The Quantification of Injectability by Mechanical Testing
Summary
Presented here is a protocol for quantitatively evaluating the injectability of a material through a syringe-needle system using a standard mechanical testing rig.
Abstract
Injectable biomaterials are becoming increasingly popular for the minimally invasive delivery of drugs and cells. These materials are typically more viscous than traditional aqueous injections and may be semi-solid, therefore, their injectability cannot be assumed. This protocol describes a method to objectively assess the injectability of these materials using a standard mechanical tester. The syringe plunger is compressed by the crosshead at a set rate, and the force is measured. The maximum or plateau force value can then be used for comparison between samples, or to an absolute force limit. This protocol can be used with any material, and any syringe and needle size or geometry. The results obtained may be used to make decisions about formulations, syringe and needle sizes early in the translational process. Further, the effects of altering formulations on injectability may be quantified, and the optimum time to inject temporally changing materials determined. This method is also suitable as a reproducible way to examine the effects of injection on a material, to study phenomena such as self-healing and filter pressing or study the effects of injection on cells. This protocol is faster and more directly applicable to injectability than rotational rheology, and requires minimal post processing to obtain key values for direct comparisons.
Introduction
Biomaterials are often studied and used as scaffolds for cell-based tissue regeneration and depots for targeted, sustained delivery of therapeutics1. Within this field, injectable biomaterials are growing in popularity as they are minimally invasive, which reduces the risk of infection, pain and scarring associated with implantation2. Further, because they are usually applied as fluids, they conform perfectly to tissue defects, and drugs and cells may be mixed into them immediately prior to the application3,4,5. As such, while injectable biomaterials may be manufactured as pre-loaded syringes, they are often prepared by clinicians directly prior to application. For example, cements begin to set once the powder and liquid phases are mixed, and so cannot be stored for long periods before use6. The characterization of these materials is thus time dependent and inextricably linked to their preparation.
Common injectable biomaterials include calcium cements, polymethyl methacrylate, bioglasses, and various polymeric hydrogels3,7. Unlike traditional injections of drugs, which have the same rheological properties as water, these injectable biomaterials are typically more viscous, non-Newtonian, may have some elastic character, and may also change over time. Therefore, the injectability of these materials cannot be assumed but must be assessed experimentally. By quantifying the force required for injection and correlating it to the ease of injection, early decisions about which biomaterial formulations, syringe, and needle sizes to take forward may be made early in the developmental process8. Such experiments may also quantify the effects of changing formulations on injectability9.
There are several methods to assess the properties of injectable materials. Rotational rheology is often utilized to assess viscosity, non-Newtonian behavior, post-shear recovery, setting time, and other properties of these materials10,11,12. Whilst this type of test is useful to establish fundamental properties of the materials, these properties do not correlate directly to injectability. For a Newtonian fluid and cylindrical syringe and needle, the injection force can be estimated from a form of the Hagen–Poiseuille equation13:
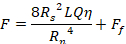
Where F is the force required for injection (N), Rs is the internal syringe radius (m), Rn is the internal needle radius (m), L is the needle length (m), Q is fluid flow rate (m3 s-1), η is the dynamic viscosity (Pa.s) and Ff is the friction force between the plunger and barrel wall (N). Thus, if the viscosity is measured via rotational rheology, the dimensions of the syringe and needle are known and the flow rate estimated, the injection force can be estimated. However, this equation does not account for the conical end of the syringe or any other geometries, such as off-center outlets, and Ff must be estimated or found experimentally by mechanical testing. Further, biomaterials are typically not Newtonian, but exhibit complex rheological properties. For a simple shear thinning fluid, the equation becomes14:
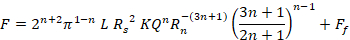
Where n is the power index (-) and K is the consistency index (Pa.sn) from the Ostwald de Waele expression: 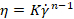 , where
, where 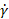 is the shear rate (s-1). The complexity vastly increases for materials whose rheological properties cannot be characterized by two values, and particularly for time-dependent materials such as setting cements. Additionally, if the material properties are shear dependent, then the material must be tested at the shear rate expected in the needle, which may far exceed the range of a rotational rheometer15.
is the shear rate (s-1). The complexity vastly increases for materials whose rheological properties cannot be characterized by two values, and particularly for time-dependent materials such as setting cements. Additionally, if the material properties are shear dependent, then the material must be tested at the shear rate expected in the needle, which may far exceed the range of a rotational rheometer15.
