Applications of Immobilization of Drosophila Tissues with Fibrin Clots for Live Imaging
Summary
This protocol describes applications of sample immobilization using Fibrin clots, limiting drifting, and allowing the addition and washout of reagents during live imaging. Samples are transferred to a drop of Fibrinogen containing culture medium on the surface of a coverslip, after which polymerization is induced by adding thrombin.
Abstract
Drosophila is an important model system to study a vast range of biological questions. Various organs and tissues from different developmental stages of the fly such as imaginal discs, the larval brain or egg chambers of adult females or the adult intestine can be extracted and kept in culture for imaging with time-lapse microscopy, providing valuable insights into cell and developmental biology. Here, we describe in detail our current protocol for the dissection of Drosophila larval brains, and then present our current approach for immobilizing and orienting larval brains and other tissues on a glass coverslip using Fibrin clots. This immobilization method only requires the addition of Fibrinogen and Thrombin to the culture medium. It is suitable for high-resolution time lapse imaging on inverted microscopes of multiple samples in the same culture dish, minimizes the lateral drifting frequently caused by movements of the microscope stage in multi-point visiting microscopy and allows for the addition and removal of reagents during the course of imaging. We also present custom-made macros that we routinely use to correct for drifting and to extract and process specific quantitative information from time-lapse analysis.
Introduction
Drosophila continues to be an important model system to study a vast range of biological questions and has excelled in advancing knowledge in many disciplines for decades. One feature that makes it particularly stand out is that it is especially well-suited for live imaging. The ability to monitor subcellular processes or the behavior of cells and tissues in real time continues to contribute to generating key concepts relevant to cell and developmental biology. Many successful protocols have been developed by different groups to maintain such Drosophila samples alive and healthy to study the behavior of the specimen and fluorescent molecules live. Organs and tissues from the fly such as imaginal discs1,2, the larval brain3,4,5,6, or egg chambers of adult females7,8,9, or the adult intestine10 for instance can be extracted and kept in culture for imaging with time-lapse microscopy. A key challenge for live imaging is to ensure that the specimen is kept close to the imaging surface for optimal resolution and immobile without compromising sample integrity that can be sensitive to mechanical impacts. To study neural stem cells, called neuroblasts or neurons in the Drosophila larval brain, for instance, keeping samples close to the imaging surface can be achieved by covering them with culture media in sealed imaging chambers11,12,13. However, this approach does not easily allow media exchange thus limiting experimental room for maneuver. Alternatively, samples can be prepared and placed on coverslips treated to increase adhesion of cells and allow multi-point visiting microscopy and extended live-cell imaging in open culture dishes, that potentially allow media exchange, but do not provide easy means to prevent sample drift or loss during media exchange14,15.
The Fibrin clot method originally developed to study meiosis in living crane-fly spermatocytes16 is specifically suited to overcome these problems. Fibrinogen is dissolved in relevant culture medium, the samples placed and oriented in a drop of this solution on an adequate glass-surface imaging chamber before Thrombin is added, resulting in the rapid formation of an insoluble Fibrin clot, a sticky fibrous mesh that adheres to the glass surface and the samples while ensuring access of the sample to the culture medium. The medium can then be exchanged without perturbing the clot or sample position. Culture medium exchange during imaging is, for instance, desirable when inhibitors are to be added as in the original method or for wash out or pulse chase experiments or when fluorescent dyes are to be added during live cell imaging while keeping cells and tissues in place. Fibrin clots are suitable for imaging of Drosophila tissues as well as individual cells13,17. Fibrin clots can further be used to help orient samples, that due to their shape or other properties would normally not be oriented in the desired way by modulating sample position within the Fibrin clot and the shape of the clot itself.
Here we provide an update on our current Fibrin clot-based imaging methods and provide tools for segmentation-based image analysis and focus on the use of this method to study neuroblast divisions in the developing fly brain. We routinely use the Fibrin clot method to follow multiple samples in different clots in the same culture dish. This allows i) imaging of subcellular events such as centrosome behavior or mRNA localization live18,19, ii) monitoring the behavior of samples upon pharmacological treatment of different genotypes under the same imaging settings using multi-point visiting microscopy20, iii) studying the effects of acute inhibition of enzymes21 and iv) minimizing drifting to study changes in the orientation of cell division of cells within their physiological environment upon targeted laser-ablation22. While unwanted effects of Thrombin and Fibrinogen and the Fibrin clot need to be empirically tested for each sample, this method is in principle suitable to immobilize any type of sample that is to be analyzed by live cell imaging and in Drosophila has been successfully used to study multiple aspects of neuroblasts biology5,19,20, but also the dynamics of cytosensor projections of female germ line stem cells23 and changes in polarity upon acute aPKC inhibition of follicle cells of Drosophila female egg chambers21.
Time-lapse fluorescent imaging results in the generation of complex time-resolved 3D data sets that require methods to extract this quantitative information. Here we describe our further development of ImageJ-based24 macros that can be used to correct for drifting in hyperstacks and custom-made ImageJ macros that allow semi-automated quantification of multi-channel fluorescence at the cell cortex developed to quantify cortical proteins in neuroblasts of Drosophila larval brains or of individual neuroblasts in primary cell culture.
Protocol
1. Preparation of reagents
NOTE: All steps in this protocol unless otherwise explained are carried out at room temperature.
- To prepare a solution of 1x PBS-BSA (0.1% w/v), dissolve 1 mg of Bovine Serum Albumin (BSA) in 1 mL of PBS. To prepare a stock solution of Thrombin 1,000 units·mL-1, dissolve 1,000 units of Thrombin in 1 mL of PBS-BSA (0.1% w/v). Make aliquots of 10 µL and store at -20 °C for up to 4 months.
- To prepare the culture medium for Drosophila brains, dissolve 1 g of Glucose in 1 L of Schneider’s medium in sterile conditions. Filter and store at 4 °C for up to 3 months.
- To prepare the Fibrinogen solution, dissolve 10 mg of Fibrinogen in 1 mL of culture medium for 20 min at room temperature or 5 min at 37 °C.
NOTE: If a different culture medium than the one prepared at step 1.2 is used and Fibrin clots already form at this step, the culture medium likely contains active Thrombin, which needs to be inactivated beforehand. A likely source of Thrombin is Fetal Bovine Serum, which can be inactivated by heating the serum to 56 °C for 30 min. - To prepare a coating solution of PBS-BSA (5% w/v), dissolve 50 mg of Bovine Serum Albumin (BSA) in 1 mL of 1x PBS.
- For the example reagent used in step 4, prepare a stock solution of 10 mM NAPP1 by dissolving 1 mg NAPP1 in 315 µL Dimethyl sulfoxide.
2. Dissection of Drosophila larval brains
NOTE: Perform this step under a dissection binocular.
