DUCT: Double Resin Casting followed by Micro-Computed Tomography for 3D Liver Analysis
Summary
Double resin casting micro-computed tomography, or DUCT, enables visualization, digitalization, and segmentation of two tubular systems simultaneously to facilitate 3D analysis of organ architecture. DUCT combines ex vivo injection of two radiopaque resins followed by micro-computed tomography scanning and segmentation of the tomographic data.
Abstract
The liver is the biggest internal organ in humans and mice, and high auto-fluorescence presents a significant challenge for assessing the three-dimensional (3D) architecture of the organ at the whole-organ level. Liver architecture is characterized by multiple branching lumenized structures, which can be filled with resin, including vascular and biliary trees, establishing a highly stereotyped pattern in the otherwise hepatocyte-rich parenchyma. This protocol describes the pipeline for performing double resin casting micro-computed tomography, or "DUCT". DUCT entails injecting the portal vein and common bile duct with two different radiopaque synthetic resins, followed by tissue fixation. Quality control by clearing one lobe, or the entire liver, with an optical clearing agent, allows for pre-screening of suitably injected samples. In the second part of the DUCT pipeline, a lobe or the whole liver can be used for micro-computed tomography (microCT) scanning, (semi-)automated segmentation, and 3D rendering of the portal venous and biliary networks. MicroCT results in 3D coordinate data for the two resins allowing for qualitative as well as quantitative analysis of the two systems and their spatial relationship. DUCT can be applied to postnatal and adult mouse liver and can be further extended to other tubular networks, for example, vascular networks and airways in the lungs.
Introduction
Organ resin casting is a technique that dates back to the 17th century1. One of the first examples of modern resin casting was performed on the human liver from an autopsy. Intrahepatic bile ducts were filled with a contrast agent mixed with gelatin, followed by imaging with an x-ray CT scan2. The aim of the DUCT technique is to visualize, digitalize and analyze two tubular resin-casted networks, in tandem, in 3D.
DUCT is based on the extensive existing knowledge of single-system liver resin casting3,4,5,6,7,8 and extends to simultaneous 3D visualization and analysis of two systems9. DUCT advanced single resin casting to double resin casting by mixing two radiopaque resins of different contrast and injecting these resins into two different networks, specifically the common bile duct and portal vein. DUCT can be applied to young postnatal mice with reproducible results as early as postnatal day 15 (P15). Compared to microscopy-based imaging techniques, the main advantage is that DUCT is faster, antibody-free, and liver tissue autofluorescence does not interfere with imaging. Further, DUCT provides quantitative data describing lumenization status, internal diameter, network connectivity, and perfusion. Differentiating between the presence of lumen-forming cells and their de facto morphogenesis into tubes is essential for analyzing organs in which ductular cells are present but do not form tubes, as can be the case in Alagille syndrome10. The main disadvantage of DUCT is the limited penetration of the resin, which is viscous and does not enter tubes with a small caliber (<5 µm). DUCT can be applied for any tubular structure after determining the injection entry point, such as the arterial and venous circulatory systems, airways, the extrahepatic bile duct, or lymphatic vessels. It could thus facilitate whole organ architecture analysis of other tissues such as lungs and pancreas.
MicroCT segmented images can be processed using commercially available imaging software, such as ImageJ, or custom-written pipelines (e.g., MATLAB). The resin-injected liver can be analyzed qualitatively for network expansion and connectivity or quantitatively for volume, length, branching, tortuosity of a single system, and the interaction between two systems such as the distance between two systems, or branchpoint dependence (does system 1 branch in proximity to system 2 branching?). The DUCT pipeline encompassing resin injection, microCT scanning, and CT data segmentation, combined with detailed quantitative analysis of architectural mechanisms of two tubular systems, could provide a standard for whole liver analysis in animal models.
Protocol
The protocol described in this study was approved and follows the animal welfare rules and regulations of the Stockholms Norra Djurförsöksetiska nämnd (Stockholm animal research ethics board). The animals used in this study were wild type or homozygous Jag1H268Q mutant mice on a mixed C3H/N and C57bl6J background. Both males and females were included in the study. The animals were used at postnatal day 15 or as adults between 3 – 8 months.
1. Double resin injections
- Preparation
- Prepare resin solution. For double system resin injections, prepare both yellow and green resins as described in the steps 1.1.2-1.1.4.
- Prepare 1 mL aliquots of diluted resin per mouse.
- Yellow Resin: Dilute yellow silicone rubber (high radiopacity) with the clear diluent in a 3:1 dilution to prepare yellow resin for injection.
- Green Resin: Mix blue silicone rubber (undetectable radiopacity) with the clear diluent in a 1:1 dilution to prepare blue resin. To generate green resin, mix the diluted blue resin with the diluted yellow resin at a ratio of 1:1. Vortex the green resin thoroughly until the color is homogeneous.
NOTE: Blue resin has very low radiopacity that is not detectable with microCT, which therefore necessitates dilution with yellow resin to create two resins with different radiopacities.
CAUTION: Resin may contain lead chromate, which is a carcinogen. It produces poisonous gases in a fire. Handle with care and dispose of the resin as hazardous waste. - Prepare two injection sets (injection set #1 and injection set #2) with tubing as described in the steps 1.1.6-1.1.11.
NOTE: For adult mice (>P30 (postnatal day 30) = P30 – 2 years), use injection set #1 (PE10 tubing with 0.6 mm outer diameter) for common bile duct injection and injection set #2 (PE50 tubing with 0.96 mm outer diameter) for portal vein injection. For young postnatal mice (up to P30), prepare two #1 injection sets, one for bile duct and one for portal vein injection (no injection set #2 as the portal vein is too narrow to accommodate PE50 tubing). - To prepare injection set #1, cut 30 cm of PE10 tubing and stretch one end of the tubing by hand by pulling it until it becomes as thin as possible (Figure 1A,B, approximately 0.15 mm diameter, non-stretched PE10 tubing is 0.6 mm diameter).
- Cut the tip of the stretched PE10 tubing diagonally to create a beveled tip (Figure 1B).
- Connect the non-stretched end of the PE10 tubing to a 30 G needle (Figure 1A, Injection set #1).
- To prepare injection set #2, cut 30 cm of PE50 tubing and stretch one end of the tubing by hand by pulling it until it becomes thin enough to fit into the portal vein (Figure 1A,B, approximately 0.7 mm, non-stretched PE50 tubing is 0.96 mm diameter).