Another quantitative method for measuring injectability involves attaching pressure and displacement sensors to a syringe while performing an injection, either by hand or using a syringe pump. This equipment is relatively inexpensive, however, requires users to generate scripts and calibration curves to convert into force data16. Further, a syringe pump may not possess sufficient torque to compress the plunger at a precise rate if high forces are required to extrude viscous or semi-solid materials. Alternatively, utilizing these sensors when injecting by hand may be useful as they can be used in a real clinical scenario, during clinical procedures17. However, this will take much longer and may introduce user bias, and will, therefore, need larger numbers of repetitions with different users to obtain reliable results. This may, thus, be more appropriate for materials that are further down the translational pipeline, or products already in clinical use.
In this protocol, a mechanical tester is used to compress the plunger at a set rate, and measure the force required to do so. This type of mechanical tester is common in materials laboratories and has been used to quantify injectability for various biomaterials18,19,20,21,22,23,24. This test can be used with any size and geometry of syringe and needle, containing any material. Further, in the case of biomaterials that are made immediately prior to the use, the exact formulation procedure that would be used in the clinic or surgery can be followed prior to testing. A further advantage of this procedure is that it is relatively fast; once the mechanical tester is set up, tens of samples can be studied in an hour, depending on extrusion speed and syringe volume. This is in contrast to rotational rheology, which typically takes at least 5 – 10 minutes per test, plus loading, equilibration and cleaning time. Using a mechanical tester produces a reliable extrusion rate equally over the plunger, which is particularly advantageous for viscous formulations or those with time dependent properties. Following testing, minimal post-processing of data is required to pull out important values for objective comparisons.
Protocol
1. Sample Preparation
- Prepare the sample and load it into the syringe.
- To simulate a pre-loaded syringe, prepare the sample in advance, load it into the syringe, and attach the needle. Store as required, until testing. This may be suitable for hydrogels and materials that do not change with time.
NOTE: For example, to prepare 2% alginate solutions, dissolve 2 g of alginic acid sodium salt in 100 mL of deionized water, by stirring at room temperature. Aspirate the solution into 5 mL syringes, and store for 24 h at room temperature. - Alternatively, to simulate an injection formulated directly prior to the application, prepare the sample in the same way it would be made in the clinic, allowing for any setting times. Load into the syringe and attach the needle. This may be suitable for cements, and materials whose properties change with time.
NOTE: For example, to prepare calcium sulfate cement, manually mix 4 g of calcium sulfate hemihydrate into 5 mL of deionized water with a spatula for 1 min. Remove the plunger from the syringe and load the cement into the syringe barrel with the spatula. Begin the mechanical testing after 4 min.
CAUTION: Needles pose a safety risk, use blunt needles if possible. If the material contains cells or other biological materials, extra care should be taken to prevent sharps injuries.
- To simulate a pre-loaded syringe, prepare the sample in advance, load it into the syringe, and attach the needle. Store as required, until testing. This may be suitable for hydrogels and materials that do not change with time.
2. Set up the mechanical tester
- Attach flat platens (for compression testing) to the mechanical tester.
- Manually equip the mechanical tester with a load cell with a maximum load of 200 N.
NOTE: A larger load cell may be used, provided it has sufficient precision at the 1 – 200 N range. Samples that are more viscous and not intended to be injected by hand may require a larger load cell. - Separate the plates, using the manual control buttons, to allow for sufficient space for the needle, syringe and plunger (around 30 cm will be sufficient).
- Create a testing protocol.
- Open the test wizard and set the test type to uniaxial compression.
- Set the pre-load. This is the measured force value at which testing will begin. 0.5 N is sufficient.
- Set the speed to pre-load to 5 mm/min. This is the speed the crosshead will move down until it encounters the pre-load.
- Set the loading to displacement control and select an appropriate test speed. 1 mm/s is an appropriate speed for a standard 5 mL syringe.
- Set an upper force limit at which to stop the test, e.g., 200 N. This is primarily for safety reasons. The test may also be stopped automatically at a given displacement, e.g., the length of the syringe.