- Use a brush to transfer L3 larvae into a 9-well borosilicate glass dish containing PBS. Stir to detach most of the fly food from the larvae and transfer to another well containing culture medium.
- Use forceps (e.g., Dumont #55) to grasp a larva across its entire diameter, in the middle of its body length (see Figure 1A,B). While holding the larva, transfer it to another well of the borosilicate plate containing 200 µL of fresh culture medium.
- Do not release the larva. To cut the larva in half across its entire diameter (Figure 1B), either grind the grasped area with lateral movement of the forceps tips (Figure 1A, top panel) or slide one tip of another pair of forceps between the two forcep tips holding the larva (Figure 1A, bottom panel). Do not cut the larva in half by pulling on one of its extremities, as it could damage the brain by pulling on it via the nerves crossing the body length (red lines in Figure 1B).
- Use forceps to hold the larva by its cuticle and another pair of forceps to peel apart the cuticle without pulling on the brain (Figure 1C). Repeat until the nerves originating from the brain are visible and the organs connecting the brain to the mouth parts can be accessed as shown in Figure 1D.
- Use forceps to hold the nerves originating from the brain. With the other pair of forceps, use either of the techniques shown in Figure 1A to cut the connection between the brain to the mouth parts, separating the brain from the rest of the cuticle (Figure 1D).
- Use forceps to hold the brain by the axons coming out of the ventral nerve cord. Pluck the organs depicted in gray in Figure 1E by gently pulling them away from the brain.
NOTE: Do not pull on the eye/antenna imaginal disks (dark yellow) to detach them from the brain. The connection between these tissues is too robust to be broken by pulling without damaging the brain. - While still grasping the nerves to hold and orient the brain, grasp the connection between the eye/antenna imaginal disks (dark yellow) and the brain with the other pair of forceps (Figure 1F). Use either of the techniques shown in Figure 1A to cut this connection without pulling on the brain. Check whether the brain is appropriately cleared of other tissues (Figure 1G) and not damaged (Figure 1H). Discard the brain if it shows signs of damage.
- Pipette the coating solution with a P20 in and out to coat the pipette tip. Pipette the brain with the coated tip along with 3 µL of medium (Figure 2A, left) and pipette it out in another well containing 200 µL of clean culture medium.
- Repeat steps 2.2–2.8 until enough brains are dissected, occasionally replacing the culture medium of the well in which the dissections are performed.
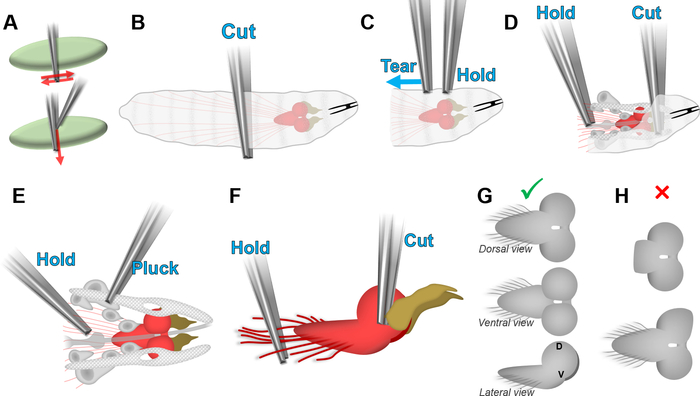
Figure 1: Dissection of D. melanogaster larval brains. (A) Technique for cutting without pulling using forceps. The sample (pale green) can be cut by being ground between the tips of the forceps (top panel) or by running a tip of a pair of forceps tip along the tips of a pair of forceps holding the sample (bottom panel). (B–F) Steps for isolating the larval brain without damaging it (see text). Red: brain. Dark yellow: eye/antenna disks. Blue text: actions to perform with the corresponding forceps. (G) Appearance of an isolated brain with no visible damage. D is the dorsal side and V is the ventral side on the lateral view. (H) Common damages caused by excessively pulling the brain during the dissection. Top panel: detachment of the ventral nerve cord. Bottom panel: deformation of a lobe. Posterior is left and anterior is right in all panels. Please click here to view a larger version of this figure.
3. Immobilization on coverslip with Fibrin clots
NOTE: Perform this step under a dissection binocular.
- Use a P20 pipette with a coated tip as in step 2.8 to transfer the dissected brains into another well of the borosilicate plate containing 200 µL of culture medium + Fibrinogen (Figure 2A).
- Pipette in one brain along with 9 µL of culture medium + Fibrinogen. With the end of the tip almost touching the cover glass bottom of a 35 mm cell culture dish, pipette out the contents (the culture medium and the brain) making the culture medium touch the coverslip immediately after exiting the pipette tip so that the brain and the medium do not slide to the sides of the tip, potentially damaging the brain (Figure 2A).
- Use one tip of a pair of forceps or a closed pair of forceps to gently push and position the brain within the culture medium + Fibrinogen drop.
NOTE: Touching the drop with an open pair of forceps can result in the culture medium getting pulled by capillarity between the forceps tips (Figure 2B). - If the ventral part of the brain has to be imaged, induce Fibrinogen clotting as follows to properly orient the brain within the clot (Figure 2C).
- Push the brain to one side of the drop. With a P1 pipette equipped with a P1 tip, pipette 1 µL of Thrombin solution, touch the edge of the drop on the opposite side of the brain with the pipette tip, and pipette out the Thrombin (Figure 2C, first drawing).
- Wait for 1–2 min for the Fibrinogen to start polymerizing, resulting in the formation of a cloudy precipitate at one side of the drop (Figure 2C, second drawing). Gently push and “tuck” the brain into the Fibrin clot, making sure that the part to image (e.g., the ventral side) is as close as possible to the coverslip without deforming the brain (Figure 2C, third drawing).
- Pipette out 1 µL of Thrombin solution close to the side of the brain not tucked into the Fibrin clot. (Figure 2C, fourth drawing).
- Wait for 2–3 min for the second Fibrin clot to set (Figure 2C, fifth drawing). Press on the edges of the clot to make it adheres more strongly to the coverslip, taking care not to deform the brain (Figure 2C, sixth and seventh drawings). If the brain appears to be too far from the coverslip, bring it closer by pressing the Fibrin clot close to the brain.
- If the dorsal part of the brain must be imaged, induce Fibrin clotting as follows (Figure 2D).
- Position the brain in the center of the drop, dorsal part facing the coverslip. With a P10 pipette equipped with a thin tip, pipette 1 µL of Thrombin solution, touch the edge of the drop on the opposite side of the brain with the pipette tip, and pipette out the Thrombin (Figure 2D, first drawing).
- Wait for 2–3 min for the Fibrinogen to start polymerizing, resulting in the formation of a cloudy, fibrous precipitate (Figure 2D, second drawing). Press on the edges of the clot to make it adhere more strongly to the coverslip, taking care not to deform the brain (Figure 2D, third and fourth drawings).
