequent tremors. By 82 hpi, PRV-Becker infected mice showed constant tremors in the inoculated leg and distinctive PRV symptoms, including intense scratching and biting of the foot. Severe inflammation was also observed in the footpad. The inflammatory response induced during PRV infection was then further characterized, including the infiltration of immune cells into tissues.
Histopathological examination of several tissues was performed, including the inoculated footpad and DRG, followed by H&E staining of paraffin-embedded tissue sections. Epidermal necrosis and severe dermal inflammation (edema and fibrin) were observed in PRV-infected foot sections (Figure 2A, panel b). The epidermis, dermis, and connective tissues of infected mice showed a massive infiltration of neutrophils (identified by multilobed nuclei) marked with black arrows. Footpads of control mice were normal (Figure 2A, panel a). PRV-infected DRG showed minimal neuronal necrosis and mixed inflammation in infected mice while the DRG of control mice were normal (Figure 2B, panels a and b). The mixed inflammation infiltrate consisted mainly of neutrophils and lymphocytes.
Next, the kinetics of inflammatory cytokine production in mouse tissue after PRV footpad inoculation were established. Levels of specific inflammatory cytokines were quantified (i.e, interleukin-6 [IL-6] and granulocyte-colony stimulating factor [G-CSF]) from several tissues collected and homogenized from control and PRV-infected mice. The results demonstrated a significant increase of G-CSF levels in the footpad and DRG compared to controls at 7 hpi and 82 hpi (Figure 3A). Significant G-CSF levels were observed at 82 hpi in spinal cord, brain, heart, and liver tissue of PRV-infected mice compared to controls. Moreover, significant IL-6 levels were detected in all tissues of PRV-infected mice compared to controls starting at 24 hpi (Figure 3B).
The footpad inoculation model was further used to investigate PRV replication and spread from the inoculated footpad to the PNS and CNS and potential correlations with neuroinflammatory response development. PRV loads were quantified in several homogenized tissues by qPCR to amplify PRV DNA. DNA concentration was then converted to PFU, as previously described10. PRV loads were detected in the footpad starting at 24 hpi (~1 x 104 PFU/mg of tissue) and in DRG starting at 60 hpi (~1 x 103 PFU/mg of tissue; data not shown).
In a moribund state (82 hpi), PRV was detected in the footpad, DRG, spinal cord, and brain, with the highest concentration of PRV in DRG (~1 x 105 PFU/mg of tissue; Figure 4). PRV infection of DRG was confirmed by indirect immunofluorescence staining of DRG cryosections. PRV infection was detected in DRG neurons using anti-PRV gB antibody. PRV glycoprotein gB was expressed during late stages of infection in the cytoplasm of infected cells. As expected, the cytoplasmic expression of PRV gB (green) was confirmed in infected DRG, while no gB was expressed in control samples (Figure 5). Cell nuclei were identified with DAPI staining (blue).
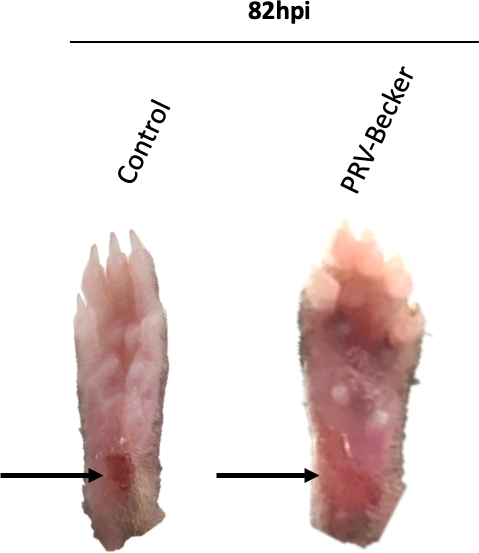
Figure 1: Representative images of mouse right hind paws after PRV inoculation. Mice are either mock-inoculated or inoculated with PRV in the abraded right hind footpad. PRV-inoculated footpad shows signs of inflammation, including redness and swelling at humane endpoint (82 hpi). The footpad of control mice appears normal with a dark red crust at the abraded site, indicating that the wound is healing. Black arrows indicate the site of abrasion. This figure has been modified from a previous publication11. Please click here to view a larger version of this figure.
