Circadian Entrainment of Drosophila Melanogaster
Summary
Here, we detail how to synchronize Drosophila to a circadian day. This is the first, and most important step necessary for studying biological rhythms and chronobiology.
Abstract
Nearly universal among organisms, circadian rhythms coordinate biological activity to earth's orbit around the sun. To identify factors creating this rhythm and to understand the resulting outputs, entrainment of model organisms to defined circadian time-points is required. Here we detail a procedure to entrain many Drosophila to a defined circadian rhythm. Furthermore, we detail post-entrainment steps to prepare samples for immunofluorescence, nucleic acid, or protein extraction-based analysis.
Introduction
Almost all organisms on Earth, from the largest down to single celled, have an internal biological clock with a cycle of about one day. This is known as the Circadian rhythm (coined in 1953 by Franz Halberg from the Latin terms circa = about/approximately and "dies" = day)1. Although components of the core clock are known and their rudimentary mechanisms of function conceptualized, there is still much to understand about how biological rhythms are maintained throughout the body. Importantly, misregulation of biological rhythms is associated with poor health outcomes including poor memory formation, sleep disorders, seasonal affect disorder, depression, bipolar disorder, diabetes, obesity, neurodegeneration, and cancer2,3,4,5.
Drosophila is a well-established model for investigation of circadian biology. Genetically and biochemically tractable, large numbers are easily entrained (as will be shown). In fact, all seven publications cited as key publications of support in awarding the Nobel Prize for the discovery of circadian rhythms leveraged these strengths of the Drosophila model6,7,8,9,10,11,12.
Additionally, we show effective strategies for collecting entrained flies for the purposes of either immunofluorescence, nucleic acid, or protein extraction-based analysis. Using these strategies, one may process and store larger amounts of samples for analysis in the future. These methods are very advantageous in that they are reproducible and can yield hundreds of entrained flies that can be a part of a large data pool.
Protocol
1. Fly food production
- Per every 1 L of water, prepare fly food consisting of 4.69 g of dried molasses, 19.70 g of dry active yeast, 87.22 g of corn meal and 7.83 g of agar.
- Combine the contents listed above in a crockpot and turn the heat to 250 °F. Mix well as ingredients are added.
- Keep the lid on the crockpot as the fly food is heating while also mixing the contents every 10 min until it reaches a rolling boil. Allow the rolling boil to continue for 20 min before turning off the heat.
- Add 83.60 mL of water and keep the lid of the crockpot off as the food cools.
- Mix and record the temperature of the food every 10 min with a glass thermometer. Avoid letting a layer of film settle on top of the food.
- Once the food has cooled to 60 °C, add 10.44 mL of Tegosept and 5.51 mL of propionic acid per liter of water added (see step 1.1).
- Mix well and turn heat up to 60 °C to prevent the food from becoming too cool.
- Pump the fly food as needed using the Droso-filler.
- For narrow vials, pump 10 mL and for 6 oz square bottom bottles pump 60 mL.
2. Collecting flies of defined age
- Monitor bottles of wild type flies stored at 25 °C for 5-7 days until large amounts of pupa (about 200 pupa) are attached to the side of the bottle. The bottles used are 6 oz Drosophila stock bottles with square bottoms.
- Clear existing adults from the bottle. Either tip the adults into a new bottle or place them in 70% ethanol. Use the dull end of a #0 paintbrush to push any remaining adults into the fly food. Be sure to wipe the paint brush down with 70% ethanol before and after uses.
- Allow cleared bottles to sit for 3 days in a 25 °C incubator to allow for the next generation to eclose. These flies will be between 0 and 3 days old.
3. Fly separation
- After 3 days, separate males from females and collect the desired number for each sex for each of the four time points. Flies can be differentiated by sex by examining genitalia; males have dark rounded genitalia whereas females have lighter, more pointed genitalia. Females are also much larger than male flies.