- Repeat steps 3.1–3.5 if several clot samples have to be immobilized on the coverslip.
- With the end of the tip positioned about 0.5 cm above the clot(s), gently pipette 390 µL of culture medium without Fibrinogen dropwise on top of the clot(s) (Figure 2B, right Petri dish). Do not add the culture medium on the sides of the clot as it may detach it from the coverslip.
- To wash out the remaining Thrombin, use a pipette to remove 300 µl of the culture medium and add 300 µl of clean culture medium, making sure that the clots remain fully immersed. Repeat twice to wash out excess Thrombin.
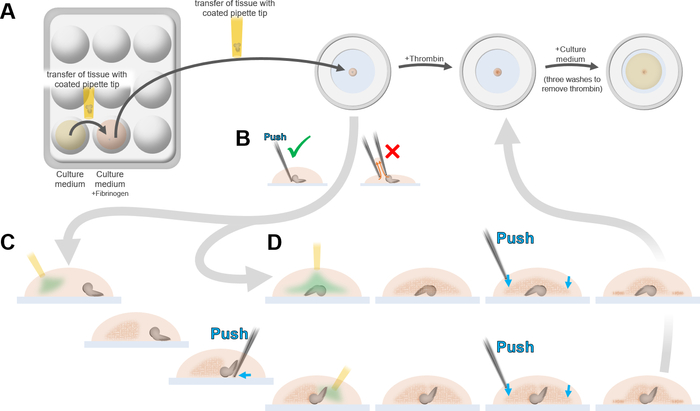
Figure 2: Immobilization of a Drosophila larval brain using Fibrin clots. (A) Using a BSA-coated Pipette tip, brains are transferred from the culture medium (yellow) into a culture medium + Fibrinogen solution (orange), then pipetted onto the coverslip (light blue) of a culture dish along with 3.5 µL of culture medium + Fibrinogen. Fibrinogen clotting is induced by addition of Thrombin to immobilize brains in dorsal or ventral position (see D and E). The secured brains in the clots are subsequently covered in culture medium without Fibrinogen and excess thrombin is removed by adding and removing culture medium three times. (B) The position of the brain within the drop of culture medium + Fibrinogen (orange) on the coverslip should only be adjusted by gently pushing it with the tip of a closed pair of forceps, or only one forceps tip. Touching the drop with on open pair of forceps can result in the culture medium getting pulled by capillarity between the tips of the forceps. (C) Steps for positioning the larval brain for imaging the ventral side (see text). (D) Steps for positioning the larval brain for imaging the dorsal side (see text). In (D) and (E), green: Thrombin. Dark orange: Fibrin clot. Pale blue: coverslip. Blue arrows: edges of the clot to be pushed against the coverslip to stabilize the clot. Please click here to view a larger version of this figure.
4. Addition of reagents during live imaging
- To avoid temperature changes causing changes of focus, keep the solution with the reagents to be added to the culture dish at the same temperature as the culture dish itself to bring it to the same temperature before adding it. If an environmental chamber is being used, leave the solution within the chamber.
- Remove the lid of the culture dish without displacing the culture dish itself. For easier removal, place the lid of the 35 mm dish upside-down on top of the dish before imaging.
- With a P1000 pipette, position the pipette tip close to the surface of the culture medium inside the culture dish and gently release the adequate volume of reagent solution onto it to reach the desired reagent concentration (e.g., 400 µL of a solution of 20 µM NAPP1 onto 400 µL of culture medium for a final concentration of 10 µM NAPP1), without generating strong fluxes that could detach the clots.
- To homogenize the solution within the culture dish, use a P200 pipette to slowly pipette a small amount of the solution in and out five times (e.g., 150 µL for a volume of 800 µL within the dish).
- Replace the lid on top of the culture dish without displacing the culture dish itself and resume imaging.
5. Washout of reagents during live imaging
- Prior to the washout, keep the washing solution at the same temperature as the culture dish.
- Remove the lid of the culture dish without displacing the culture dish itself.
- With a P200 or P1000 pipette, slowly remove some culture medium from the culture dish without exposing the top of the clot (e.g., remove 600 µL from 800 µL of culture medium within the dish).
- To dilute the reagent, with a P1000 pipette, position the pipette tip close to the surface of the culture medium inside the culture dish and slowly release the washing solution (e.g., add 1 mL of washing solution to the 200 µL of culture medium left within the dish for a dilution factor of 6).
- Repeat steps 5.3 and 5.4 until the reagent is diluted as required (e.g., another three similar rounds will result in a dilution factor of 1296, reducing the initial concentration of 10 µM NAPP1 to 7.7 nM).
- Replace the lid on top of the culture dish without displacing the culture dish itself and resume imaging.
6. Correction of the xyz drifting on tridimensional and/or multichannel time-lapses
- Preparation
- Download and install ImageJ24. Download the TurboReg25 and MultiStackReg26 plugins and place them inside the plugins folder of ImageJ.
- Download the MultiHyperStackReg (Supplemental coding file 1) and AutoHyperStackReg ImageJ macros (Supplemental coding file 2). To install a macro, either place the .ijm file inside the plugins folder of ImageJ or add the contents of the .ijm file to the ImageJ/macros/StartupMacros.txt file.
- On ImageJ, open a time-lapse movie including several slices and/or several channels (henceforth referred to as “hyperstack”, Figure 3A,4A). If the time-lapse only includes one slice and one channel, the alignment can directly be performed using MultiStackReg.
- Correction of lateral drift using MultiHyperStackReg
- Decide which channel and/or slice of the hyperstack should be used as a reference for alignment. To generate a one-channel, one-slice reference stack (henceforth referred to as “reference stack”), click on Image | Duplicate…, tick the Duplicate hyperstack checkbox, type the relevant channel number in Channels (c): (e.g., replace 1–2 with 1) and/or the relevant channel number in Slices (z): (e.g., replace 1–15 with 8), and ensure that Frames: (t) includes all frames (e.g., 1–50) before clicking on OK (Figure 3B).
- Alternatively to step 6.2.1, if a projection of multiple slices is preferred for the one-channel, one-slice reference stack, click on Image | Stacks | Z Project…, select the first and last slices and type of projection, ensure that All time frames is ticked and click on OK. If the time-lapse has multiple channels either delete the irrelevant channels (Image | Stacks | Delete Slice) from the resulting projection or duplicate the chosen reference channel (Image | Duplicate) (Figure 3B).
- Optionally, crop the reference stack to use only one part as the image as a reference by selecting the rectangle tool in the toolbar, tracing a rectangle over the region of interest, and clicking on Image | Crop.
- To start MultiStackReg, click on Plugins | Registration | MultiStackReg. In the pop-up menu Stack 1:, select the name of the one-channel, one-slice stack generated in the previous steps. In the pop-up menu Transformation:, select Translation; tick the Save Transformation File checkbox and ignore all the other fields.