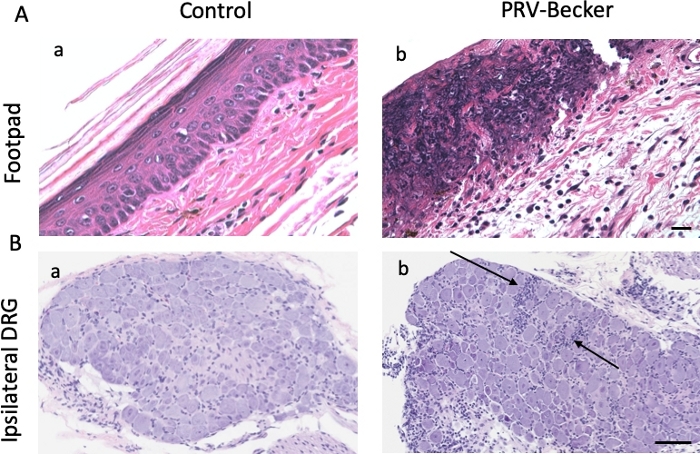
Figure 2: Histopathological findings in footpad and DRG after PRV footpad inoculation. Hematoxylin and eosin (H&E) staining of (A) mouse inoculated footpads and (B) and ipsilateral DRG from control (panel a) and PRV-infected (panel b) mice at 82 hpi. Histopathological manifestations observed in PRV-infected tissues (epidermal and neuronal necrosis and neutrophil infiltration) are absent from all examined mock-infected mice. Results are representative of three biological replicates for a given type of tissue. Black arrows indicate representative areas of inflammation with immune cell infiltration. Scale bars (50 μm) are indicated for each picture. This figure has been modified from11. Please click here to view a larger version of this figure.
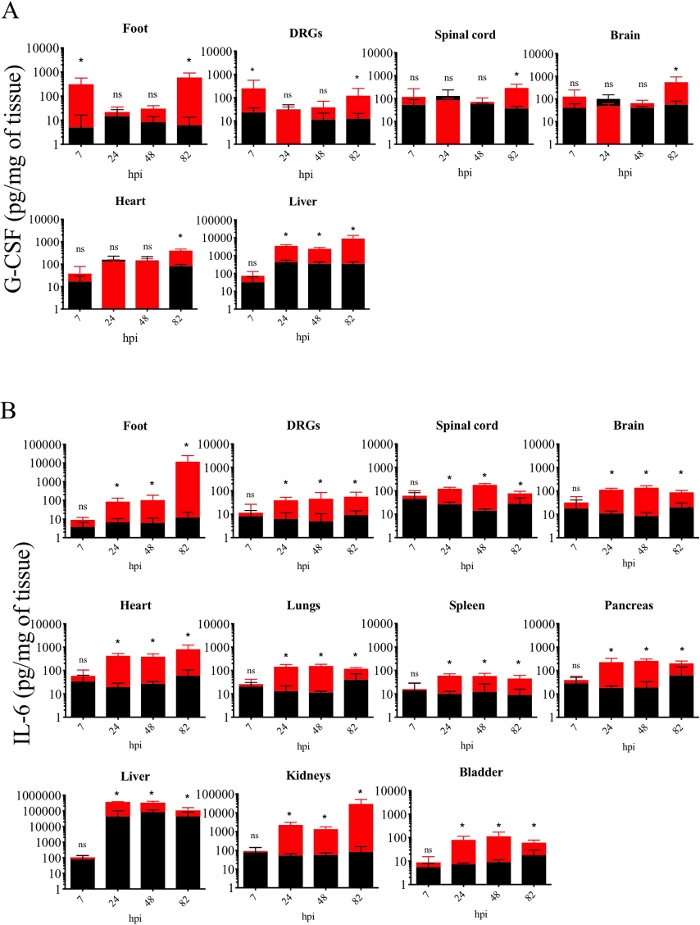
Figure 3: Kinetics of inflammatory cytokine production in homogenized mouse tissues after PRV footpad inoculation. (A) G-CSF and (B) IL-6 protein levels detected in PRV-infected (red) and control (black) mouse homogenized tissues at different hpi. Protein levels are quantified by ELISA and expressed as picogram (pg) per milligram (mg) of homogenized tissue (n = 5 per group, *p < 0.05, ns = not significant). This figure has been modified from a previous publication12. Please click here to view a larger version of this figure.