- Cut the tip of the stretched PE50 tubing diagonally to create a beveled tip (Figure 1B).
- Connect the non-stretched end of the PE50 tubing to a 23 G needle (Figure 1A, injection set #2).
NOTE: Each injection set can only be used for one injection since the resin will harden in the syringe and tubing. Adjust the size of the stretched and beveled tip based on the size of the common bile duct and portal vein of the mouse, depending on its age, genotype, and phenotype. When unsure about the size and fit of the tubing, insert the tubing in the appropriate duct or vessel after the incision and before the tubing is filled with resin. If the tubing is too wide to fit in the duct/vessel, stretch it further. - Anesthetize the animal by isoflurane inhalation (first 4% in the induction chamber and ~2% using the nose cone).
- Place the mouse on a ventilated bench on a dissection pad while the mouse is breathing isoflurane through the nose cone. Verify that the animal is unconscious by an institute/IRB-recommended method, e.g., by pinching one of the paws.
CAUTION: Isoflurane may cause drowsiness or dizziness upon inhalation and may cause damage to the cardiovascular system and central nervous system through prolonged or repeated exposure. Do not inhale it. Handle the substance on a ventilated bench and in well-ventilated areas.
NOTE: It is possible to use another anesthetizing method, as long as it is compatible with cardiac perfusion. - Once the animal is unconscious, spray the ventral side with 70% ethanol to prevent interference from fur.
- Using skin scissors, cut the skin, fascia, and muscle layer starting at the midline of the abdominal cavity and cut through to the thoracic cavity to expose the internal organs.
- Grab the xiphoid process with forceps to lift the sternum and cut the diaphragm and rib cage on both sides to expose the heart and lungs.
- Remove the rib cage by cutting through the ribs on the left and right sides of the ribcage with scissors. Take extra care not to damage the liver as this will result in leakage of the resin.
- Using straight forceps, pull the heart towards the liver and cut away the right atrium.
- Insert a butterfly needle (23 G), connected to a peristaltic perfusion pump, into the left ventricle. Perfuse the mouse transcardially with Hanks balanced salt solution (HBSS) and heparin (1 U/g of mouse body weight). Perfuse for 3 min with a perfusion rate of 5 mL/min.
NOTE: If the mouse is being perfused correctly, the internal organs will become pale, especially the liver. If, instead, the lungs are turning white, the needle is inserted into the right ventricle and should be repositioned. After perfusion, the mouse is exsanguinated and can be removed from the nose cone and isoflurane. - Turn off the isoflurane pump.
- Resin injection – Biliary system resin casting
- Move the mouse to the dissection microscope, abdomen up, tail towards the experimenter, and head away from the experimenter. The anatomical landmarks of interest are depicted in Figure 2A.
- Locate the inferior vena cava (Figure 2A) by moving the intestine to the side. Use spring scissors to make a small transversal incision in the inferior vena cava to allow the release of hepatic vascular pressure (Figure 2Bi).
- Expose the common bile duct and portal vein as described in steps 1.2.4- 1.2.6.
- Move the intestine and pancreas to the right side (of the experimenter) using a phosphate-buffered saline (PBS)-wetted cotton swab.
- Flip the ventral side of the liver towards the heart using the PBS-wetted cotton swab to expose the visceral surface and the hilar region.
- Locate the common bile duct that runs from the hilar region across the pancreas and into the intestine at the sphincter of Oddi (Figure 2Bii, common bile duct outlined with the yellow dotted line, black arrowheads point to Sphincter of Oddi).
NOTE: Make sure the liver is moist throughout the procedure by sprinkling it with PBS. - Clear surrounding tissue (area ~ 5 mm) from the common bile duct using straight forceps. Place silk suture thread (size 4-0, 0.17 mm, 3 – 5 cm long) under the common bile duct (Figure 2Bii) and tie a loose overhand knot around the common bile duct (Figure 2Biii).
NOTE: Choose an area for the knot halfway between the hilar region and the sphincter of Oddi and at some distance from the portal vein so that after the suture is tightened around the common bile duct, it will not interfere with the portal vein injection. - Hold the spring scissors flat against the common bile duct to make an oblique incision to the common bile duct at the spot where the common bile duct enters the pancreas and intestine next to the sphincter of Oddi. (Figure 2Biv), yellow dotted line outlines the sphincter of Oddi region, emphasized by black arrowheads).
NOTE: This is a crucial step. Make an oblique and not a transversal cut, and make an incision; do not sever the bile duct. Cutting through the entire bile duct makes insertion of the tubing very challenging. - Just before use, mix 1 mL of the yellow resin with 50 µL of the curing agent (by filling and emptying the 1 mL syringe) and fill a 1 mL Luer syringe with the resin – curing agent mixture.
- Connect the filled syringe with the tubing (set #1). Press the plunger to fill the tubing completely. Ensure that the resin/curing agent mix drips from the tip of the tubing.
NOTE: Avoid and remove bubbles in the syringe and tubing for best results. - Using forceps, straighten the area around the common bile duct incision and insert the tubing into the opening in the common bile duct (Figure 2Bv), with the longest edge of the beveled tip downwards towards the dorsal side of the bile duct. This orientation ensures that resin can exit the tubing opening, which is facing upwards, into the duct (Figure 2Bv).
- Tighten the silk thread knot to secure the tubing inside the common bile duct (Figure 2Bvi).
- Inject the resin into the common bile duct. Observe the gall bladder and the individual liver lobes.
- Massage the liver with a PBS-wetted cotton swab to help spread the resin equally. Resin-filled bile ducts' terminal branches (in wild-type mice) are faintly visible at the liver surface.
NOTE: Expected time to fill the liver is 30 -100 s. - Stop injecting the resin when dots of resin appear at the surface of the liver (Figure 2Ci, blue arrowheads) or when resistance is met.
NOTE: Work as fast as possible since the resin begins to harden after adding the resin curing agent. The working time from adding the curing agent is approximately 15 min. - Remove the tubing by pulling it out of the common bile duct and quickly tighten the silk knot using forceps to prevent the resin from leaking out. Cut away the loose ends of the silk suture, so they do not interfere with the portal vein injection.
- Dispose of the tubing containing resin and the remaining resin into the hazardous waste and the needle into the sharps waste.