- Use a CO2 anesthesia pad to effectively separate the flies and differentiate between the sexes. Move the flies with paint brushes to avoid killing them.
- Collect 100 males and 100 females for each time point, with 50 males per vial and 100 females per vial. Males tend to be more aggressive and their social interactions lead to a lot of deaths when there are 100 individuals in a vial. The females-up to 100 individuals- are unaffected while contained in the vial.
- Perform the collections at the following time points: ZT1, ZT7, ZT13 and ZT19 (Table 1). Note that collections done in the dark (ZT12-ZT24) are light sensitive whereas ZT0-ZT11 collections are done during lights-on times and room lights can be on. Please note that two separate incubators are used with inverse 12 hour light patterns to allow for all collections to occur during the day and not overnight.
- Use excess flies to create new bottles of flies for future entrainment. Place 25 females with 5-7 males in each new bottle and place in the incubator at 25 °C.
4. Fly incubation
- Allow the flies to stay in light regulated incubators for 3-5 days to allow circadian entrainment to occur. Ensure that incubators are light-tight because even small amounts of light pollution will disturb entrainment.
5. Immunofluorescence fixation
- After entrainment, prepare new vials of fixation solution for samples that will be used for immunofluorescence. Prior to removing the flies from the incubator, add 4.8 mL of 4% formaldehyde diluted in 1x PBS + 0.1% Tween-20 to each new narrow vial for every 100 flies that are to be fixated. Each vial will house 100 male or 100 female Circadian entrained flies. Place the narrow vials in ice.
6. Immunofluorescence collection
- When collecting the flies from the incubator for immunofluorescence, remove the bottle cap and quickly invert the bottle into the funnel. Gently tap the flies into the solution via the funnel to help guide the flies into the vial; combine two tubes of the 50 males for a total of 100 males in one vial and use a single tube of 100 females for the other vial.
- Perform ZT13 and ZT19 collections in the dark; use a red light in order to see as drosophila are far less sensitive to these light wavelengths and are therefore less prone to light pollution13. Cryptochrome protein, in particular, is especially sensitive to blue light, which must be avoided14.
- To reduce light exposure, ensure the room of collection is light tight with any sources of light blocked out or covered. Wrap these vials in foil so that ZT13 and ZT19 flies are not exposed to light when placed on the nutating mixer in the following step. ZT1 and ZT7 flies are not light sensitive and can be placed on the mixer without foil covering.
7. Nutating mixer and storage
- After the flies have been collected, tape the top of the vials containing fixative to avoid spillage and place them on the nutating mixer at 165 RPM at 4 °C for 4 h. The flies are no longer light sensitive after this fixation step, so foil may be removed to verify the solution is moving and flies are being submerged in the fixative.
- After removing the fly containing vials from the nutating mixer remove the formaldehyde and wash three times with 3,000 µL of 1x PBS, inverting the vial with each wash.
- Store the vials bearing immunofluorescence samples at 4 °C to await future immunofluorescence15.
8. Collection for protein extraction
- Prepare four 50 mL tubes and a Dewar containing liquid nitrogen for preservation of samples for protein extraction
- For collecting flies for protein or nucleic acid extraction, transfer flies from the bottles to the tube in the same manner as in step 6.1 and quickly cap the tube to prevent the release of flies and place the tube into liquid nitrogen to snap freeze.
9. Storage for protein extraction
- Store snap frozen samples for protein extraction at -80 °C. These can be processed according to the protein extraction protocol appropriate for downstream analysis, including immunoblotting16.
Representative Results
Controlled circadian entrainment allows researchers to examine biology at specific time points throughout the circadian day using the ZT1-ZT19 timing schedules or to add time-points as necessary. Here we use light and darkness to entrain flies to circadian cycles and verify entrainment by immunoblotting and immunofluorescence analysis of the period protein, a marker for circadian entrainment (Figure 1). Upon correct entrainment, period proteins should have a characteristic intensity and mobility pattern (Figure 1A) and should be visible at specific locations in the ZT1 brain (Figure 1B). Although other variables, including food and temperature, can influence circadian entrainment, light is most simple and reliable to control17. For the purpose of these methods, incubator temperatures are kept constant, relying on cerebral clock neurons that are influenced by light for entrainment18.