NOTE: Other types of Transformation (see25). - Click on OK. Select a location and name to save the alignment file and click on Save. On the reference stack window, wait for the frames to stop moving automatically, indicating that the registration is over (Figure 3B).
- Check on the reference stack to see whether the alignment is satisfying. If not, repeat steps 6.2.1–6.2.5 with a different reference slice, channel and/or cropping. Close the reference stack.
- Select the hyperstack window and run the MultiHyperStackReg macro. Select the transformation file created in step 6.2.5 and click on Open. Wait for the alignment to be applied to every channel and/or slice of the hyperstack, at which point a new window with the suffix “_aligned” will open (Figure 3C).
- Correction of focus drift using MultiHyperStackReg
- Click on Image | Properties…, copy the value displayed in Pixel Width. To orthogonally reslice the one-channel hyperstack with a one-pixel spacing, click on Image | Stacks | Reslice [/]…, paste the pixel width value into the Output Spacing: numeric field; select Top in the Start At: popup menu, tick Avoid Interpolation and click on OK.
NOTE: In case memory has to be freed on ImageJ, the hyperstack can be closed following the reslice. - Select the new window generated by the reslice (henceforth referred to as “resliced hyperstack”, Figure 4B). If the resliced hyperstack has multiple channels, select a reference channel to perform the alignment and delete the other channels by selecting them in the channel scroll bar; click on Image | Stacks | Delete Slice, select Channel in the Delete Current popup menu and click on OK.
- Generate a single-slice resliced hyperstack (henceforth referred to as “resliced reference stack”), either by duplicating a single slice of the resliced hyperstack (see step 6.2.1) or by projecting several slices of the resliced hyperstack (see step 6.2.2) (Figure 4C).
- Optionally, crop the resliced reference stack to use only one part as the image as a reference (see step 6.2.3).
- Perform the image registration on the resliced reference stack with MutiStackReg (see steps 6.2.4–6.2.6) (Figure 4C).
- Select the resliced hyperstack window and run the MultiHyperStackReg macro. Select the transformation file created in step 6.3.5, click on Open and wait for the alignment to be applied to every channel and/or slice of the hyperstack, at which point a new window with the suffix “_aligned” will open (henceforth referred to as “resliced reference stack”, Figure 4D). Close the original resliced hyperstack.
- To convert the resliced aligned hyperstack to the xy view of the original hyperstack, click on Stacks | Reslice [/]…, select Top in the Start At: popup menu, tick Avoid Interpolation and click on OK. Once a new window with the final focus-aligned hyperstack opens (Figure 4E), close the resliced aligned hyperstack.
- Click on Image | Properties…, copy the value displayed in Pixel Width. To orthogonally reslice the one-channel hyperstack with a one-pixel spacing, click on Image | Stacks | Reslice [/]…, paste the pixel width value into the Output Spacing: numeric field; select Top in the Start At: popup menu, tick Avoid Interpolation and click on OK.
- To automatically correct the lateral drift and focus drift, run the AutoHyperStackReg macro. Select the reference channel, if applicable, and whether to correct the lateral and/or focus drift. Click on OK.
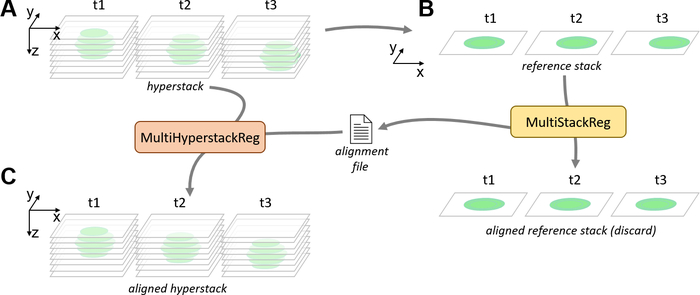
Figure 3: Pipeline for the correction of lateral drift with MultiHyperstackReg. (A) Lateral and focus drifts can be observed on a hyperstack containing several slices and timepoints. (B) A single slice, single channel reference stack is generated from the hyperstack, either by duplication or Z projection. The reference stack is aligned by the MultiStackReg plugin, generating an alignment file. (C) The MultiHyperstackReg macro is used to apply the alignment file to the hyperstack, resulting in an aligned hyperstack for which the lateral drift is corrected. Please click here to view a larger version of this figure.
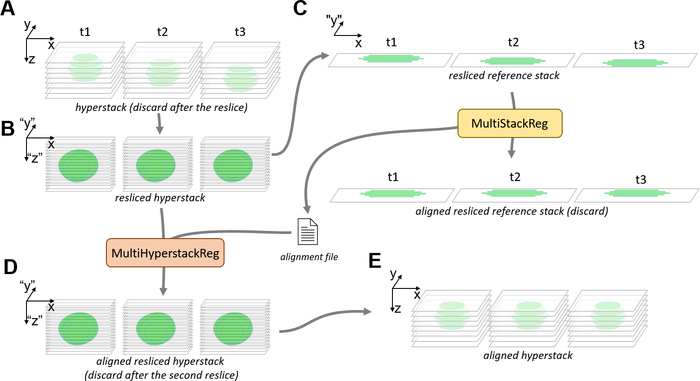
Figure 4: Pipeline for the correction of focus drift with MultiHyperstackReg. (A) Focus drifts can be observed on a hyperstack containing several slices and timepoints. (B) The hyperstack is orthogonally resliced from the top, along each line of pixels along the Y-axis, without interpolation. This results in a resliced hyperstack containing the same information as the hyperstack, with its y and z coordinates switched. The focus drift (in z) of the original hyperstack occurs as a drift in y on the resliced hyperstack. (C) A single slice, single channel resliced reference stack is generated from the resliced hyperstack, either by duplication or Z projection. The resliced reference stack is aligned by the MultiStackReg plugin, generating an alignment file. (D) The MultiHyperstackReg macro is used to apply the alignment file to the resliced hyperstack, resulting in an aligned resliced hyperstack for which the focus drift (in y) is corrected. (E) A second orthogonal reslice restores the original coordinates of the hyperstack, which now no longer presents a focus drift (in z). Please click here to view a larger version of this figure.
7. Cortical signal measurement with rotating linescans
- Download the Rotating Linescans ImageJ macro (Supplemental coding file 3).
- Open a time-lapse in ImageJ. Position the frames scrollbar to the first frame and, in case the time-lapse contains several slices, position the slices scrollbar to the slice on which the signal will be measured.
- Tracking mode
- Select the straight-line tool in the toolbar. Trace a line across the cortical zone to be measured (Figure 5A), making sure that the line is roughly perpendicular to the cortex and that the line is long enough to cross the cortex at every next time point (Figure 5B, first and second panel).