- Resin injection – Portal vein resin casting
- Clear the portal vein (area ~5 mm) from its surrounding tissue about 2 cm from its entry to the liver using straight forceps. Place silk suture thread (size 4-0, 0.17 mm, 3 – 5 cm long) under the cleared area of the portal vein (Figure 2Ci) and tie a loose overhand knot (Figure 2Cii).
- Make a longitudinal incision in the portal vein distal to the liver and the knot (Figure 2Ciii).
- Mix 1 mL of the green resin with 50 μL of the curing agent (by filling up and emptying a 1 mL syringe) and fill the 1 mL Luer syringe with the resin-curing agent mixture.
- Connect the filled syringe with the tubing (set #2 for >P30 mice, new set #1 for <P30 mice). Press the plunger to fill the tubing completely. Ensure that the resin drips from the tip of the tubing.
NOTE: Avoid and remove bubbles in the syringe and tubing for best results. - Using forceps, straighten the portal vein by pulling the surrounding tissue towards the experimenter and insert the tubing with the longest edge of the beveled tip towards the dorsal side of the vessel (Figure 2Ciii).
- Tighten the silk thread to secure the tubing in the portal vein.
- Inject the resin into the portal vein. Observe the blood vessels filling up with resin. Massage the liver with a PBS-wetted cotton swab to help spread the resin equally (Figure 2Civ).
- Stop injecting the resin when all blood vessels are filled (ends of portal veins are visible at the liver periphery) or when resistance is met.
NOTE: Work fast since the resin begins to harden after the addition of the curing agent. The working time from adding the curing agent is approximately 15 min for injection set#1 and 25 min for injection set #2. - Remove the tubing by pulling out the tubing from the portal vein and quickly tighten the silk knot using forceps to prevent the resin from leaking out.
- Dispose of the tubing containing resin and the remaining resin into the hazardous waste and the needle into the sharps waste.
- Liver dissection and fixation
- Dissect out the whole liver by cutting it away from the surrounding tissue and the diaphragm.
- Gently place the whole liver in an empty 50 mL conical tube with the ventral side facing up and the dorsal side resting on the wall of the conical tube to prevent deformation of the liver. Store the conical tube horizontally overnight at 4 °C for the resin to harden completely.
NOTE: Any container that is big enough to fit the liver can be used instead of 50 mL conical tube. Using a flat bottom container will increase the likelihood that the liver is not deformed. - Separate the liver into individual lobes. Make a selection of the lobes that will be used for microCT analysis (1.4.4-1.4.5) or quality control (1.4.6-1.4.8). Optionally use the whole liver for both optical clearing and microCT without separating it into individual lobes.
NOTE: Liver architecture of the biliary and vascular systems is different in each lobe, and matched lobe analyses are therefore required. The optical clearing does not interfere with microCT scanning, and the lobe(s) used for quality control can be subsequently scanned with microCT. Conversely, samples scanned with microCT can be subsequently optically cleared for comparison. The whole liver analysis is thus possible. In wild-type mice (C3H/C57bl6 genetic background), the right medial lobe is filled first by resin injections, making this a suitable lobe for microCT analysis, with the highest reproducibility. The left lateral lobe is the biggest lobe, in which it is therefore straightforward to pre-screen for injection quality. The selection of the lobe (or whole liver) used for quality control and microCT scanning is dependent on the animal model and research question. - In a ventilated hood, prepare 4% formaldehyde solution (10 -20 mL per tube). Fix the lobes used for microCT with 4% formaldehyde overnight at 4 °C and afterward wash once with PBS (10 -20 mL per tube).
- Collect formaldehyde waste in a separate container. Keep the liver lobes in PBS at 4 °C for short-term storage or 70% ethanol for long-term storage. Proceed with section 2: Micro-computed tomography.
CAUTION: Formaldehyde causes acute toxicity if swallowed, inhaled, or in contact with skin. Formaldehyde is a flammable liquid and vapor. It causes severe skin burns and eye damage. May cause an allergic skin reaction. Fatal if inhaled (concentrated or in powder). May cause respiratory irritation. Suspected of causing genetic defects. May cause cancer. Causes damage to organs (eyes, central nervous system). Precautionary statements – keep away from heat, hot surfaces, sparks, open flames, and other ignition sources. No smoking. Wear protective gloves and protective clothing. In case of spillage, immediately take off all clothing that was contaminated. If in contact with the skin: rinse the contaminated area with water. If inhaled: remove person to fresh air and monitor breathing if comfortable. Immediately call a poison center/ doctor. If in eyes: rinse eyes with water for several minutes. If present and possible, remove contact lenses. - For quality control, fix the remaining lobes (at least one) with 50% methanol (mixed with deionized water) for a minimum of 4 h, rocking at room temperature, followed by 100% methanol overnight rocking at room temperature. Collect methanol waste in a separate container.
CAUTION: Methanol causes acute toxicity if swallowed, inhaled, or in contact with skin. Methanol is a flammable liquid and vapor. Wear protective gloves and clothing. Avoid breathing when handling and keep away from fire and heat. Wash skin thoroughly after using. In case of spillage, immediately take off all contaminated clothing. Rinse skin with water. If inhaled: Remove person to fresh air and keep comfortable for breathing. If inhaled, swallowed, or exposed, call a poison center/ doctor. - In a ventilated hood, prepare benzyl alcohol and benzyl benzoate (BA: BB, 1:2) (5 -10 mL per lobe) solution.
- Place the liver lobe in a 15- or 50- mL polypropylene tube (depending on the size of the lobe) containing BABB solution. Leave it rocking at room temperature until transparent. This step can take between 2-16 h depending on the size of the lobe.
NOTE: The BABB solution dissolves certain types of plastic. It is safe to store the samples in polypropylene tubes or glass containers.
CAUTION: Benzyl benzoate can cause acute toxicity when swallowed. It is toxic to aquatic life with long-lasting effects. Precautionary statements: wash skin thoroughly after handling. It is harmful when swallowed, in contact with skin, or inhaled. Avoid breathing fume/vapors. Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. If swallowed – call a poison center/doctor if unwell Use in ventilated areas. Collect spillage. Dispose of contents/container to an approved waste disposal plant. - Inspect the quality of the injection. Only well-injected liver should be scanned with microCT.
2. Micro-computed tomography
- Sample preparation
- Prepare 1% agarose gel by mixing 100 mL of distilled water and 1 g of agarose powder. Place the mixture in a microwave and boil the solution until the agarose powder dissolves.
