Optimizing the Setup and Conditions for Ex Vivo Electroretinogram to Study Retina Function in Small and Large Eyes
Summary
Modification of existing multielectrode array or patch clamp equipment makes the ex vivo electroretinogram more widely accessible. Improved methods to record and maintain ex vivo light responses facilitate the study of photoreceptor and ON-bipolar cell function in the healthy retina, animal models of eye diseases, and human donor retinas.
Abstract
Measurements of retinal neuronal light responses are critical to investigating the physiology of the healthy retina, determining pathological changes in retinal diseases, and testing therapeutic interventions. The ex vivo electroretinogram (ERG) allows the quantification of contributions from individual cell types in the isolated retina by addition of specific pharmacological agents and evaluation of tissue-intrinsic changes independently of systemic influences. Retinal light responses can be measured using a specialized ex vivo ERG specimen holder and recording setup, modified from existing patch clamp or microelectrode array equipment. Particularly, the study of ON-bipolar cells, but also of photoreceptors, has been hampered by the slow but progressive decline of light responses in the ex vivo ERG over time. Increased perfusion speed and adjustment of the perfusate temperature improve ex vivo retinal function and maximize response amplitude and stability. The ex vivo ERG uniquely allows the study of individual retinal neuronal cell types. In addition, improvements to maximize response amplitudes and stability allow the investigation of light responses in retina samples from large animals, as well as human donor eyes, making the ex vivo ERG a valuable addition to the repertoire of techniques used to investigate retinal function.
Introduction
Electroretinography measures retinal function in response to light1. It is integral to studying retinal physiology and pathophysiology, and measuring the success of therapies for retinal diseases. The in vivo ERG is widely used to assess retinal function in intact organisms, but it has significant limitations2,3. Amongst these, the quantitative analysis of individual retinal cell types in the in vivo ERG is hampered, since it records the sum of potential changes, and therefore overlaying responses, from all retinal cells to light stimuli4. Furthermore, it does not readily allow addition of drugs to the retina, is vulnerable to systemic influences, and has a relatively low signal-to-noise ratio. These disadvantages are eliminated in the ex vivo ERG that investigates the function of the isolated retina2,3,5,6. The ex vivo ERG allows the recording of large and stable responses from specific retinal cell types by addition of pharmacological inhibitors and easy evaluation of therapeutic agents, which can be added to the superfusate. At the same time, it removes influences of systemic effects and eliminates physiological noise (e.g., heartbeat or breathing).
In the ex vivo ERG, retinas or retinal samples are isolated and mounted photoreceptor-side up on the dome of the specimen holder3,5. The specimen holder is assembled, connected to a perfusion system that supplies the retina with heated, oxygenated media, and placed onto the stage of a microscope, which has been modified to deliver computer-controlled light stimuli. To record the responses elicited by light, the specimen holder is connected to an amplifier, digitizer, and recording system (Figure 1). This technique allows isolation of responses from rod and cone photoreceptors, ON-bipolar cells, and Müller glia by changing the parameters of the light stimuli and adding pharmacological agents.
An existing patch clamp or multi-electrode array (MEA) setup can be converted to record ex vivo ERG, either in conjunction with a commercially available ex vivo ERG adapter or a custom polycarbonate computer numerical control (CNC)-machined specimen holder, to measure light responses in retinas from small animal models, such as mice. This modification increases the accessibility of ex vivo ERG while minimizing the need for specialized equipment. The design of the specimen holder simplifies the mounting technique and integrates electrodes, eliminating the need for manipulation of microelectrodes compared to previously reported transretinal ex vivo ERG methods7. The perfusion rate and temperature inside the specimen holder are important factors that affect the response properties from photoreceptors and ON-bipolar cells. By adjusting these conditions, the ex vivo ERG can be reliably recorded from the isolated mouse retina over prolonged periods of time. Optimized experimental conditions allow ex vivo ERG recordings in retinal punches from larger retinas, including large animal eyes and human donor eyes8.