3. Set up the clamping system
- Attach two sets of clamps to two stands, with grips large enough to securely ensconce the chosen syringe.
- Place the grips between the crosshead and baseplate, with enough space below the grips for the syringe and needle.
- Line up the centers of the two grips, and line these up with the center of the crosshead.
NOTE: Alignment of the clamp grips with each other and the center of the crosshead may take some time and iteration to achieve, but is important to acquire high quality data. - Ensure the clamps are secured firmly so that there is no movement in the clamps when a downward force is applied.
- Place a dish onto the bottom plate to collect the extruded material.
4. Run the injectability protocol
- Insert the syringe into the clamp grips and close them. The grips should hold the syringe in place, but allow it to move up and down without resistance.
- Ensure the syringe and plunger are perpendicular to the crosshead. This ensures that only uniaxial compression of the material will be measured.
NOTE: An empty syringe should be used to check steps 4.1 and 4.2. - Lower the top plate to a position just above the plunger, using the manual movement buttons.
NOTE: It may be possible to select a ‘Start position’ in the mechanical tester protocol, such that the original position above the plunger is reached automatically and is consistent throughout testing. - Zero the measured force by clicking ‘Zero Force’.
- Run the testing protocol by pressing ‘Run’.
CAUTION: The experimenter should always be present to observe each trial, and ready to activate the emergency stop in case of a mishap. - Raise the plates to a sufficient height, using the manual movement buttons, such that the syringe can be removed.
- Repeat step 4 for each sample.
NOTE: At this point, the syringe and extruded sample can be discarded if no further analysis is required, but may be kept in order to examine filter pressing, self-healing, the effects on cells, etc.
5. Data collection
- Save the data from each trial in a format from which a table of force and displacement values can be generated (.txt, .xls, .xlsx).
- Plot the results from each trial, with displacement on the x-axis and force on the y-axis.
- Read the maximum force (if it exists) and plateau force from the graphs.
Representative Results
The set-up of the mechanical tester and clamping system is shown in Figure 1A. This protocol generates a table and graph of force versus displacement for each tested sample. A typical force displacement curve consists of three sections (Figure 1B): an initial gradient, as the plunger overcomes friction from the barrel and the material is accelerated, a force maximum, and a plateau, as the material is extruded at a steady state.
However, a distinct maximum only exists where the plateau force is lower than the force required to accelerate the plunger. As such, peaks are only seen for inviscid samples passing through wide needles. For viscous samples passing through a more narrow orifice, the force needed to inject the sample at constant speed is greater than the force required to overcome friction in the barrel and accelerate the material, and no distinct peak is seen (Figure 1C). For highly viscous samples or very narrow needles, the force required to extrude the material may be so great that the syringe buckles and fails, often with very little extrusion of the material (Figure 1D). If the material being injected contains particles or is undergoing setting, such as cement, filter pressing (preferential expulsion of the liquid phase) or bulk setting may occur, leading to incomplete injection (Figure 1E).
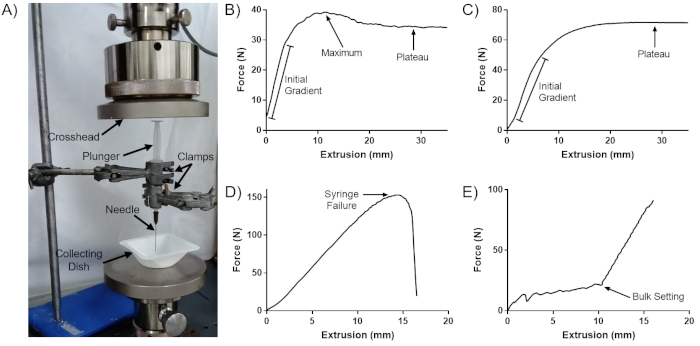
Figure 1: Sample curves generated by this protocol. (A) Set up of the mechanical tester for this protocol. (B) Typical force-extrusion curve. (C) Force-extrusion curve with no distinct maximum peak. (D) Force-extrusion curve for syringe failure. (E) Force-extrusion curve for a setting cement. This figure is adapted from Robinson et al.8. Please click here to view a larger version of this figure.