- Temper the 1% agarose gel to ~40 °C to avoid thermal damage to the liver sample.
- Place the lobe(s) of interest in a 15 mL conical tube and fill it with the 1% agarose gel to approximately 2/3 of the total volume of the tube. This step minimizes undesired sample motion during CT measurement.
- CT measurement
NOTE: The following steps are CT device-dependent, and specific settings and actions may differ for different CT devices and manufacturers. In this protocol, the micro-computed tomography scanner used was equipped with a nanofocus X-ray tube (180 kV/15 W), and flat-panel dynamic 41|100 (4048 px x 4048 px, pixel size 100 mm with binning 2) for the CT measurements.- Mount the 15 mL conical tube with the sample on the rotational stage of a CT device and allow it to thermally adapt to the measurement chamber for at least 1 h.
- When the sample is thermally adapted, center it in the Field of View (FOV).
- Optimize the source-sample and sample-detector distances (SSD, SDD) to reach sufficient voxel resolution, e.g., 12 µm for an adult murine liver sample or 6.5 µm for <P30 liver, corresponding to dimensions of FOV (for beforehand specified hardware) 24.3 mm × 24.3 mm and 13.2 mm × 13.2 mm respectively.
- Set the acquisition parameters (i.e., accelerating voltage and current, exposure, binning, averaging) following the CT device manufacturer's recommendations to reach a sufficient level of detected signal.These parameters are not only CT device-dependent but also sample-dependent and should be optimized for each sample. In Hankeova et al.9, the settings were: 80 kV accelerating voltage, 160 µA accelerating current, 400 ms exposure time, and 2000 images.
- Start a CT measurement. Use a dedicated tomographic reconstruction software to reconstruct the CT data.
3. Analysis and data segmentation
NOTE: The following steps are image-processing software-dependent; specific settings and actions may differ depending on the software used.
- Load the CT data: Select File > Import > Command. A further selection is dependent on the specific format of the CT data to be processed.
- Use the function Surface Determination on the top panel to segment the resin in the data using global thresholding. In the dialog window, determine the threshold value with histogram evaluation by setting the position of the red "Isovalue" line to segment only the resin-filled vessels (i.e., encompassed by the yellow line in the presented "Preview panel") (Figure 3A).
- On the left panel, select the module Create ROI from Volume/CAD/Mesh to create a region of interest (ROI) of the resin-filled vessels. In the dialog window, use the option Create ROI(s) from Solid, select the name of the processed volume and confirm.
- Eliminate erroneous segmentation of noise clusters in the background region of this ROI. Mark this ROI on the right panel, right-click on it and select the module Split ROI.
- In the dialog window, set the Minimum Volume [voxel] parameter to exclude all the noise particles – this value is experiment- and data-dependent and must be optimized for each sample to be analyzed (Figure 3B).
- Create smooth, continuous, and solid canal masks without artifacts in the resin-cast ROI – e.g., presence of air bubbles or resin leakage.
- On the left panel, use the module Smoothing, set the Smoothing Strength parameter to 1 or 2 (depending on the individual data, when higher values might lead to model deformation, especially when dealing with fine structures). If needed, run this process twice (Figure 3C).
- Identify and separate the individual tubular systems in the segmented resin mask.
- Create a separate ROI for the system filled with the more absorptive resin (the yellow resin used for the common bile duct injection) with higher intensity values in the CT data. Follow the procedure described in step 3.2. (Figure 3Di).
- Mark the new ROI and the resin mask ROI, right-click and select Subtract ROI(S) and subtract the new ROI from the resin mask ROI to create a new ROI for the remaining tubular system (Figure 3Dii, iii).
- Export the resulting ROIs for both tubular systems in various formats, based on operator preferences, for subsequent processing in different software. Further, process the resulting ROIs in a volume graphics software to export the final visualization in the form of an image or a video.
Representative Results
What to do
Successful double resin injection is achieved when both the intrahepatic bile ducts and portal vein vasculature are well filled. As a quality control step, clearing one lobe (for example, the left lateral lobe) allows for verification of a successful injection, followed by imaging of lobes of interest. The optically cleared lobe can be scanned later using microCT; hence it is possible to optically clear the whole liver. In well-injected mouse liver, the portal vein vasculature should be filled with resin until the liver periphery and resin should be visible in side-branches (Figure 4), and this architecture is faithfully recapitulated in microCT scanned and segmented data. Further, well-injected intrahepatic bile ducts should be visible next to the main portal vein branches extending almost to the periphery, and resin should be visible in the major side branches. If the control lobe passes the quality control step, the lobes of interest (included the optically cleared one) can be scanned with microCT. The result of the segmented data from a well-injected liver is shown for a P15 mouse (Figure 4A,B) and an adult mouse (Figure 4C,D).
What not to do
Intact liver tissue is a prerequisite for successful injection. Take extra care when cutting the abdominal cavity and diaphragm not to accidentally nick the liver tissue. If there is physical damage to the liver during this procedure, the resin is very likely to leak out during portal vein injection (Figure 5A). It is not possible to achieve a good injection of the vascular system if the liver is physically damaged.
One of the common mistakes is underfilling the liver with resin that can lead to challenges for visualization or analysis. One of the causes for system underfilling is resin hardening prematurely in the needle or the tip of the tubing before the injection is completed (Figure 5B, blue arrowheads, brackets depict large bubbles). A good practice is to use one injection set per animal and work fast after the curing agent is added to the resin. If the resin hardens during the injection (which can be observed by a half-filled system, here exemplified with a half-filled portal vein vasculature) remove the tubing, cut the tip of the tubing (always diagonally to create a beveled tip), and push the plunger. If resin begins to drip again, carefully re-insert the tubing and secure it with the suture. If the resin has hardened in the needle, replace the tubing completely, fill it with resin (avoiding bubbles), carefully re-insert the tubing, and secure it with the suture. It can be challenging to replace the tubing, especially in young postnatal mice <P30, as the tissue is more fragile. Another cause of poor resin filling of portal vein vasculature can be insufficient transcardial perfusion (Figure 5C, blue arrowheads denote blood visible in terminal branches). This can be observed when the tips of the vessels are filled with blood instead of resin. To avoid this, ensure that the portal vein (outside the liver) does not contain any blood before the injection. The third cause of underfilled liver is when the tubing is inserted too deep into the liver and enters a branch towards one of the lobes. To prevent this, insert the tubing at a minimum of 0.5 cm from the entry to the liver.