Protocol
All experiments using mice were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Studies Committee at the University of Utah. Pig eyes for demonstration of this video were obtained postmortem from the slaughterhouse (Sustainable Swine Resources, Johnsonville). Eyes were obtained from human donors after brain or cardiac death with consent for research use through the Utah Lions Eye Bank, the San Diego Eye Bank or Lifesharing, which are fully accredited by the FDA, the Association of Organ Procurement Organizations (AOPO) and the Eye Banks of America Association. The use of human donor eyes had exempt status at the University of Utah (IRB no. 00106658) and ScrippsHealth IRB (IRB no. 16-6781).
1. Setting up the ex vivo ERG
- To convert a multielectrode array setup, connect the ex vivo ERG specimen holder via a head stage to a differential amplifier, which is plugged into the analog input of the interface board of the multielectrode array system. Use the recording software for the multielectrode array to record and store the input data from the ex vivo ERG. Set the gain of the differential amplifier to 100 and add an additional 10x voltage amplification depending on the digitizer specifications. Set the low pass filter to 100 Hz.
- To convert a patch clamp setup, connect the ex vivo ERG specimen holder via a head stage to a differential amplifier, which is connected to the head stage of the patch clamp amplifier. Use the patch clamp system software and digitizer to record and store the input data from the ex vivo ERG. Set the gain of the differential amplifier to 100 and apply an additional 10x voltage amplification via the patch clamp head stage. Set the low pass filter to 100 Hz.
- Connect LEDs with the appropriate wavelengths (e.g., approximately 530 nm to elicit rod photoreceptor and ON-bipolar cell responses) to the microscope. Control the LEDs with recording software that enables the triggering of light stimuli. To control light stimuli, use an LED driver controlled by analog outputs from a digitizer.
- Calibrate the light output of the LEDs at the position of the retina in the specimen holder using a photodiode. If necessary, insert neutral density filters into the light path to dim the light intensity.
- Use a commercially available or custom built ex vivo ERG specimen holder.
NOTE: Drawings for CNC machining from polycarbonate can be obtained from the authors upon request. - To prepare the electrode, insert an Ag/AgCl pellet electrode into a threaded luer connector. Fill the inside of the luer connector with hot glue and insert a 2 mm socket into the hot glue from the non-threaded side. Solder a silver wire of the EP1 electrode to the 2 mm socket. Screw the finished electrode, with an o-ring on the thread, into the electrode ports of the ex vivo ERG specimen holder.
NOTE: Electrodes can be used for a long time. If the pellet surface accumulates dirt and/or gets dark (this can cause high offset voltage and/or electrical drift), it can be "polished" using fine sandpaper. - At least 1 day prior to experimentation, glue filter papers onto the domes of the ex vivo ERG specimen holder using epoxy glue, ensuring that the glue does not obstruct the electrode channel (see 3 for video).
2. Animal preparation
- Dark adapt animals for at least 6 h or overnight.
3. Equipment preparation
- Fill the perfusion line of the ex vivo ERG with Ames' medium bubbled with 5% carbon dioxide and 95% oxygen. Connect the perfusion line to a heating element with a DC current source or heat controller near the specimen holder to warm the Ames' medium, so that the retina is kept at approximately 35-38 °C.
- Fill both halves of the ex vivo ERG specimen holder with electrode solution, seal the perfusion line with luer stoppers, connect the electrodes, and assemble the specimen holder with four screws (see 3 for video).
- Check the resistance and offset voltage between the electrodes in the assembled specimen holder by inserting the probes of the multimeter into the electrodes.
NOTE: If there are no blockages, and electrodes are in good condition, the resistance should be below 100 kΩ and offset voltage below 5 mV. - Connect the assembled specimen holder to the perfusion line and place onto the stage of a microscope set up to deliver light flashes. Ensure that the specimen holder and perfusion line do not contain any air bubbles.
4. Tissue preparation
- Sacrifice the animal with CO2 and immediately enucleate the eyes, or obtain large animal or human donor eyes.
- Clean the globe of the remaining connective tissue and extraocular muscles. Trim off the optic nerve.
- On filter paper, carefully place a small incision with a razor blade along the ora serrata, approximately 1 mm from the limbus in the mouse eye. Insert fine vannas scissors into the incision and cut along the limbus to remove the anterior portion of the eye with the lens.