Discussion
Mechanical testing is perhaps the simplest and most reliable way to quantify injectability. A key advantage of this protocol is that no special equipment is required, other than the mechanical tester, which is common in materials laboratories. This protocol is highly versatile; any material, needle gauge and syringe size can be used, provided the syringe can be accommodated by the clamps. This has been verified in this protocol for syringes up to 10 mL. Further, the material can be prepared exactly as it would for the real-world application25. Finally, this procedure is very fast, taking only up to a few minutes per sample, allowing tens of samples to be processed per hour.
For samples that give typical curves, two values can be extracted: the maximum force and the plateau force curves. The maximum force is arguably more objective and can be extracted computationally from the data table for each sample. Conversely, the plateau force may be more representative, as this will be the force experienced for the greatest amount of time and, as an average, is less affected by curves with large fluctuations. These fluctuations may be caused by air bubbles or particles in the material causing intermittent changes as they are extruded, or by low instrument precision for small force measurements. However, it is notable that, for many samples, there is no maximum force peak, and so the maximum and plateau value are the same. Objective comparisons between injection forces can be made so long as a consistent value is used.
The data obtained can be used in several ways. The injectability force values may be compared to ease of injection, to establish which formulations, syringe and needle sizes are viable for translation8. Alternatively, comparing between samples allows for the quantification of changes to formulations on injectability. For example, in cements, changing the viscosity of the liquid phase, the particle size distribution, and adding additives such as citrate to alter the colloidal properties, can have large changes in injectability9. These tests may also inform formulation protocol for cements, for example mixing time, time to loading and time to application, for optimum injection and post-injection performance. In addition, this method may be used to test the initial feasibility of novel bioinks for 3D printing.
This protocol can be modified in several ways. The clamp system may be replaced with a bespoke 3D printed construct to hold the syringe, which may make it easier to ensure the syringe and plunger are perpendicular to the crosshead, and the syringe held securely. The needle can be replaced with a cannula or any device that extrudes material by compression of a plunger and can be of any size and geometry. In order to increase the fidelity of the results, the tip of the needle can be placed into a tissue or hydrogel, in order to more accurately simulate clinical injection. However, this adds further complexities to the protocol, as tissue/gel composition and needle depth must be kept constant. Further, this protocol utilizes displacement-controlled extrusion, to measure the force required to inject at the specified speed. Alternatively, the injection force can be specified, and the amount of extrusion can be measured against time. This may be useful for materials with time dependent properties, such as cements. For example, by using a correlation between injection force and ease of injectability to select a force8, this protocol may be used to establish whether the entire volume of cement can be injected with this speed prior to setting. Finally, this protocol can easily be combined with other experiments, in order to test the effect of injection on the material properties and examine phenomena such as filter pressing and self-healing, or the effect of injection on cells.
The main limitation of this protocol is that a universal mechanical tester is required. While these are common in materials testing labs, they are expensive to purchase if the user cannot access one. Further, the mechanical tester provides uniaxial compression at either a set force or rate of displacement, whereas the applied force and injection speed may vary over the course of injection by hand. This protocol is also unsuitable for replicating some real world injections, such as injections into complex tissues in theatre, or injecting at different angles. To quantify the force of injection in the clinic, force and displacement transducers may be a better method.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by the EPSRC CDT for Formulation Engineering in the School of Chemical Engineering at the University of Birmingham, UK, Grant reference EP/L015153/1, and the Royal Centre for Defence Medicine.
Materials
| Alginic Acid Sodium Salt | Sigma | A2033-100G | |
| Blunt Needles | Needlez | NB19G1.5 | Any size may be used, depending on application |
| Calcium Sulphate Hemihydrate | Acros Organics | 22441.296 | |
| Clamp stand | Eisco | MTST5 | Two required |
| Clamps | R&L Enterprises | 41 | Two required, should have flat tops |
| Syringes | BD | 307731 | Any size can be used, depending on application |
| Universal Mechanical Tester | Zwick Roell | Z030 |
References
- Webber, M. J., Appel, E. A., Meijer, E. W., Langer, R. Supramolecular biomaterials. Nature Materials. 15, 13-26 (2015).