Conversely, one, or both, of the systems become overfilled with resin (Figure 5D). It is necessary to visually monitor the liver throughout the injection. Biliary system casting with resin is more challenging than portal vein resin casting since resin-filled ducts are only faintly visible on the liver surface, and it is difficult to assess when the system is nearly full and when to stop. When small yellow resin dots appear on the liver surface (Figure 2Ci, blue arrowhead), this is a sign that the biliary system is completely filled, and the resin is starting to leak out of the ducts. Minor resin leakage can be manually corrected during microCT data segmentation (Figure 5D, right panels).
If the injection pressure is too high, this can cause vessels or ducts to rupture (Figure 5E), irreversibly damaging vessel or duct architecture. The liver will not be suitable for microCT scanning or analysis. To avoid resin overfilling, optimize the right volume and pressure used for injection in each mouse model. When working with mice that have been challenged with a toxic diet, genetic modification, or liver injury that affects the biliary or venous systems, or liver stiffness, the injection pressure and volume may need to be adjusted as volume and pressure tolerated may be different from the wild type mice. This protocol describes the manual injection of the two systems, but it is possible to connect the syringe to a pump to standardize the injection pressure. Bubbles are another very common injection artifact that leads to sparse filling of the tubular networks (Figure 5F–H, blue arrowheads). To avoid bubble formation, make sure that the syringe and tubing do not contain any bubbles, are completely filled with resin, and the resin is dripping from the tip of the tubing before injection. Small bubbles that appear as negative areas on the microCT data can be manually corrected during post-processing steps, although this is laborious.
Fresh is the best
Using fresh yellow resin is a crucial factor, significantly affecting the contrast of the two resins and the microCT data segmentation. When the freshly opened resin is used (Figure 6A) there is a clear difference in contrast between the yellow resin-injected bile ducts (bright white) and the green resin-injected portal veins (bright grey). Liver that is injected with fresh resin is easily processed using automated global thresholding. With prolonged storage, the resin precipitates, and the contrast diminishes. After 3 months of storage, the contrast can still be sufficient to distinguish the portal vein from the bile duct (Figure 6B), but precipitation affects the mixing of the two resins, which is visible as a heterogeneous opacity in the filled portal vein (Fig 6B, blue arrowheads). Heterogeneous contrast negatively affects the automated thresholding and necessitates manual corrections, which increases the processing time. If the resin is older than six months, the contrast has degraded to a point at which it is not possible to distinguish the yellow-injected bile duct from the green-injected portal vein based on their contrast alone (Figure 6C). In this case, the bile duct and portal vein must be segmented manually based on their diameter and position in the hilar region and followed manually throughout the entire microCT data. This procedure is extremely time-consuming and best avoided.
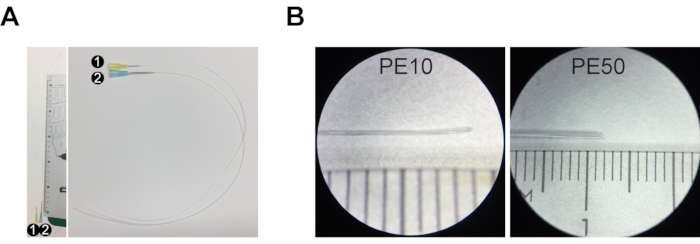
Figure 1: Injection set for resin casting. (A) Injection set #1 comprises a 30 G needle and PE10 tubing that is ~30 cm long. Injection set #2 is composed of a 23 G needle and PE50 tubing ~30 cm long. (B) The tip of the tubing is stretched and cut at an angle to create a beveled tip. The ruler in A and B is a centimeter ruler, with major increments of 1 cm, intermediate increments of 5 mm, and minor increments of 1 mm. Please click here to view a larger version of this figure.
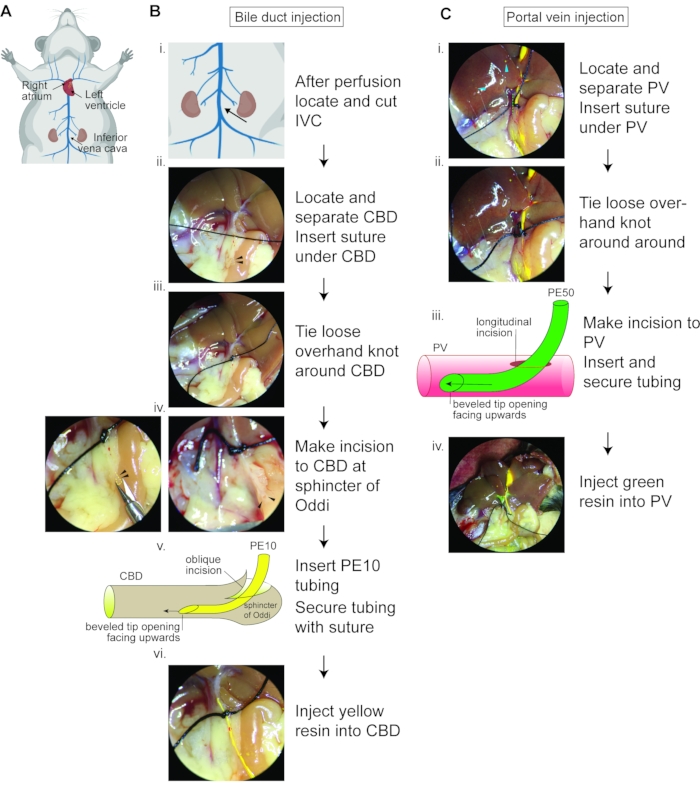
Figure 2: Double resin casting flow chart. (A) Schematic showing the murine venous circulatory system and the heart with highlighted right atrium, which should be cut away before perfusion, and the left ventricle into which the needle should be inserted for perfusion to wash away the blood from the circulatory system. The inferior vena cava should be severed under the kidneys to relieve vascular pressure. (B) Bile duct resin injection flow chart. (i) Zoom image of (A) depicting where to sever the IVC. (ii) Image depicting the common bile duct (yellow dotted line) from the liver hilar region to the sphincter of Oddi (black arrowheads), with suture thread under the cleared common bile duct. (iii) Suitable position for loose overhand knot around common bile duct. (iv) The yellow dotted line and black arrowheads label the sphincter of Oddi, demonstrating the oblique angle for incision and how the opening should appear after the oblique angle incision. (v) Schematic demonstrating the orientation of the PE10 tubing bevel opening (upwards) upon insertion. (vi) Appearance of yellow resin being injected; resin should easily pass the loosely tied knot. IVC, inferior vena cava; CBD, common bile duct. (C) Portal vein resin injection flow chart. (i) The green dotted line marks the portal vein from the hilar region. The blue arrowheads label the overfilled biliary system. (ii) Suitable location for loose overhand knot around portal vein. (iii) Schematic demonstrating the bevel opening (upward) upon insertion. (iv) Appearance of liver upon injection of green resin and yellow resin; note the resin-filled blood vessel in the liver periphery. PV, portal vein. Figure 2A was created with Biorender.com. Please click here to view a larger version of this figure.