- Move the eye cup into a dish containing Ames' medium. Grasp the sclera with fine forceps, taking care to not touch the retina. Insert vannas scissors between the retina and the sclera and cut the sclera from the peripheral toward the central part. Take care not to touch or damage the retina.
- Immobilize the sclera by holding one side of the incision with vannas scissors against the bottom of the dissection dish. Grasp the sclera with forceps on the other side of the incision. Remove the sclera without touching or damaging the retina by pulling apart the sclera on either side of the incision to allow isolation of the retina with minimal damage to the tissue.
- Remove the anterior segment with the lens from the fellow eye and store the eye cup at room temperature in Ames' solution bubbled with 5% carbon dioxide and 95% oxygen.
NOTE: Functional light responses from eyes stored in this way can be obtained for several hours. - In large eyes, including human donor eyes, clean the globe of the remaining connective tissue and remove the anterior segment and lens, similarly to the procedure described for the mouse eye. Use a scalpel to make a cut approximately 3 mm from the limbus. Insert curved dissection scissors into the incision and cut along the limbus to remove the anterior portion of the eye with the lens. Obtain retinal specimens for ex vivo electroretinography with a 3-6 mm disposable biopsy punch.
5. Mounting the tissue on the specimen holder
- Place the lower half of the specimen holder into a large Petri dish and fill with oxygenated Ames' medium so the dome of the specimen holder is just submerged.
- Carefully grasp the edge of the retina with fine forceps and transfer the retina onto the dome of the ex vivo specimen holder, photoreceptor side facing up.
- Lift the specimen holder from the Ames' solution, taking care that the retina stays in place.
- Completely dry the plate of the specimen holder to minimize noise, electrical crosstalk, and signal shunting.
- Assemble both halves of the specimen holder with four screws and connect the perfusion line. Dry the electrode in the lower half of the specimen holder and connect the anode cable to the inner retinal side and the cathode cable to the photoreceptor side.
- Perfuse the specimen holder with at least 1 mL/min of oxygenated Ames' medium for 10-20 min to allow time for light responses to stabilize.
6. Recording retinal neuronal cell function
- Record response families by exposing the retina to light flashes of increasing intensity. For example, record mouse rod photoreceptor response families with light intensities ranging from approximately 10 to 1,000 photons/µm2 and those from mouse ON-bipolar cells with light intensities ranging from approximately 0.6 to 20 photons/µm2.
- Measure photoreceptor light responses (a-wave) in the presence of 100 µM barium chloride, which blocks potassium channels in Müller glial cells, and 40 µM DL-AP4, which blocks glutamatergic signal transmission to ON-bipolar cells (Figure 2B).
- To isolate the b-wave, which originates from the function of the ON-bipolar cells, first record combined light responses from both photoreceptor and ON-bipolar cells in the presence of barium chloride alone (Figure 2A). Then, perfuse for 5-10 min with Ames' medium containing barium chloride, as well as DL-AP4, and record photoreceptor responses to the same light stimuli as before (Figure 2B). Subtract photoreceptor responses from combined photoreceptor and ON-bipolar cell responses, thus calculating ON-bipolar cell light responses alone (Figure 2C).
7. Optimizing ON-bipolar cell function
NOTE: The b-wave, which originates from the ON-bipolar cells, is highly sensitive to the temperature in the specimen holder and the perfusion rate.
- Maintain a perfusion rate of at least 0.5 mL/min to obtain the b-wave.
NOTE: A higher perfusion rate of 1-2 mL/min is preferable to maintain a large and stable response from ON-bipolar cells. - Ensure that for a given perfusion rate, the temperature in the specimen holder near the retina is close to body temperature (i.e., approximately 35-38 °C).
NOTE: It is important adjust the temperature of the perfusate, which is heated before it reaches the ex vivo ERG specimen holder, so that it is within the optimal temperature range at the retina. - Store eye cups for later experimentation protected from light and in oxygenated Ames' medium at room temperature to maintain normal a- and b-waves for several hours.