- Double-click the line tool icon in the toolbar to open the Line Width window. Increase the width of the line as much as needed while keeping the intersection between the line and the cortex a straight line (Figure 5B, third panel).
- Start the Rotating Linescans macro. Set the rotation range (Figure 5E) to a low value (about 10°) if the orientation of the cortical zone to be measured does not change much from one timepoint to the next. Set the Measure Around value to 0 pixels to only measure the signal on the line where the maximal signal intensity is detected, or to higher values to also measure the signal around this line.
NOTE: The Measure Around value will only affect the signal measurement after detection of the cortex and will not affect the detection itself. - On the Find Cortex on Channel: popup menu, select the channel on which the detection will be performed. To visualize where the cortex has been detected at each timepoint, keep the Display Position… checkbox ticked (recommended). Keep the Recenter and reorient… checkbox ticked. Click on OK.
- Once the Done, Results Copied to Clipboard status is displayed in the ImageJ window status, scroll through the time-lapse to check whether the detected cortex positions (yellow overlay) and the measured area (cyan overlay) are satisfying. If not, repeat steps 7.3.1–7.3.4 with a different line position, length, or width and different settings in the Rotating Linescans options window.
- Paste the measurements into a spreadsheet software.
NOTE: Columns correspond to channels in the order of their appearance in the time-lapse and lines correspond to frames.
- Non-tracking mode
- Select the straight-line tool in the toolbar. Trace a line (indicated in cyan, Figure 5A) across the cortical zone to be measured, making sure that the line is roughly perpendicular to the cortex and that the line is long enough to cross the cortex at every time point (Figure 5C, first and second panel).
- Double-click on the line tool icon in the toolbar to open the Line Width window. Increase the width of the line as much as needed while keeping the intersection between the line and the cortex a straight line (Figure 5C, third panel).
- Start the Rotating Linescans macro. Set the rotation range (Figure 5E) accordingly to the changes of orientation of the cortical zone to be measured throughout the entire time-lapse. Set the Measure Around value to 0 pixels to only measure the signal on the line where the maximal signal intensity is detected, or to higher values to also measure the signal around this line.
NOTE: The Measure Around value will only affect the signal measurement after detection of the cortex and will not affect the detection itself. - On the Find Cortex on Channel: popup menu, select the channel on which the detection will be performed. To visualize where the cortex has been detected at each timepoint, keep the Display positions… checkbox ticked (recommended). Untick the Recenter and Reorient… checkbox. Click on OK.
- Once Done, Results Copied to Clipboard is displayed in the ImageJ window status, scroll through the time-lapse to check whether the detected cortex positions (yellow overlay) and the measured area (cyan overlay) are satisfying. If not, repeat the previous steps with a different line position, length, or width and different settings in the Rotating Linescans options window.
- Paste the measurements into a spreadsheet software.
NOTE: Columns correspond to channels in the order of their appearance in the time-lapse and lines correspond to frames.
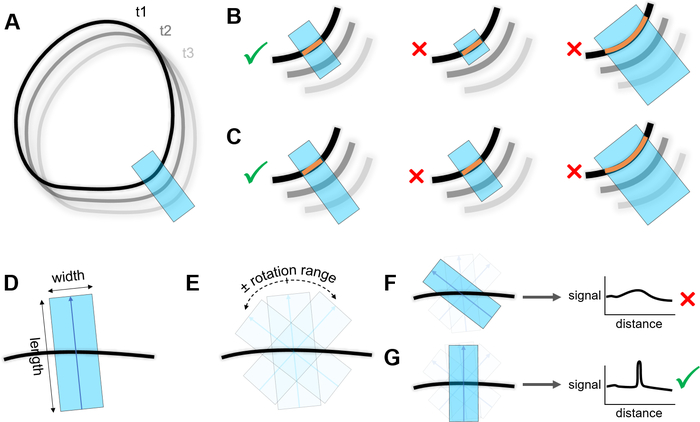
Figure 5: Measurement of cortical signal with rotating linescan. (A) A line (cyan) is traced perpendicularly to the cortex (black) where the signal will be measured. Different shades of gray show the cell position changing over three timepoints (t1, t2, t3). (B) Left: correct positioning of the line in tracking mode. Middle: incorrect positioning of the line in tracking mode as there is no overlap with the cortex at the next timepoint. Right: overly wide line resulting in the intersection (orange line) between the cortex and the line not being straight. (C) Left: correct positioning of the line in non-tracking mode. Middle: incorrect positioning of the line in non-tracking mode as there is no overlap with the cortex at every timepoint. Right: overly wide line resulting in the intersection (orange line) between the cortex and the line not being straight. (D) Length and width of the scanning line. (E) Linescans are performed within a range of orientations around the original orientation shown in (D) defined by the “rotation range” parameter, at an interval defined by the “rotation step” parameter. (F) Non-optimal orientation of the scanning line relative to the cortex, resulting in a low signal measured along the line. (G) The optimal orientation of the line is defined as the one resulting in the highest peak of signal intensity measured along the line. Please click here to view a larger version of this figure.
Representative Results
Immobilization of Drosophila tissues with Fibrin clots and culture medium exchange during live imaging.
After being dissected following the procedure presented in step 2 (Figure 1) and immobilized in Fibrin clots on the same coverslip following the procedure presented in step 3 (Figure 2), larval brains expressing GFP-tagged Bazooka (Baz::GFP), the fly ortholog of the polarity protein Par-3, were imaged for 30 min in time-lapse multi-position confocal imaging. Baz::GFP is combined with apkcas4, an allele that allows acute inhibition of atypical protein kinase C (aPKC) through the addition of the small ATP analog 1-NAPP120. 1-NAPP1 was therefore added to the culture medium following the procedure presented in step 4 and live imaging was resumed for 2.5 h. Control brains carrying a wild-type version of the kinase aPKC did not react to the ATP analog, whereas brains carrying additionally an analog-sensitive mutation of aPKC (apkcas4) displayed a contraction of the neuroepithelium and bright clusters of Baz in neuroblasts and their progeny (Figure 6, Video 1). Neuroblasts keep on dividing throughout the time-lapse, indicating that the tissue remains healthy, and brains show little drift despite the culture medium change, indicating that they are well immobilized. Similarly, after being dissected following the procedure presented in27, ovarioles expressing Baz::GFP were immobilized in Fibrin clots on the same coverslip and imaged for 8 min, after which application of 1-NAPP1 induced the contraction of apkcas4 mutant follicular cells but not of controls (Figure 7, Video 2).
Correction of lateral and focus drift using MultiHyperstackReg.
A hyperstack displaying both lateral and focus drift (Video 3, left panel) was first corrected for lateral drift (step 6.2, Figure 3, Video 3, middle panel), then focus drift (step 6.3, Figure 4, Video 3, right panel) using the MultiStackReg plugin and the MultiHyperstackReg macro, resulting in a substantial reduction of the movements observed in the original hyperstack.