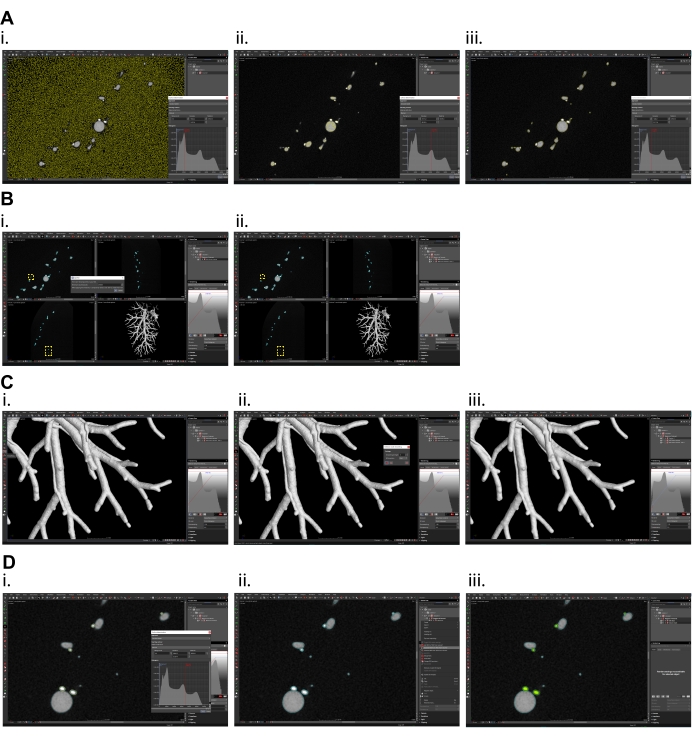
Figure 3: Micro CT data processing in volume graphics software. (A) Surface determination, (i) overestimated isovalue (current preview of selection is shown in yellow color), (ii) underestimated isovalue, (iii) optimal selection of isovalue for proper surface determination of portal vein and bile ducts. (B) Splitting region of interest (ROI) created by surface determination, (i) set the value in dialog window high enough that only one segment (the largest one) will remain, (ii) in yellow frames the smaller (excluded) particles are shown. (C) Surface smoothing of the data, (i) smoothing function is on the left panel, (ii) set the smoothing strength to 1 (max. 2) and create new smoothed ROI, (iii) smoothed data. (D) Separation of individual tubular systems, (i) in surface determination function set the isovalue so that only the biliary system is included in selection (current preview of selection is shown in yellow color), (ii) mark the ROI of both systems and ROI of only the biliary system and subtract biliary system ROI from ROI of both systems, (iii) Portal vein shown in grey, the biliary system shown in green. Please click here to view a larger version of this figure.
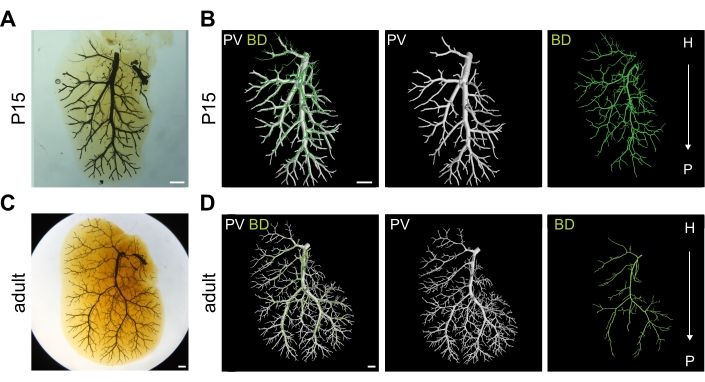
Figure 4: Well-injected bile duct (BD) and portal vein (PV) systems. (A) Optically cleared right medial lobe (RML) of postnatal day 15 (P15) liver injected with two resins into the two systems. Scale bar 1 mm. (B) 3D rendering of P15 RML shown in (A) depicting portal vein vasculature in white and biliary system in green. Scale bar 1 mm. (C) Optically cleared RML of adult liver injected with two resins into the two systems. Scale bar 1 mm. (D) 3D rendering of adult RML shown in (C) depicting portal vein vasculature in white and biliary system in green. H = hilar, P = peripheral. Scale bar 1 mm. Panels A, B, D are adapted with permission from Hankeova et al.9. Please click here to view a larger version of this figure.
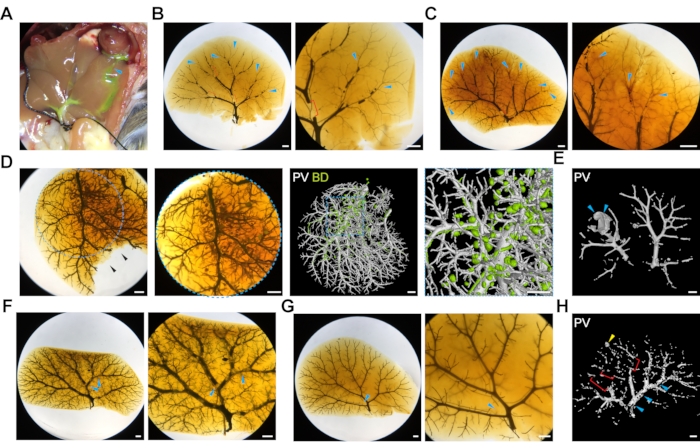
Figure 5: Common challenges of double resin liver injections. (A) The image depicts a liver that was accidentally nicked during the initial opening of the abdominal cavity, and the resin is leaking through the cut (blue arrowhead). (B) Poorly injected portal vein system due to resin hardening. Blue arrowheads label empty terminal branches, and red brackets label large bubbles. Scale bar 1 mm. (C) Poorly injected portal vein system due to poor transcardial perfusion. Blue arrowheads label blood visible in the terminal branches. Scale bar 1 mm. (D) Overfilled biliary system manifested by isolated balls of resin. The left panels show the optically cleared liver, and the right panels show the 3D microCT rendered image. The blue dotted outlines depict zoom-in regions. The black arrowheads label a part of the liver that was damaged during the optical clearing after microCT scanning. Scale bar 1 mm. (E) High pressure during resin injection can cause rupture of the portal vein (the animal in this panel carries a Jag1H268Q mutation), marked by blue arrowheads. Scale bar 1 mm. (F) Bubbles in the resin during portal vein injection (blue arrowheads) and (G) biliary system injection (blue arrowhead), scale bar 1 mm. (H) MicroCT scan of bubbles (blue arrowhead), poorly filled terminal branches (red brackets) and resin leakage (yellow arrowhead), scale bar 1 mm. Please click here to view a larger version of this figure.