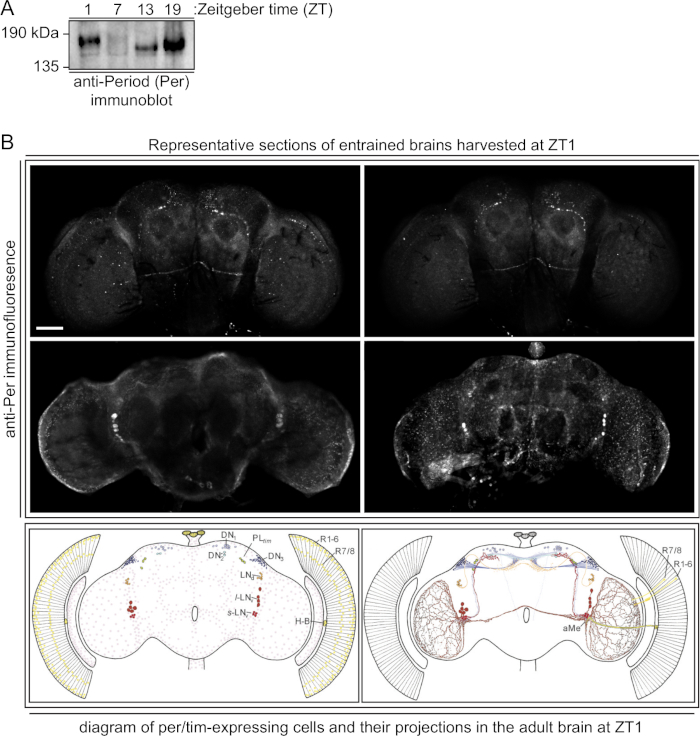
Figure 1: Verification of entrainment. (A) Immunoblotting of whole cell extracts prepared from heads of entrained flies shows canonical patterns of period protein mobility and intensity19. 1.4 female heads from each of the indicated Zeitgeber times (ZT) were analyzed using an anti-Per antibody. (B) Immunofluorescence of entrained brains collected at ZT, where the Period protein is found in a characteristic pattern (bottom panels, recreated from Helfrich-Forster20). Shown are images taken from different sections of the brain, capturing all neurons expected to contain Period protein at ZT1. Scale bar is 40 µm. Please click here to view a larger version of this figure.
| ZT1 | ZT7 | ZT13 | ZT19 |
| Light between 10 am – 10 pm | Light between 10 am – 10 pm | Dark between 9 am – 9 pm | Dark between 9 am – 9 pm |
| Collected at 11 am after 1 hour in light | Collected at 5 pm after 7 hours in light | Collected at 10 am after 1 hour in dark | Collected at 4 pm after 7 hours in dark |
Table 1: ZT1-ZT19 Circadian Rhythm Timing Schedules.
Discussion
Researchers utilize this entrainment protocol with success and consistency. This procedure allows the fixation of a large sampling pool that can be stored for future analysis. Additionally, this strategy preserves the neurological patterns induced by entrainment for future examination.
Fixation for storage is a major component of the entrainment process as it helps to stabilize brain tissue and it allows for more time to analyze each brain from the data pool thus minimizing waste from brains that lose viability due to age21. The main goal is to circadian entrain as many flies as possible so that there is continuous inventory available for head dissections and ultimately immunofluorescence or protein extraction to observe the findings and determine if results are of high confidence. To ensure that circadian entrainment is preserved through fixation, it is integral that any source of light pollution is eliminated. The fixation process allows for Drosophila to be stored while maintaining its neurological "timestamp" so that they can be dissected later and analyzed with no noticeable differences to flies that are dissected and have undergone immunofluorescence immediately after entrainment. For the purposes of fixation prior to immunofluorescence, the lab has determined with consistency that flies are viable at least up to 1 month. Fixations for western blot protein extraction render the brains viable indefinitely when stored at -80 °C.