Representative Results
Ex vivo ERG enables recording of reproducible and stable photoreceptor and ON-bipolar cell light responses, for example, from the mouse retina (Figure 2A–C). Recording of photoreceptor responses from human donor retinas is possible with up to 5 h postmortem delay of enucleation (Figure 2D) and of ON-bipolar cell responses with a <20 min enucleation delay (Figure 2E). Important parameters to obtain large responses include a careful dissection technique, high perfusion rate, and perfusion temperature close to physiological values (35-38 °C in the mammalian retina). Under these conditions, response amplitudes and kinetics in both cell types were relatively stable over time but slowly declined approximately 40-45 min after retinas were mounted on the specimen holder (Figure 3).
Compared to photoreceptors, ON-bipolar cell function is more easily disrupted, for example, by damage to the retina during dissection and mounting or by a drop in the temperature and/or perfusion speed. While reduced temperature in the specimen holder greatly slowed the kinetics of both photoreceptors and ON-bipolar cells, it decreased the amplitude of the b-wave but not the a-wave (Figure 4A). Conversely, slowing the perfusion rate from 2.1 mL/min to 0.6 mL/min reduced the amplitudes of both photoreceptor and ON-bipolar cell responses but did not affect the implicit time (time from stimulus onset to response peak) of either the a- or the b-wave (Figure 4B). Cessation of perfusion for 10 min followed by reperfusion resulted in a complete loss of ON-bipolar cell function with preserved photoreceptor responses (Figure 5).
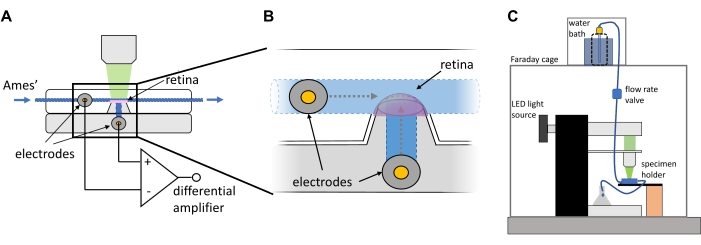
Figure 1: Ex vivo electroretinogram specimen holder and recording setup. (A,B) The ex vivo ERG specimen holder comprises a dome to mount the isolated retina, which is connected to a perfusion line to continuously deliver Ames' medium. Electrodes are connected through narrow channels to both the photoreceptor side of the retina via the perfusion line and the inner retina through the filter paper glued to the dome. These electrodes are connected to a differential amplifier, which enables measurement of potential differences in the retina in response to light stimuli. (C) The specimen holder is placed onto the stage of a microscope, which has been modified to deliver light flashes and connected to the perfusion line, which delivers heated, oxygenated Ames' medium by gravity. The entire recording setup is shielded by a Faraday cage to minimize electrical noise. This figure has been modified from 9. Abbreviation: ERG = electroretinogram. Please click here to view a larger version of this figure.
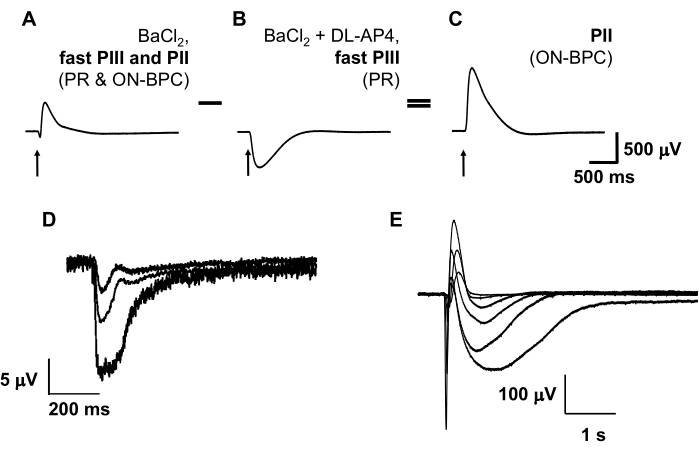
Figure 2: Example traces of ex vivo photoreceptor and ON-bipolar cell responses. Addition of pharmacological agents to the perfusate allows quantification of contributions from individual retinal cell types to the ex vivo electroretinogram. Photoreceptor (PR) light responses are isolated in the presence of 100 µM barium chloride (BaCl2), a blocker of K+ channels expressed by Müller glial cells, as well as 40 µM DL-AP4, a glutamate receptor blocker, which inhibits signal transmission from photoreceptors to ON-bipolar cells (B). ON-bipolar cell (ON-BPC) function (C) is determined by subtracting the photoreceptor component (B) from the combined photoreceptor and ON-bipolar cell response in the presence of barium chloride alone (A). Photoreceptor light responses can be obtained from human donor retinas with a death to enucleation delay of <5 h (D), whereas retinas enucleated within 20 min of death frequently also give ON-bipolar cell responses (E) (see 8 for further information). Figure 2A–C is modified from 9. Abbreviations: PR = photoreceptor; ON-BPC = ON-bipolar cell. Please click here to view a larger version of this figure.