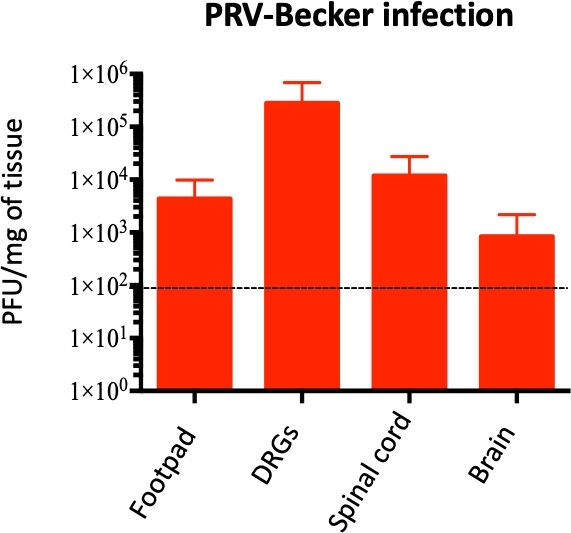
Figure 4: Quantification of PRV genome in mouse homogenized tissues. PRV DNA is quantified in homogenized mouse tissues by qPCR using PRV UL54 primers. PRV loads are expressed as plaque forming units (PFU) per mg of tissue. PRV DNA is detected only in the foot, DRG, spinal cord, and brain (and not in other tissues), n = 10 per group. Dotted line shows the detection limit. This figure has been modified from a previous publication11. Please click here to view a larger version of this figure.
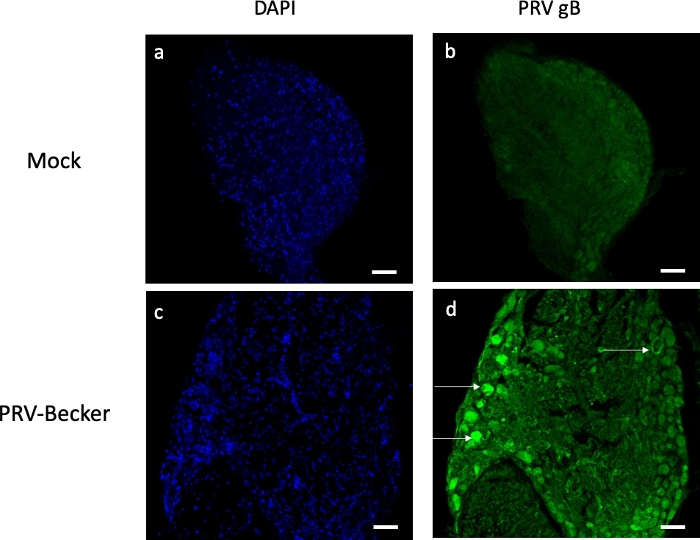
Figure 5: Assessment of PRV infection in DRG neurons by immunofluorescence staining. Confocal Z-stack images of mock- and PRV-infected DRG neurons after immunofluorescence staining using a mouse antibody specific for PRV gB (green). Cell nuclei are stained with DAPI (blue, panels a and c). Panel d shows several PRV-infected neurons expressing gB (white arrows). No gB expression is detected in the control DRG sections (panel b). Scale bars (50 μm) are indicated for each picture. Please click here to view a larger version of this figure.