- Mathew, A. P., Uthaman, S., Cho, K. -. H., Cho, C. -. S., Park, I. -. K. Injectable hydrogels for delivering biotherapeutic molecules. International Journal of Biological Macromolecules. 110, 17-29 (2018).
- Zhou, H., et al. Injectable biomaterials for translational medicine. Materials Today. 28, 81-97 (2019).
- Alves, H. L. R., dos Santos, L. A., Bergmann, C. P. Injectability evaluation of tricalcium phosphate bone cement. Journal of Materials Science: Materials in Medicine. 19, 2241-2246 (2008).
- Yu, L., Ding, J. Injectable hydrogels as unique biomedical materials. Chemical Society Reviews. 37, 1473 (2008).
- Pawelec, K. M., Planell, J. A. . Bone Repair Biomaterials: Regeneration and Clinical Applications. , (2019).
- Fernandez de Grado, G., et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. Journal of Tissue Engineering. 9, 204173141877681 (2018).
- Robinson, T. E., et al. Filling the Gap: A Correlation between Objective and Subjective Measures of Injectability. Advanced Healthcare Materials. , 1901521 (2020).
- O’Neill, R., et al. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomaterialia. 50, 1-19 (2017).
- Gantar, A., et al. Injectable and self-healing dynamic hydrogel containing bioactive glass nanoparticles as a potential biomaterial for bone regeneration. RSC Advances. 6, 69156-69166 (2016).
- Ramin, M. A., Latxague, L., Sindhu, K. R., Chassande, O., Barthélémy, P. Low molecular weight hydrogels derived from urea based-bolaamphiphiles as new injectable biomaterials. Biomaterials. 145, 72-80 (2017).
- Ren, K., He, C., Xiao, C., Li, G., Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for tissue engineering. Biomaterials. 51, 238-249 (2015).
- Burckbuchler, V., et al. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. European Journal of Pharmaceutics and Biopharmaceutics. 76, 351-356 (2010).
- Allmendinger, A., et al. Rheological characterization and injection forces of concentrated protein formulations: An alternative predictive model for non-Newtonian solutions. European Journal of Pharmaceutics and Biopharmaceutics. 87, 318-328 (2014).
- Davison, P. F. The Effect of Hydrodynamic Shear on the Deoxyribonucleic Acid from T2 and T4 Bacteriophages. Proceedings of the National Academy of Sciences of the United States of America. 45, 1560-1568 (1959).
- Chen, M. H., et al. Methods to Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomaterials Science and Engineering. 3, 3146-3160 (2017).
- Krebs, J., et al. Clinical measurements of cement injection pressure during vertebroplasty. Spine. 30, (2005).
- Bohner, M., Baroud, G. Injectability of calcium phosphate pastes. Biomaterials. 26, 1553-1563 (2005).
- Gbureck, U., Barralet, J. E., Spatz, K., Grover, L. M., Thull, R. Ionic Modification of Calcium Phosphate Cement Viscosity. Part I: Hypodermic Injection and Strength Improvement of Apatite Cement. Biomaterials. 25, 2187-2195 (2004).
- Habib, M., Baroud, G., Galea, L., Bohner, M. Evaluation of the ultrasonication process for injectability of hydraulic calcium phosphate pastes. Acta Biomaterialia. 8, 1164-1168 (2012).
- Martin, B. C., Minner, E. J., Wiseman, S. L., Klank, R. L., Gilbert, R. J. Agarose and methylcellulose hydrogel blends for nerve regeneration applications. Journal of Neural Engineering. 5, 221-231 (2008).
- Borzacchiello, A., Russo, L., Malle, B. M., Schwach-Abdellaoui, K., Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. BioMed Research International. 2015, 871218 (2015).
- Zhao, L., Weir, M. D., Xu, H. H. K. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 31, 6502-6510 (2010).
- Ji, D. -. Y., Kuo, T. -. F., Wu, H. -. D., Yang, J. -. C., Lee, S. -. Y. A novel injectable chitosan/polyglutamate polyelectrolyte complex hydrogel with hydroxyapatite for soft-tissue augmentation. Carbohydrate Polymers. 89, 1123-1130 (2012).
- Vaishya, R., Chauhan, M., Vaish, A. Bone cement. Journal of Clinical Orthopaedics and Trauma. 4, 157-163 (2013).