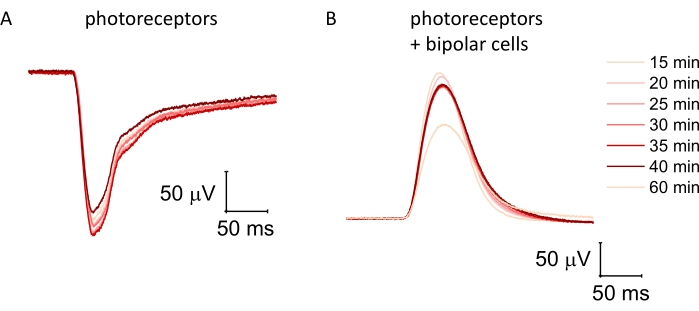
Figure 3: Stability of photoreceptor and ON-bipolar cell function in the ex vivo electroretinogram over time. (A) Light responses were recorded every minute from photoreceptors alone in the presence of both 100 µM barium chloride and 40 µM DL-AP4. (B) Dim light flashes in the presence of 100 µM barium chloride alone are heavily dominated by ON-bipolar cell function, although they contain a small photoreceptor component. Light responses from isolated retinas typically stabilize after perfusing the ex vivo specimen holder for 15-20 min, and were stable for at least 20-25 min before starting to decline. Please click here to view a larger version of this figure.
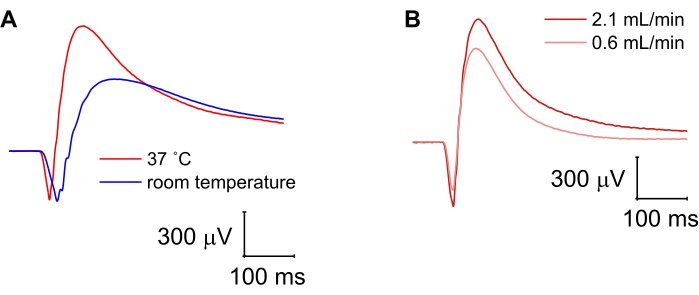
Figure 4: Combined photoreceptor and ON-bipolar cell responses at different temperatures and perfusion speeds. (A) Lowering of the temperature inside the specimen holder from 37 °C to room temperature greatly slowed photoreceptor and ON-bipolar cell kinetics but only reduced the ON-bipolar cell amplitude in the mixed photoreceptor and ON-bipolar cell response. (B) Reduction of the perfusion rate from 2.1 mL/min to 0.6 mL/min resulted in decreased photoreceptor and ON-bipolar cell amplitudes but no change in the response kinetics. Please click here to view a larger version of this figure.
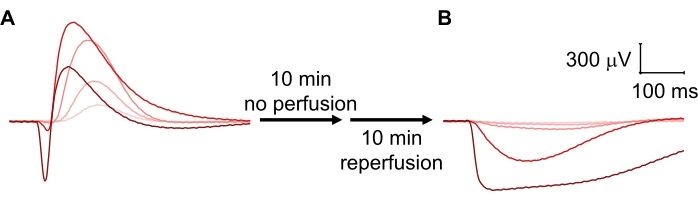
Figure 5: ON-bipolar cell responses are more sensitive to cessation of perfusion. (A) Large photoreceptor and ON-bipolar cell responses were recorded following perfusion with 2.1 mL/min for 20 min. (B) After cessation of perfusion for 10 min followed by reperfusion for 10 min at 2.1 mL/min, photoreceptor responses were present, whereas ON-bipolar cells responses were completely lost. Please click here to view a larger version of this figure.