Cortical signal measurement using rotating linescans
A neuroblast expressing Baz::GFP (green) and the transmembrane protein CD4 tagged with an infrared fluorescent protein (CD4::mIFP, red) was imaged during one mitosis. The CD4::mIFP signal was used as a reference to detect the cortex at various parts of the neuroblast with linescans (step 7, Figure 5, Figure 8A–C) and the Baz::GFP signal was measured at the detected positions Figure 8D). During their division, neuroblasts transiently polarized by establishing distinct and opposite cortical domains: the apical pole and the basal pole. Baz defines the apical pole and, consistently, the Baz::GFP signal increased at the apical pole of the neuroblast and decreased at the basal pole during the division (Figure 8C,D). The position of the cortex was satisfyingly tracked throughout the time-lapse (Figure 8C, Video 4). The Drosophila lines and genotypes are referenced in Supplemental Table 1–2.
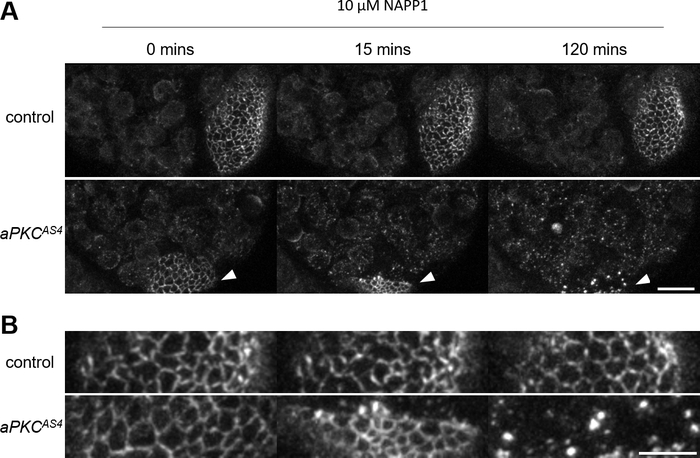
Figure 6: Application of an ATP analog on immobilized larval brains during live imaging. (A) Live imaging of two larval brains expressing Baz::GFP immobilized on the same coverslip. The culture medium is changed to a concentration of 10 µM of the ATP analog 1-NAPP1 at 0 min. Control brains do not visibly react to the ATP analog whereas brains carrying an analog sensitive mutation of aPKC (aPKCAS4) display a contraction of the neuroepithelium (arrowheads) and bright clusters of Baz in neuroblasts and their progeny. Maximal intensity projection of 33 slices for a depth of 23 µM. Scale bar: 20 µM. (B) Magnification of the neuroepithelium. Scale bar: 10 µM. Please click here to view a larger version of this figure.
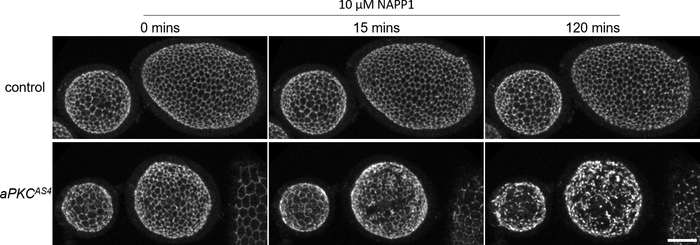
Figure 7: Application of an ATP analog on immobilized egg chambers during live imaging. Live imaging of two ovarioles expressing Baz::GFP immobilized on the same coverslip. The culture medium is changed to a concentration of 10 µM of the ATP analog 1-NAPP1 at 0 min. Control egg chambers do not visibly react to the ATP analog whereas egg chambers carrying an analog sensitive mutation of aPKC (aPKCAS4) display a contraction of the epithelium (arrowheads) eventually leading to the apparent breakdown of adherens junctions. Maximal intensity projection of 33 slices for a depth of 27 µM. Scale bar: 20 µM. Please click here to view a larger version of this figure.
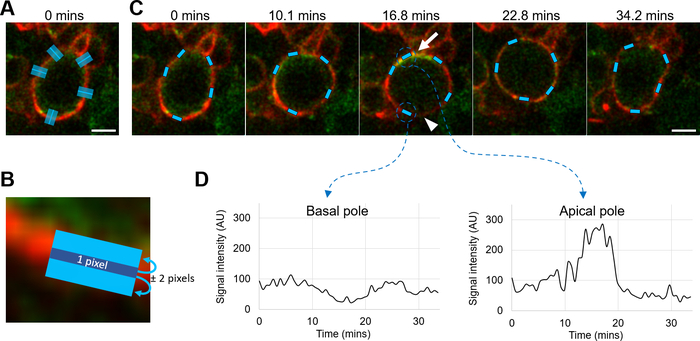
Figure 8: Measurement of the cortical signal using rotating linescans. (A) Live D. melanogaster neuroblast expressing Baz::GFP (green) and CD4::mIFP (red). Cyan lines: initial scanning lines traced perpendicularly to various cortical zones to measure. Scale bar: 5 µM. (B) Identification of the cortex (dark blue line) using cortical linescans and CD4::mIFP (red) as the reference channel. Cyan line: area defined by the “Measure around” parameter (here, 2 pixels), where the signal is measured after cortex detection. Scale bar: 2 µm. (C) Cyan: cortical areas identified during a neuroblast division by the rotating linescans method from the initial scanning lines displayed in (A), using CD4::mIFP (red) as the reference channel. Scale bar: 5 µM. Arrow: apical pole. Arrowhead: basal pole. (D) Measurement of the Baz::GFP signal intensity over time in the two corresponding zones identified by rotating linescans displayed in (C). During mitosis, Baz::GFP gets transiently enriched at the apical pole of the neuroblast and is depleted from the opposite basal pole. Please click here to view a larger version of this figure.
Video 1: Application of media exchange on immobilized larval brains during live imaging to add an inhibitor, presented in Figure 6, scale bar: 20 µm. Please click here to download this video.
Video 2: Application of media exchange on immobilized egg chambers during live imaging to add an inhibitor, presented in Figure 7, scale bar: 20 µm. Please click here to download this video.
Video 3: Correction of lateral and focus drift using MultiHyperStackReg. Live imaging of a neuroblast expressing the membrane probe PH::GFP. Xy: single focal plane. Xz: orthogonal reslice. Left: before correction of the drift. Middle: after correction of the lateral drift. Right: after correction the lateral and focus drift, scale bar: 10 µm. Please click here to download this video.
Video 4: Detection of the cortex using rotating linescans, presented in Figure 8, scale bar: 5 µm. Please click here to download this video.
Supplemental Table 1: Genotypes of imaged Drosophila tissues. Please click here to download this table.
Supplemental Table 2: Origin of transgenes used. Please click here to download this table.
Supplemental Coding File 1: Origin of transgenes used. Please click here to download this file.