Another critical protocol step is the sexing of the flies. It is important that this step is done accurately as having both sexes in the same vial prior to fixation can lead to mating, which will yield new flies that are of younger age and corrupt protein analysis if males are accidentally examined instead of females or vice versa. Additionally, when sexing it is important to remove larvae specimens that are at times attached to females. This prevents the development of new progeny inside the female vial that could potentially corrupt results.
The next step for the entrainment protocol may be with items related to data analysis. The focus of the protocol is protein localization, but if there are other variables that are impacted by circadian entrainment, they must be explored through new avenues, often requiring protein or nucleic acid extraction. Additionally, there are other proteins of the brain that may still be analyzed via this protocol. The experiments associated with the protocol analyzed certain proteins but the list of genes and proteins that play a role in circadian biology has not been exhausted. The protocol is effective in accomplishing the goal of establishing a circadian rhythm, however, the applications are wide-ranging.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Special thanks to the University of Missouri-Kansas City and the Jeffrey L. Price laboratory.
Materials
| 100-1000uL pipette | Eppendorf | ES-1000 | |
| 10-100uL pipette | Eppendorf | ES-100 | |
| 16% Paraformaldehyde Solution | 15710 | ||
| 1X PBS | Caisson Labs | PBL01-6X100ML | |
| Agar | Fisher Scientific | BP1423500 | |
| Anesthesia Filter Connection Kit | World Precision Instruments | EZ-251A | |
| Corn meal | Genesee Scientific | 62-100 | |
| Dried Molasses | Food Service Direct | OT280504 | |
| Droso-filler Food Pump | geneseesci.com | 59-169 | |
| Drosophila Stock bottles, 6 oz square bottom w/ Flugs | geneseesci.com | 32-130BF | |
| Drosophila vials, Narrow K-Resin super bulk | geneseesci.com | 32-118SB | |
| Dry active yeast | Genesee Scientific | 62-103 | |
| Ethanol | IBI Scientific | IB15720 | |
| EZ Basic Anesthesia System | World Precision Instruments | EZ-175 | |
| Falcon Centrifuge tubes | Corning | 352097 | |
| Falcon round bottom tubes | Corning | 352057 | |
| Fine point Sharpie marker | Sharpie | 30001 | |
| Fisherbrand Nutating Mixer | Fisher Scientific | 88-861-043 | |
| Flugs-Narrow Plastic Vials | Genesee Scientific | 49-102 | |
| Glass Thermometer | Cole-Palmer | EW-08008-12 | |
| Liquid nitrogen hose | Thermo Scientific | 398202 | |
| Liquid nitrogen tank-Dewar | Cooper Surgical Inc | 900109-1 | |
| Liquid nitrogen transfer vessel | Electron Mircoscopy Sciences | 61891-02 | |
| Paintbrushes(Red Sable) Size #0 | Electro Microscopy Sciences | 66100-00 | This is used to separate the flies via sex without causing injury. |
| Plastic funnel | Plews and Edelmann | 570-75-062 | |
| Polarizing light microscope | Microscope Central | 1100100402241 | Used to more clearly view Drosophila during sexing |
| ProPette Pipette Tips | MTC Bio Incorporated | P5200-100U | |
| ProPette Pipette Tips | MTC Bio Incorporated | P5200-1M | |
| ProPette Pipette Tips | MTC Bio Incorporated | P5200-5M | |
| Propionic Acid | Sigma Aldrich | P1386-1L | |
| Rayon Balls | Genesee Scientific | 51-100 | |
| Reynolds wrap standard aluminum foil | Staples | 1381273 | |
| Roaster Oven (Crockpot) | Hamilton Beach | 32950 | |
| Scotch 810 Magic Tape | Electron Microscopy Sciences | 77300 | |
| Spray bottle with trigger | US Plastic | 66446 | Used to spray ethanol to clean work bend areas |
| Tegosept | Genesee Scientific | 20-258 | |
| Thermo Scientific Drosophila Incubator | Thermo Scientific | 3990FL | |
| Thermo Scientific Revco 4 degree Lab fridge | ThermoFisher Scientific | REL7504D | |
| Thermo Scientific Revco Lab Freezer | ThermoFisher Scientific | REL7504A | |
| Tween 20 | Anatrace | T1003-1-GA |
References
- Halberg, F. Some physiological and clinical aspects of 24-hour periodicity. The Journal-Lancet. 73 (1), 20-32 (1953).