Discussion
The footpad inoculation model described here is useful to investigate the initiation and development of neuroinflammatory responses during alphaherpesvirus infection. Moreover, this in vivo model is used to establish the kinetics of replication and spread of alphaherpesvirus from the PNS to CNS. This is an alternate to other inoculation models, such as the flank skin inoculation model, which relies on deep dermal scratching13, or the intracranial route, which directly introduces the virus into the CNS14,15,16. As a result, with the footpad model, it is possible to obtain a more detailed assessment of viral kinetics of replication and spread with associated local and distant immunopathological processes in the nervous system11,12.
In this protocol, the abrasion of the footpad and subsequent viral inoculation are crucial steps. Indeed, the stratum corneum needs to be completely removed after adequate abrasion in order to expose the stratum basale to viral inoculum for successful infection. However, the abrasion must be gentle and not induce bleeding, as this helps prevent infection of the blood circulation. The detached stratum corneum can be visualized by H&E staining under light microscopy. The corneocytes present in the stratum corneum are flat, eosinophilic cells that lack nuclei. The volume of the viral inoculum (20 μL droplet) has been optimized to ensure that the droplet stays on the footpad and covers the abraded site. Gently rubbing the inoculum droplet onto the abraded footpad is essential for efficient viral penetration. It is recommended to wait until the footpad is completely dry to stop the anesthesia and place the animal in a new cage. This step will avoid the mouse from licking the viral inoculum off the abraded footpad. It is recommended to process a maximum of three mice at once, using a nose cone that is set to expose them simultaneously to anesthesia.
While performing the mouse dissection, it is important to make cuts parallel to the vertebrae column, which prevents damage to the spinal cord and associated DRG. It also is suggested to remove as much fat, muscle, and soft tissue as possible to reduce accidental cutting into the spinal column and facilitate a better grip on the column segment with forceps before cutting down the midline.
It is also recommended to perform transverse cuts through the vertebrae column between discs in order to produce cleaned column segments and limit the risk of damaging DRG pairs. The meninges surrounding the spinal cord and covering the DRG must be completely removed to facilitate the identification and extraction of DRG. The DRG should be removed carefully from the spinal column without damage from the forceps. It is important that DRG remain intact for H&E and immunofluorescence staining. Timing is critical for efficiency of this in vivo experiment, and the mouse dissection and spinal cord/DRG extraction should be performed consecutively in order to collect tissue that is as fresh as possible.
The tissue homogenization method described here has been optimized to ensure efficient disruption of a large number of heterogenous tissue samples. It is paramount to standardize the amount of tissue used, which allows direct comparison of ELISA and qPCR results between samples. For instance, it is suggested to weigh 100 mg of tissue for each homogenization procedure. Each tissue sample must be homogenized in its entirety, and leftovers should not be freeze-thawed. It is important to autoclave the steel beads and forceps before use to avoid any contamination during homogenization. A volume of 500 μL is optimal to ensure complete homogenization of a 100 mg tissue sample. It is important to note that this limited volume allows only for the processing of three to four ELISA kits per sample.
Using the footpad inoculation model, it was demonstrated that PRV infection in mice induces severe inflammation, characterized by neutrophil infiltration in the footpad and DRG. High concentrations of inflammatory cytokines G-CSF and IL-6 were also detected in many homogenized tissues using ELISA. In addition, a strong correlation was found between PRV gene and protein expression (by qPCR and IF staining) in DRG and the production of both pro-inflammatory cytokines.