Supplemental Coding File 2: Origin of transgenes used. Please click here to download this file.
Supplemental Coding File 3: Origin of transgenes used. Please click here to download this file.
Discussion
Live imaging of whole mount D. melanogaster larval brains provides the opportunity to observe asymmetric neural stem cell divisions in conditions close to a physiological context. The first part of our protocol introduces our approach on the dissection of larval brains. As already stated13, a critical aspect of the preparation is to avoid damaging the brain. The most challenging aspect of this is to separate the brain from the neighboring imaginal disks without pulling excessively on the brain. Our approach to “cutting without pulling” is to perform either a grinding movement with the tips of one pair of forceps, or to slide a forceps tips along two other forceps tips holding the connection between tissues (Figure 1A) whereas Lerit et al. describe saw-like movements with a dissecting pin. We advise the experimenter to try all approaches and to adopt the best-suited one for themself. We also suggest using BSA- or FCS-coated pipette tips rather than dissection tools to transfer isolated brains. The coating prevents tissues from sticking to the plastic and allows the safe transfer of tissues while always keeping them completely immersed, without subjecting them to a possible transient deformation as they stick to the dissection tool during the transfer. Coated tips have other advantages to allow the transfer of several brains at once, which is particularly useful when it is critical to control the residency time of the samples within a particular medium; they are suitable for the transfer of other tissues, even fragile ones as, for example, fat bodies; they can accommodate a wide range of different sample sizes by using larger tips or cutting the extremity of the tip.
Next, this protocol describes the use of Fibrinogen clotting to immobilize larval brains on the coverslip of a culture dish. A brain is oriented within a drop of culture medium + Fibrinogen, after which clotting is induced by the addition of Thrombin. Fibrin formation is gradual, providing a time window during which the orientation of the sample can be fine-tuned, if necessary (Figure 2C). If a slight compression of the sample is required – which we advise against in the case of larval brains – it can also be finely adjusted by pressing the clot more or less close to the sample during steps 3.4.4 and 3.5.2. The main advantage of this Fibrinogen clotting over the protocol described in Lerit et al., in which brains are mounted between a gas permeable membrane and a coverslip, is the ability to replace the culture medium during live imaging. A possible advantage of using a gas permeable membrane over our protocol could be that it provides an optimal oxygenation of the samples, which could be more limited with our approach as brains are separated from the air by the clot and some medium. For this reason, although we did not assess the effect of the amount of culture medium within the culture dish, we suggest limiting the amount of culture medium on top of the clots while keeping the clots completely immersed.
As the manipulation of the clots can be experimentally difficult and time-consuming, we recommend that the experimenter first practices manipulating clots without samples. Modulating the volume of the drop of culture medium + Fibrinogen on the coverslip, the concentration of Fibrinogen or the volume and concentration of Thrombin could help making the preparation easier and could be important when adapting the protocol to other types of tissues. With enough experience manipulating the clots, several brains can be immobilized in one clot if necessary, although it complicates the fine-tuning of the orientation. Several clots can be formed on the coverslip, allowing, for example, to image samples of different genotypes. It is also possible to expose different clots on the same coverslip to different culture media, although care has to be taken never to mix the drops of culture medium covering the different clots. Once larval brains are immobilized and ready for live imaging, we advise to follow the recommendations of Lerit et al.13 to avoid excessive photodamage. We only add to these recommendations to not only maintain the brains at 25 °C during live imaging with a stage incubator, but also to preheat this stage for at least 30 min before the start of imaging. In our hands, failing to do so systematically results in a strong focus drift.
It can be advantageous in some contexts to be able to perform correlated microscopy and fix and immunostain a sample after live imaging. However, a limitation of using Fibrin clots is that it is nearly impossible to mechanically isolate a brain from a clot without damaging it. A possible way to overcome this limitation could be to develop methods to degrade Fibrin clots with Plasmin or to induce clot disassembly by addition of a peptide mimicking the knobs allowing Fibrin polymerization28. We typically use Fibrin clots on samples ranging from single cells to 200 µM long tissues, but we could also successfully immobilize in clots and image brains from adult bumblebees over 3 mm wide. The upper limit of the sample size that can be immobilized in clots remains to be determined. Similarly, although we usually image immobilized samples for 4 to 5 h, we successfully imaged neuroblasts in primary culture for up to 3 days, indicating that the clot at least does not interfere with longer term viability. On the contrary, clots can facilitate the regular replacement of the medium, a requirement for longer term imaging, without the need for devices such as peristaltic pumps for this purpose. While we have not measured the permeability parameters of fibrin clots, the fibrous gel-like nature of the clots appears to be penetrable by cell permeable molecules. We found that Latrunculin A, Colcemid or 1-NAPP1, HALO-tag ligands and other standard dyes used in cell biology reach the cells in the fibrin clot without any problems and have so far not encountered molecules that would be retained by the clot, which, however, is a possibility that needs to be empirically tested.
In conclusion, using Fibrin clots provide a reliable and relatively easy to implement a way of immobilizing living tissues for live imaging. Beyond its usefulness in limiting lateral drifting, the ability to change the culture medium during live imaging has proven invaluable for our chemical genetics approach to studying the asymmetric cell division of D. melanogaster neuroblasts21. We anticipate that this technique will be beneficial to studies in a wide range of different tissues, particularly if they involve chemical genetics or, for example, protein self-labeling29.
Our MultiHyperStackReg macro relies on the TurboReg ImageJ plugin, which aligns successive frames or slices of a stack, and the MultiStackReg ImageJ plugin, which allows to apply the transformations to other stacks. MultiHyperStackReg simply allows to apply these transformations to hyperstacks (stacks with at least four dimensions). As the use of MultiHyperStackReg, as we describe it, requires a lot of actions and can get quite time consuming, we also wrote the AutoHyperStackReg macro, which automatically performs all the steps described in steps 6.2 and 6.3. However, contrary to MultiHyperStackReg, AutoHyperStackReg currently lacks the possibility to calculate the alignment of a stack based on a subregion of this stack, an option that we found is sometimes crucial for a satisfying alignment. Another limitation of AutoHyperStackReg is that its use is restricted to translations and not the other transformations proposed by TurboReg and MultiStackReg, whereas MultiHyperStackReg can apply to a hyperstack any transformation type should the user need it. Future releases will implement these functions and will be available on GitHub30. Finally, our rotating linescan macro provides an easy way to quickly measure cortical signals in time lapses, even if the cortex changes its position or orientation over time. Whether its tracking mode or its non-tracking mode is best suited for analysis depends on the quality and consistency of the cortical signal. The tracking mode is easier to use as the user does not need to consider where the cortex will be for every timepoint and can accommodate large changes of position and orientation over time. However, it is only suitable if the cortical signal remains strong enough for detection during the entire movie: a failure to detect anything else than the cortex (e.g., a bright cytoplasmic compartment close to the cortex) can compromise the detection for every following timepoint (e.g., the cytoplasmic compartment moves away from the cortex and the cortical linescan follows it for the rest of the time lapse). The non-tracking mode does not have this pitfall as the detection is limited to the line originally drawn by the user (e.g., a bright cytoplasmic compartment may cause the detection to fail for a few timepoints, but as the cytoplasmic compartment moves away from the detection zone, proper detection of the cortex resumes), but does not behave well if the cortex position or orientation changes a lot over time. The next feature (which will be available on GitHub30) to be developed for the tracking mode will be the option to only analyze specified timepoints and to define a reference timepoint (rather than the first timepoint) before which and after which the detection will be performed, which will allow the user to at least avoid problematic timepoints.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Work in the Januschke laboratory is supported by Wellcome trust grants 100031/Z/12/A and 1000032/Z/12/A. The tissue imaging facility is supported by the grant WT101468 from Wellcome.