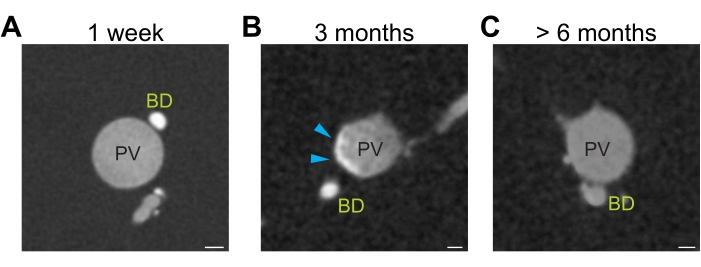
Figure 6: Differential resin contrast. (A) Freshly opened yellow resin generates sufficient contrast to distinguish resin injected portal vein (grey) and bile ducts (white). (B) Three months storage of yellow resin lead to precipitation of the resin resulting in heterogeneous opacity (grey-white portal vein, blue arrowhead). (C) Prolonged storage (>6 months) of yellow resin diminishes the contrast between the portal vein (grey) and bile ducts (grey). Scale bar 100 µm. Please click here to view a larger version of this figure.
Discussion
Several critical steps determine DUCT success, from sample preparation to the parameters of the CT device. To achieve the best results, well contrasted, well-injected, and bubble-free resin should be used to allow straightforward digital processing with automated thresholding to obtain 3D data, images, and movies. With training and following this protocol, 90% of injections are successful and result in reproducible data. It is important to use fresh yellow resin to achieve the best contrast between the two injected systems. The yellow resin has a very strong radiopacity, while the blue resin has undetectable radiopacity. Top results are achieved within the first three months after opening a new yellow resin bottle. With time, resin precipitates, and after longer storage (>6 months), the yellow and the green resins will no longer be distinguishable in CT scans. Images with poor contrast necessitate extensive and time-consuming manual tracing and segmentation of the two systems. Next, well-stretched tubing is indispensable to fit into the common bile duct of adult mice and common bile duct and portal vein of postnatal mice. The entry point for the injection must be created with care. If the common bile duct is cut open transversally, it is likely to detach from the surrounding tissue, preventing successful entry of the tubing. This step is especially delicate for postnatal mice in which the common bile duct retracts and "curls up" if it has detached from its surrounding tissue, making insertion of the tubing extremely challenging. The common bile duct entry and injection may require some practice. While preparing the tubing with resin and throughout injection, avoid bubble formation as bubbles will create negative space in the CT images and require time-consuming manual correction. It is important to gently massage the liver by rolling over its surface with a wetted cotton swab during and after the injection procedure as this facilitates even resin spreading. After the completion of injection and removal of the tubing, the silk suture knot must be tightened quickly and carefully, so the resin does not flow out of the liver before it polymerizes completely. For successful microCT imaging, the sample must be properly fixed in place with agarose and thermally adapted to eliminate movement artifacts in the CT data. The acquisition settings are also of key importance, which should be optimized to reach an adequate spatial resolution to resolve fine structures.
Technical modifications to the injection procedure can be made to achieve injection in younger mice. Currently, resin casting of younger mouse livers is limited by the availability of sufficiently thin tubing, with PE10 being the smallest commercially available tubing. Tanimizu et al. successfully injected carbon ink into embryonic day 17 (E17) common bile duct using glass capillaries11. Future testing of whether resin can be delivered via glass capillary would therefore be of interest. DUCT was further adapted to inject other tubular systems such as the airways and pulmonary artery vasculature of the lungs9. The double resin injection could also be modified to be used with other commercially available resins, or this protocol could be used for injections with carbon ink.
One of the main limiting factors of the DUCT pipeline is the resin viscosity. DUCT can only be used for resin casting of tubular structures above a diameter of 5 µm. In this data set, the resin could penetrate tubes with the smallest diameter of 5 µm9. This size limitation precludes the analysis of fine ductules and small capillaries. To further advance the DUCT pipeline to smaller caliber vessels, other commercially available resins should be tested, or the development of new low-viscosity radiopaque agents may improve the lumen penetration.
In Hankeova et al.9, DUCT was compared to two other commonly used techniques, double carbon ink injections followed by tissue clearing and standard photography, and iDISCO+ with staining of the blood vessels with alpha-smooth muscle cell actin and bile ducts with cytokeratin 7, followed by 3D imaging9. DUCT outperformed the other two methods in terms of dual analysis (which was challenging for iDISCO+ due to high liver autofluorescence), 3D imaging, and quantification (not possible with carbon ink injection), and lumenization (DUCT provides data for the internal lumen architecture and system perfusion). As mentioned above, the main limitation of DUCT is the minimum lumen size that can be injected and analyzed (5 µm limit), a parameter in which both carbon ink injection and iDISCO+ performed better. DUCT is superior to single system resin casting3,5,6 because it allows analysis of each injected system separately and also facilitates dual 3D investigation to study the architectural relationship between the two systems.