This model is suitable to compare the kinetics of viral replication/spread as well as neuroinflammatory responses among different alphaherpesvirus infections. For instance, regarding VZV, the restricted host-specificity and lack of clinical disease have limited the use of animal models17. Therefore, the mouse footpad PRV inoculation model may represent a new animal model for the study of the cellular and molecular mechanisms responsible for neuropathic pruritus in patients with post-herpetic lesions. Based on the similarities in clinical signs, pathogenesis, and genomes between VZV and PRV, it is believed that this mouse model will enhance the understanding of VZV pathogenesis and lead to the developments in innovative therapeutic strategies.
Finally, the model will guide research on peripheral neuropathies, such as multiple sclerosis and associated viral-induced damage to the PNS18. The pathogenesis of several neurotropic viruses (i.e., rabies virus, poliovirus, West Nile virus, Zika virus), which are known to infect the PNS, can also be studied using this model19,20,21,22. The footpad inoculation model can be used as a possible cost-effective tool for drug development. For instance, it may serve as a platform to screen and test the efficacy of anti-inflammatory and antiviral drugs designed to prevent viral-induced peripheral neuropathies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Charles River laboratories for their excellent technical support executing the histopathology analyses. This work was funded by National Institute of Neurological Disorders and Stroke (NINDS) (RO1 NS033506 and RO1 NS060699). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| Antibody anti-PRV gB | Made by the lab | 1/500 dilution | |
| Aqua-hold2 pap pen red | Fisher scientific | 2886909 | |
| Compact emery boards-24 count (100/180 grit nail files) | Revlon | ||
| Complete EDTA-free Protease Inhibitor Cocktail | Sigma-Aldrich | 11836170001 | |
| C57BL/6 mice (5-7 weeks) | The Jackson Laboratories | ||
| DAPI solution (1mg/ml) | Fisher scientific | 62248 | 1/1000 dilution |
| Disposable sterile polystyrene petri dish 100 x 15 mm | Sigma-Aldrich | P5731500 | |
| Dulbecco's Modified Eagle Medium (DMEM) | Hyclone, GE Healthcare life Sciences | SH30022 | |
| Dulbecco's Phophate Buffer Saline (PBS) solution | Hyclone, GE Healthcare life Sciences | SH30028 | |
| Fetal bovine serum (FBS) | Hyclone, GE Healthcare life Sciences | SH30088 | |
| Fine curved scissors stainless steel | FST | 14095-11 | |
| Fluoromount-G mounting media | Fisher scientific | 0100-01 | |
| Formalin solution, neutral buffered 10% | Sigma-Aldrich | HT501128 | |
| Isothesia Isoflurane | Henry Schein | NDC 11695-6776-2 | |
| Microcentrifuge tube 2ml | Denville Scientific | 1000945 | |
| Microtube 1.5ml | SARSTEDT | 72692005 | |
| Negative goat serum | Vector | S-1000 | |
| Penicillin/Streptomycin | Gibco | 154022 | |
| Precision Glide needle 18G | BD | 305196 | |
| Razor blades steel back | Personna | 9412071 | |
| RNA lysis buffer (RLT) | Qiagen | 79216 | |
| Stainless Steel Beads, 5 mm | Qiagen | 69989 | |
| Superfrost/plus microscopic slides | Fisher scientific | 12-550-15 | |
| Tissue lyser LT | Qiagen | 69980 | |
| Tissue-Tek OCT | Sakura | 4583 | |
| 488 (goat anti-mouse) | Life Technologies | A11029 | 1/2000 dilution |
References
- Field, H. J., Hill, T. J. The pathogenesis of pseudorabies in mice following peripheral inoculation. Journal of General Virology. 23 (2), 145-157 (1974).
- Engel, J. P., Madigan, T. C., Peterson, G. M. The transneuronal spread phenotype of herpes simplex virus type 1 infection of the mouse hind footpad. Journal of Virology. 71 (3), 2425-2435 (1997).