Discussion
Originally developed in 1865 by Holmgren to measure retinal light responses from the amphibian retina10, technical constraints initially prevented the ERG from being widely used. Nevertheless, seminal studies by Ragnar Granit and others identified the cellular origins of the ERG and measured photoreceptor and ON-bipolar cell responses ex vivo11,12,13. Since then, refined methods have allowed more widespread use of ex vivo ERG recordings14,15, although response amplitudes, particularly those from ON-bipolar cells, remained comparatively small16. To overcome these limitations, retinal function is today more commonly measured with in vivo ERG, despite experimental constraints and more complicated interpretation of the recorded waveforms. Although the ex vivo ERG attempts to replicate physiological conditions as closely as possible, it nevertheless measures retinal function in an artificial environment and in the absence of systemic factors and pathological changes. However, when combined with the in vivo ERG, this limitation can be used to answer important questions, such as whether changes in retinal function in disease are intrinsic to retinal cells or caused by systemic changes17.
Recently, newly designed ex vivo ERG specimen holders have simplified the methodology, making the ex vivo ERG accessible to a broader research community2,3,5,18. Modifications to existing patch clamp or multielectrode array equipment described in this protocol will enable more laboratories to perform ex vivo ERG with minimal financial investments and space requirements. In particular, recent developments in ex vivo ERG technology have amplified the responsiveness of mammalian isolated retinas and resulted in a superior signal-to-noise ratio, which remains good despite additional amplification steps described in this protocol. Nevertheless, the decline of the light responses, mainly from ON-bipolar cells19, has perhaps hampered the more widespread use of the ex vivo ERG. The use of Ames' or Locke's medium results in larger photoreceptor and ON-bipolar cell responses, making them preferable to, for example, HEPES-buffered Ringer solution for ex vivo ERG3. Other laboratories stabilized ex vivo ON-bipolar cell function by supplementing with glutamine or glutamate19. This report demonstrates how to obtain large and stable light responses from isolated retinas, including from human donor eyes, as previously reported8.
Important experimental parameters include the temperature of the retina, which should be kept within the physiological range, and a rapid perfusion rate. The most noticeable effects of lowering the temperature in the ex vivo ERG specimen holder are slower response kinetics in both photoreceptors and ON-bipolar cells and an attenuated ON-bipolar cell amplitude. Although lowering the temperature appears to have little effect on the photoreceptor amplitude in the combined response from photoreceptors and ON-bipolar cells, this may be an artifact due to differential changes in the response kinetics from both cell types.
A sufficient perfusion rate appears to be especially critical for retinal function, most likely to supply oxygen and nutrients and remove waste products. While both photoreceptor and ON-bipolar cell amplitudes are somewhat diminished by a moderate reduction in the perfusion rate, even a short cessation of perfusion abolished ON-bipolar but not photoreceptor function. This implies that photoreceptor responses are more robust and may be more easily preserved in eyes that undergo experimentation with a significant delay, such as human donor eyes8. In this context, it is notable that ON-bipolar cell function is not diminished by storing eye cups in oxygenated Ames' medium for several hours. We therefore hypothesize that the loss of ON-bipolar cell function when the perfusion in the ex vivo specimen holder is stopped may be due to rapid depletion of oxygen and other nutrients in the small volume surrounding the retina in the specimen holder. This is supported by reports that a short delay from death to enucleation is critical to record photoreceptor and, particularly, ON-bipolar cell responses from human retinas, and that postmortem hypoxia is the most likely candidate for irreversible damage to retinal neuronal function8. While experimental parameters for ex vivo ERG were optimized in the mouse retina, they nevertheless successfully improved recording conditions for retinal specimens from large animals and human donor eyes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Eye Institute grants EY02665 and EY031706 and International Retinal Research Foundation to Dr. Vinberg, National Institutes of Health Core Grant (EY014800), and an Unrestricted Grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah. Dr. Frans Vinberg is also a recipient of a Research to Prevent Blindness/Dr. H. James and Carole Free Career Development Award, and Dr. Silke Becker of an ARVO EyeFind grant. We thank Dr. Anne Hanneken from The Scripps Research Institute for providing the donor eye used for recordings shown in Figure 2E.