DUCT can be applied to study any two tubular networks in 3D. As proof of principle, DUCT was used to visualize the liver biliary and portal vein systems and the pulmonary artery vasculature and airways in lung9. The intrahepatic bile ducts develop adjacent to the portal vein, and the portal vein provides a structural template and signaling center that regulates the growth and differentiation of the biliary tree12. In Hankeova et al.9, DUCT explored biliary regeneration in a mouse model for the human pediatric disease Alagille syndrome. DUCT revealed previously unreported architectural mechanisms that the biliary system used to achieve a wild-type-like volume9. The Alagille syndrome mice utilized two different strategies: (1) in the hilar and central regions of the liver, the biliary system increased its branching, and (2) in the liver periphery, the de novo-generated bile ducts were highly tortuous. These two factors combined to yield a near-normal biliary system volume, despite the abnormal architecture. Furthermore, DUCT detected abnormal bile duct branching that occurred independent of portal vein branching and bile ducts forming connecting bridges between two portal veins9. These phenotypes would be impossible to detect in single resin casting and could be misinterpreted in 2D histological sections as bile duct proliferation. DUCT thus provides data describing the 3D architecture of two tubular networks at the whole organ or lobe level with the possibility of qualitative and in-depth quantitative analysis. DUCT could be a new standard for postnatal liver development and liver regeneration analyses in different animal models.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Kari Huppert and Stacey Huppert for their expertise and help regarding bile duct cannulation and their laboratory hospitality. We also thank Nadja Schultz and Charlotte L. Mattsson for their help with common bile duct cannulation.
We thank the following Granting Agencies for their support:
For work in ERA Lab: Karolinska Institutet (2-560/2015-280), Stockholms Läns Landsting (CIMED (2-538/2014-29)), Ragnar Söderbergs stiftelse (Swedish Foundations' Starting Grant), European Association for the Study of the Liver (Daniel Alagille Award), Swedish Heart-Lung Foundation (20170723), and Vetenskapsrådet (2019-01350).
For work in JK Lab: We acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110). J.K. thanks to the support of grant FSI-S-20-6353.
Materials
| 1.5 mL SafeSeal micro tubes | Sarstedt | 72.706 | |
| 23 G butterfly needle with tubing | BD bioscience | 367283 | |
| 25 G needle | BD bioscience | 305122 | |
| 30 G needle | BD bioscience | 305106 | |
| Agarose | Top-Bio | P045 | |
| Benzyl alcohol | Sigma Aldrich | 108006 | |
| Benzyl benzoate | Sigma Aldrich | B6630 | |
| Corning 50 mL tubes | Sigma Aldrich | CLS430829-500EA | polypropylene |
| Cotton swabs | Medicarier | 60406 | |
| Dissection Microscope | Leica Camera AG | Leica M60 | |
| Dulbecco's phosphate-buffered saline | ThermoFisher Scientific | 14190144 | |
| Ethanol 70% | VWR | 83801.41 | |
| Falcon tube 15 mL | Verkon | 331.850.084.006 | |
| Forceps curved | Fine Science Tools | 11051-10 | Fine Graefe 10 cm curved |
| Forceps straight | Fine Science Tools | 11050-10 | Fine Graefe 10 cm straight |
| Formaldehyde solution | Sigma Aldrich | F8775 | |
| GE Phoenix v|tome|x L 240 | Waygate Technologoies | micro computed tomography scanner | |
| Hanks' Balanced Salt Solution | ThermoFisher Scientific | 14025092 | |
| Heparin | Leo Pharma | B01AB01 | 5000 IE/mL |
| Isolfurane | Baxter | FDG9623 | |
| Methanol | ThermoFisher Scientific | 11413413 | |
| MICROFIL | Flowtech | MV-122 | synthetic resin yellow |
| MICROFIL | Flowtech | MV-120 | synthetic resin blue |
| MICROFIL | Flowtech | MV-diluent | clear resin diluent |
| Pasteur pipette | Verkon | 130.690.424.503 | |
| Peristaltic pump | AgnThos | 010.6131.M20 | |
| phoenix datos|x 2.0 software | Baker Hughes | CT data reconstruction software | |
| Rocker | VWR | 444-0142 | |
| Silk suture | AgnThos | 14757 | Black silk, 4-0, sterile, 100 m |
| Skin scissor | Fine Science Tools | 14058-09 | Iris straight tip 9 cm |
| Spring scissor | Fine Science Tools | 15000-03 | Vannas micro, straight tip 2 mm |
| Syringe 1 mL Luer | BD bioscience | 303172 | |
| Tubing PE10 | BD bioscience | 427401 | |
| Tubing PE50 | BD bioscience | 427411 | |
| VG Studio MAX 3.3 software | Volume Graphics GmbH | CT data processing and analysis software |
References
- Narat, J. K., Loef, J. A., Narat, M. On the preparation of multicolored corrosion specimens. The Anatomical Record. 64, 155-160 (1936).
- Ludwig, J., et al. Anatomy of the human biliary system studied by quantitative computer-aided three-dimensional imaging techniques. Hepatology. 27, 893-899 (1998).
- Masyuk, T. V., Ritman, E. L., LaRusso, N. F. Quantitative assessment of the rat intrahepatic biliary system by three-dimensional reconstruction. American Journal of Pathology. 158, 2079-2088 (2001).
- Masyuk, T. V., Ritman, E. L., LaRusso, N. F. Hepatic artery and portal vein remodeling in rat liver: Vascular response to selective cholangiocyte proliferation. American Journal of Pathology. 162, 1175-1182 (2003).
- Sparks, E. E., et al. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 51, 1391-1400 (2010).
- Cuervo, H., et al. Endothelial notch signaling is essential to prevent hepatic vascular malformations in mice. Hepatology. 64, 1302-1316 (2016).
- Thakurdas, S. M., et al. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi). Hepatology. 63, 550-565 (2016).
- Walter, T. J., Sparks, E. E., Huppert, S. S. 3-Dimensional resin casting and imaging of mouse portal vein or intrahepatic bile duct system. Journal of Visualized Experiments: JoVE. (68), e4272 (2012).
- Hankeova, S., et al. DUCT reveals architectural mechanisms contributing to bile duct recovery in a mouse model for Alagille syndrome. Elife. 1, 1-29 (2021).
- Andersson, E. R., et al. Mouse model of Alagille syndrome and mechanisms of Jagged1 missense mutations. Gastroenterology. 154, 1080-1095 (2018).
- Tanimizu, N., et al. Intrahepatic bile ducts are developed through formation of homogeneous continuous luminal network and its dynamic rearrangement in mice. Hepatology. 64, 175-188 (2016).
- Ober, E. A., Lemaigre, F. P. Development of the liver: Insights into organ and tissue morphogenesis. Journal of Hepatology. 68, 1049-1062 (2018).