- Guedon, J. M., et al. Neuronal changes induced by Varicella Zoster Virus in a rat model of postherpetic neuralgia. Virology. 482, 167-180 (2015).
- Pomeranz, L. E., Reynolds, A. E., Hengartner, C. J. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiology and Molecular Biology Reviews. 69 (3), 462-500 (2005).
- Wittmann, G., Rziha, H. J., Knipe, D. M., Howley, P. M. Aujeszky’s disease (pseudorabies) in pigs. Herpesvirus diseases of cattle, horses and pigs. 9, 230-325 (1989).
- Leman, A. D., Glock, R. D., Mengeling, W. L., Penny, R. H. C., Scholl, E., Straw, B. . Diseases of swine, 6th ed. , 209-223 (1986).
- Sleigh, J. N., Weir, G. A., Schiavo, G. A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Research Notes. 9, 82 (2016).
- Sands, S. A., Leung-Toung, R., Wang, Y., Connelly, J., LeVine, S. M. Enhanced Histochemical Detection of Iron in Paraffin Sections of Mouse Central Nervous System Tissue: Application in the APP/PS1 Mouse Model of Alzheimer’s Disease. ASN Neuro. 8 (5), (2016).
- Cardiff, R. D., Miller, C. H., Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor Protocols. 2014 (6), 655-658 (2014).
- Koyuncu, O. O., MacGibeny, M. A., Hogue, I. B., Enquist, L. W. Compartmented neuronal cultures reveal two distinct mechanisms for alpha herpesvirus escape from genome silencing. PLoS pathogens. 13 (10), 1006608 (2017).
- Laval, K., Vernejoul, J. B., Van Cleemput, J., Koyuncu, O. O., Enquist, L. W. Virulent Pseudorabies Virus Infection Induces a Specific and Lethal Systemic Inflammatory Response in Mice. Journal of Virology. 92 (24), 01614-01618 (2018).
- Laval, K., Van Cleemput, J., Vernejoul, J. B., Enquist, L. W. Alphaherpesvirus infection of mice primes PNS neurons to an inflammatory state regulated by TLR2 and type I IFN signaling. PLoS Pathogens. 15 (11), 1008087 (2019).
- Brittle, E. E., Reynolds, A. E., Enquist, L. W. Two modes of pseudorabies virus neuroinvasion and lethality in mice. Journal of Virology. 78 (23), 12951-12963 (2004).
- Mancini, M., Vidal, S. M. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mammalian Genome: Official Journal of the International Mammalian Genome Society. 29 (7-8), 425-445 (2018).
- Kopp, S. J., et al. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proceedings of the National Academy of Sciences of the United States of America. 106 (42), 17916-17920 (2009).
- Wang, J. P., et al. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. Journal of Virology. 86 (4), 2273-2281 (2012).
- Haberthur, K., Messaoudi, I. Animal models of varicella zoster virus infection. Pathogens. 2 (2), 364-382 (2013).
- Sarova-Pinhas, I., Achiron, A., Gilad, R., Lampl, Y. Peripheral neuropathy in multiple sclerosis: a clinical and electrophysiologic study. Acta Neurologica Scandinavia. 91 (4), 234-238 (1995).
- MacGibeny, M. A., Koyuncu, O. O., Wirblich, C., Schnell, M. J., Enquist, L. W. Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. PLoS Pathogens. 14 (7), 1007188 (2018).
- Hunsperger, E. A., Roehrig, J. T. Temporal analyses of the neuropathogenesis of a West Nile virus infection in mice. Journal of Neurovirology. 12 (2), 129-139 (2006).
- Swartwout, B. K., et al. Zika Virus Persistently and Productively Infects Primary Adult Sensory Neurons In Vitro. Pathogens. 6 (4), 49 (2017).
- Racaniello, V. R. One hundred years of poliovirus pathogenesis. Virology. 344 (1), 9-16 (2006).