Materials
| Albumin bovine serum, BSA | Merck | 5470 | |
| D-(+)-Glucose | Sigma | G7021 | |
| Dimethyl sulfoxide | Sigma | D2650 | |
| Dumont #55 Forceps | Fine Science Tools | 11255-20 | |
| Fetal Bovie Serum, suitable for cell culture | Sigma | F7524 | |
| Fibrinogen from human plasma | Sigma | F3879 | |
| FluoroDish Cell Culture Dish – 35mm, 23 mm well | World Precision Instruments | FD35-100 | |
| PP1 Analog (1NA-PP1) | Merck Millipore | 529579 | |
| Pyrex spot plate with nine depressions | Sigma | CLS722085-18EA | |
| Schneider's Insect Medium | Sigma | S0146 | |
| Thrombin from bovine plasma | Merck | T4648 |
References
- Restrepo, S., Zartman, J. J., Basler, K. Cultivation and live imaging of Drosophila imaginal discs. Methods in Molecular Biology. 11478, 203-213 (2016).
- Tsao, C. K., Ku, H. Y., Lee, Y. M., Huang, Y. F., Sun, Y. H. Long term ex vivo culture and live imaging of Drosophila larval imaginal discs. PloS One. 11 (9), 0163744 (2016).
- Cabernard, C., Doe, C. Q. Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila. Developmental Cell. 17 (1), 134-141 (2009).
- Basto, R., et al. Flies without centrioles. Cell. 125 (7), 1375-1386 (2006).
- Januschke, J., Gonzalez, C. The interphase microtubule aster is a determinant of asymmetric division orientation in Drosophila neuroblasts. The Journal of Cell Biology. 188 (5), 693-706 (2010).
- Rusan, N. M., Peifer, M. A role for a novel centrosome cycle in asymmetric cell division. Journal of Cell Biology. 177 (1), 13-20 (2007).
- Dai, W., Montell, D. J. Live imaging of border cell migration in Drosophila. Methods in Molecular Biology. 1407, 153-168 (2016).
- Haigo, S. L., Bilder, D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 331 (6020), 1071-1074 (2011).
- Tissot, N., et al. Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte. Nature Communications. 8, 15168 (2017).
- Martin, J. L., et al. Long-term live imaging of the Drosophila adult midgut reveals real-time dynamics of division, differentiation and loss. eLife. , (2018).
- Cabernard, C., Doe, C. Q. Live imaging of neuroblast lineages within intact larval brains in Drosophila. Cold Spring Harbor Protocols. 2013 (10), 970-977 (2013).
- Sabado, V., Nagoshi, E. Single-cell resolution fluorescence live imaging of Drosophila circadian clocks in larval brain culture. Journal of Visualized Experiments: JoVE. (131), (2018).
- Lerit, D. A., Plevock, K. M., Rusan, N. M. Live imaging of Drosophila larval neuroblasts. Journal of Visualized Experiments: JoVE. (89), (2014).
- Egger, B., van Giesen, L., Moraru, M., Sprecher, S. G. In vitro imaging of primary neural cell culture from Drosophila. Nature Protocols. 8 (5), 958-965 (2013).
- Homem, C. C., Reichardt, I., Berger, C., Lendl, T., Knoblich, J. A. Long-term live cell imaging and automated 4D analysis of drosophila neuroblast lineages. PloS One. 8 (11), 79588 (2013).
- Forer, A., Pickett-Heaps, J. D. Cytochalasin D and latrunculin affect chromosome behaviour during meiosis in crane-fly spermatocytes. Chromosome Research: An iIternational Journal On the Molecular, Supramolecular and Evolutionary Aspects of Chromosome Biology. 6 (7), 533-549 (1998).
- Pampalona, J., Januschke, J., Sampaio, P., Gonzalez, C. Time-lapse recording of centrosomes and other organelles in Drosophila neuroblasts. Methods in Cell Biology. 129, 301-315 (2015).
- Ramat, A., Hannaford, M., Januschke, J. Maintenance of miranda localization in Drosophila neuroblasts involves interaction with the cognate mRNA. Current Biology: CB. 27 (14), 2101-2111 (2017).
- Rebollo, E., et al. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing drosophila neural stem cells. Developmental Cell. 12 (3), 467-474 (2007).
- Hannaford, M. R., Ramat, A., Loyer, N., Januschke, J. aPKC-mediated displacement and actomyosin-mediated retention polarize Miranda in Drosophila neuroblasts. eLife. 7, 166 (2018).
- Hannaford, M., Loyer, N., Tonelli, F., Zoltner, M., Januschke, J. A chemical-genetics approach to study the role of atypical Protein Kinase C in Drosophila. Development. 146 (2), (2019).
- Loyer, N., Januschke, J. The last-born daughter cell contributes to division orientation of Drosophila larval neuroblasts. Nature Communications. 9 (1), 3745 (2018).
- Wilcockson, S. G., Ashe, H. L. Drosophila ovarian germline stem cell cytocensor projections dynamically receive and attenuate BMP signaling. Developmental Cell. 50 (3), 296-312 (2019).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 9 (7), 671-675 (2012).
- Thevenaz, P., Ruttimann, U. E., Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Transactions on Image Processing. 7 (1), 27-41 (1998).
- Prasad, M., Montell, D. J. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Developmental Cell. 12 (6), 997-1005 (2007).
- Chernysh, I. N., Nagaswami, C., Purohit, P. K., Weisel, J. W. Fibrin clots are equilibrium polymers that can be remodeled without proteolytic digestion. Scientific Reports. 2, 879 (2012).
- Liss, V., Barlag, B., Nietschke, M., Hensel, M. Self-labelling enzymes as universal tags for fluorescence microscopy, super-resolution microscopy and electron microscopy. Scientific Reports. 5, 17740 (2015).
- . ImageJ macros [software] Available from: https://github.com/nicolasloyer?tab=repositories (2020)