Materials
| 2 mm socket | WPI | 2026-10 | materials to prepare electrode |
| Ag/AgCl Electrode | World Precision Instruments | EP1 | materials to prepare electrode |
| Ames' medium | Sigma Aldrich | A1420 | perfusion media |
| barium chloride | Sigma Aldrich | B0750 | potassium channel blocker |
| DL-AP4 | Tocris | 0101 | broad spectrum glutamatergic antagonist |
| OcuScience Ex Vivo ERG Adapter | OcuScience | n/a | ex vivo ERG specimen holder |
| Threaded luer connector | McMaster-Carr | 51525K222 or 51525K223 | materials to prepare electrode |
References
- Perlman, I., Kolb, H., Fernandez, E., Nelson, R. . Webvision: The Organization of the Retina and Visual System. , (1995).
- Bonezzi, P. J., Tarchick, M. J., Renna, J. M. Ex vivo electroretinograms made easy: performing ERGs using 3D printed components. Journal of Physiology. 598 (21), 4821-4842 (2020).
- Vinberg, F., Kefalov, V. Simultaneous ex vivo functional testing of two retinas by in vivo electroretinogram system. Journal of Visualized Experiments. (99), e52855 (2015).
- Heckenlively, J. R., Arden, G. B. . Principles and Practice of Clinical Electrophysiology of Vision. , (2006).
- Vinberg, F., Kolesnikov, A. V., Kefalov, V. J. Ex vivo ERG analysis of photoreceptors using an in vivo ERG system. Vision Research. 101, 108-117 (2014).
- Winkler, B. S. Calcium and the fast and slow components of P3 of the electroretinogram of the isolated rat retina. Vision Research. 14 (1), 9-15 (1974).
- Green, D. G., Kapousta-Bruneau, N. V. A dissection of the electroretinogram from the isolated rat retina with microelectrodes and drugs. Visual Neuroscience. 16 (4), 727-741 (1999).
- Abbas, F., et al. Revival of light signalling in the postmortem mouse and human retina. Nature. , (2022).
- Becker, S., Carroll, L. S., Vinberg, F. Diabetic photoreceptors: Mechanisms underlying changes in structure and function. Visual Neuroscience. 37, (2020).
- Kantola, L., Piccolino, M., Wade, N. J. The action of light on the retina: Translation and commentary of Holmgren (1866). Journal of the History of the Neurosciences. 28 (4), 399-415 (2019).
- Frank, R. N., Dowling, J. E. Rhodopsin photoproducts: effects on electroretinogram sensitivity in isolated perfused rat retina. Science. 161 (3840), 487-489 (1968).
- Hamasaki, D. I. The effect of sodium ion concentration on the electroretinogram of the isolated retina of the frog. Journal of Physiology. 167 (1), 156-168 (1963).
- Granit, R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. Journal of Physiology. 77 (3), 207-239 (1933).
- Donner, K., Hemila, S., Koskelainen, A. Temperature-dependence of rod photoresponses from the aspartate-treated retina of the frog (Rana temporaria). Acta Physiologica Scandinavica. 134 (4), 535-541 (1988).
- Green, D. G., Kapousta-Bruneau, N. V. Electrophysiological properties of a new isolated rat retina preparation. Vision Research. 39 (13), 2165-2177 (1999).
- Luke, M., et al. The isolated perfused bovine retina–a sensitive tool for pharmacological research on retinal function. Brain Research Protocols. 16 (1-3), 27-36 (2005).
- Becker, S., Carroll, L. S., Vinberg, F. Rod phototransduction and light signal transmission during type 2 diabetes. BMJ Open Diabetes Research and Care. 8 (1), 001571 (2020).
- Nymark, S., Haldin, C., Tenhu, H., Koskelainen, A. A new method for measuring free drug concentration: retinal tissue as a biosensor. Investigative Ophthalmology & Visual Science. 47 (6), 2583-2588 (2006).
- Winkler, B. S., Kapousta-Bruneau, N., Arnold, M. J., Green, D. G. Effects of inhibiting glutamine synthetase and blocking glutamate uptake on b-wave generation in the isolated rat retina. Visual Neuroscience. 16 (2), 345-353 (1999).