- Smarr, B. L., Jennings, K. J., Driscoll, J. R., Kriegsfeld, L. J. A time to remember: the role of circadian clocks in learning and memory. Behavioral Neuroscience. 128 (3), 283-303 (2014).
- Leng, Y., Musiek, E. S., Hu, K., Cappuccio, F. P., Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. The Lancet. Neurology. 18 (3), 307-318 (2019).
- Hood, S., Amir, S. Neurodegeneration and the Circadian Clock. Frontiers in aging Neuroscience. 9, 170 (2017).
- Sulli, G., Lam, M. T. Y., Panda, S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends in Cancer. 5 (8), 475-494 (2019).
- Zehring, W. A., et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 39 (2 Pt 1), 369-376 (1984).
- Bargiello, T. A., Jackson, F. R., Young, M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 312 (5996), 752-754 (1984).
- Siwicki, K. K., Eastman, C., Petersen, G., Rosbash, M., Hall, J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1 (2), 141-150 (1988).
- Hardin, P. E., Hall, J. C., Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 343 (6258), 536-540 (1990).
- Liu, X., et al. The period gene encodes a predominantly nuclear protein in adult Drosophila. Journal of Neuroscience. 12 (7), 2735-2744 (1992).
- Vosshall, L. B., Price, J. L., Sehgal, A., Saez, L., Young, M. W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science (New York, N.Y). 263 (5153), 1606-1609 (1994).
- Price, J. L., et al. double-time is a novel Drosophila clock gene that regulates period protein accumulation. Cell. 94 (1), 83-95 (1998).
- Hanai, S., Hamasaka, Y., Ishida, N. Circadian entrainment to red light in Drosophila: requirement of Rhodopsin 1 and Rhodopsin 6. Neuroreport. 19 (14), 1441-1444 (2008).
- Vinayak, P., et al. Exquisite light sensitivity of Drosophila melanogaster cryptochrome. PLoS Genetics. 9 (7), e1003615 (2013).
- Kelly, S. M., Elchert, A., Kahl, M. Dissection and Immunofluorescent Staining of Mushroom Body and Photoreceptor Neurons in Adult Drosophila melanogaster Brains. Journal of Visualized Experiments. (129), e56174 (2017).
- Au-Eslami, A., Au-Lujan, J. Western Blotting: Sample Preparation to Detection. Journal of Visualized Experiments. (44), e2359 (2010).
- Fan, J. Y., Muskus, M. J., Price, J. L. Entrainment of the Drosophila circadian clock: more heat than light. Science’s signal Transduction Knowledge Environment. (413), pe65 (2007).
- Miyasako, Y., Umezaki, Y., Tomioka, K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. Journal of Biological Rhythms. 22 (2), 115-126 (2007).
- Edery, I., Zwiebel, L. J., Dembinska, M. E., Rosbash, M. Temporal phosphorylation of the Drosophila period protein. Proceedings of the National Academy of Sciences of the United States of America. 91 (6), 2260-2264 (1994).
- Helfrich-Forster, C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microscopy Research and Technique. 62 (2), 94-102 (2003).
- Price, J. L. Genetic screens for clock mutants in Drosophila. Methods in Enzymology. 393, 35-60 (2005).

