Rodent Heart and Brain Tissue Preparation for Digital Macro Photography after Ischemia-reperfusion
Summary
Presented here is a protocol for the standardized methodology of rodent tissue preparation after the ischemia-reperfusion experiment and guidelines for establishing lighting and camera setups for high-resolution image acquisition. This method is applicable to all experimental small-animal organ photography.
Abstract
Macro photography is applicable for imaging various tissue samples at high magnification to perform qualitative and quantitative analyses. Tissue preparation and subsequent image capture are steps performed immediately after the ischemia-reperfusion (IR) experiment and must be performed in a timely manner and with appropriate care. For the evaluation of IR-induced damage in the heart and brain, this paper describes 2,3,5-triphenyl-2H-tetrazolium chloride (TTC)-based staining followed by macro photography. Scientific macro photography requires controlled lighting and an appropriate imaging setup. The standardized methodology ensures high-quality, detailed digital images even if a combination of an inexpensive up-to-date digital camera and macro lens is used. Proper techniques and potential mistakes in sample preparation and image acquisition are discussed, and examples of the influence of correct and incorrect setups on image quality are provided. Specific tips are provided on how to avoid common mistakes, such as overstaining, improper sample storage, and suboptimal lighting conditions. This paper shows the appropriate methodology for rat heart and brain tissue slicing and staining and provides guidelines for establishing lighting and camera setups and photography techniques for high-resolution image acquisition.
Introduction
For decades, photography and analysis of heart and brain tissue specimens have been an important part of life science experiments. Science and innovation progress drives the development of expensive microscopes capable of superresolution. Photomicrographs are obtained in a well-controlled light environment following detailed instructions. In contrast, macro photography (at 1:2 or greater magnification) is frequently performed in an uncontrolled light environment using inappropriate imaging setups. Often, the techniques of sample preparation and camera setup need to be substantially optimized. As a result, macro photographs of limited quality have been widely published in scientific journals. Insufficient image resolution and contrast limit the possibilities of precise image quantification in IR studies.
Experimental procedures of myocardial1,2 and brain3,4 infarctions have been described in detail. The purpose of this study is to provide a step-by-step guide on how to set up a system for photography and standardized analysis of rodent heart and brain tissue specimens after infarction experiments. This includes tissue slicing, staining, and macro photography of heart and brain samples. The preparation of tissue specimens is an essential part of the experiment, and the planimetric image analysis results depend highly on the quality of the obtained images5.
These methods are particularly useful for performing measurements and image planimetric analysis in rodent tissues and could be of value for general scientific macro photography. In addition, the high quality and consistency of images allow automated analysis of digital photographs to be performed, which helps to save time, avoid user input, and minimize the risk of errors or bias during image analysis. This will result in the generation of robust and reliable data and increase the translation of preclinical discoveries into novel antiischemic treatments in clinics.
Protocol
The experimental procedures were performed in accordance with the guidelines of the European Community and local laws and policies (Directive 2010/63/EU), and all the procedures were approved by the Food and Veterinary Service, Riga, Latvia.
1. Heart staining and slicing
NOTE: Techniques described in this protocol can be used after both Langendorff-perfused isolated rat or mouse heart6,7 and in vivo rat heart IR injury assays8,9,10,11. For staining after an in vivo IR injury assay, it is assumed that the heart is excised, mounted on a cannula, and briefly perfused in the Langendorff perfusion mode.
- Detach the heart cannula from the syringe filled with Krebs-Henseleit solution and connect it to a syringe filled with a warm (37 °C) solution of 0.1% methylene blue in Krebs-Henseleit solution. Use a 5 mL syringe for rat hearts and a 1-2 mL syringe for mouse hearts.
NOTE: An alternative is to fill the pressure- or flow-controlled (e.g., Langendorff) apparatus with a blue dye-containing solution. During the detachment and mounting procedure, it is essential not to leave any air bubbles in the cannula and not loosen the suture used for coronary artery reocclusion. - Further perfuse rat hearts with 4 mL of the methylene blue solution at a rate of ~4 mL/min and perfuse mouse hearts with 1 mL of methylene blue solution at a rate of ~0.5-1 mL/min.
NOTE: Based on experience, both techniques are safe and provide adequate staining; however, using a pressure-controlled pump/hydrostatic pressure system is a more time-consuming but safer option against overstaining for novice scientists. - Disconnect the cannula from the syringe and remove the heart from the cannula.
- Remove excess methylene blue by gentle rolling of the heart on tissue paper. Loosen the ligature around the coronary artery by opening the hemostatic forceps and removing the plastic tubing from the surgical suture only after removing excess methylene blue.
NOTE: At this stage, it is possible to place the mouse heart in a small plastic bag or a 5 mL centrifuge microtube in the freezer (-20 °C) for up to 5-10 min. Maximal freezing time should be determined experimentally in each laboratory. Short-term freezing of a mouse heart can help a novice experimenter cut it into 1-mm-thick slices. The freezing of rat hearts is not recommended. Overfreezing for more than 10 min at -20 °C must be avoided. - Place the stained rat heart in a stainless-steel matrix (see the Table of Materials) for heart slicing (Figure 1A). Then, cut the ventricles of the heart into 2-mm-thick slices (aim for 6-7 slices of an adult rat heart). For mouse hearts, cut the ventricles of the heart into 1.5-mm-thick slices (aim for at least 4 slices of an adult mouse heart).
NOTE: Slicing matrix-compatible razor blades must be used. In general, compatible single-edge razor blades (e.g., thickness of up to 0.01 inch (0.254 mm)) can be used for slicing rat hearts. Double-edge razor blades are generally used for mouse hearts and are usually up to 0.004 inches (0.1 mm) in thickness.
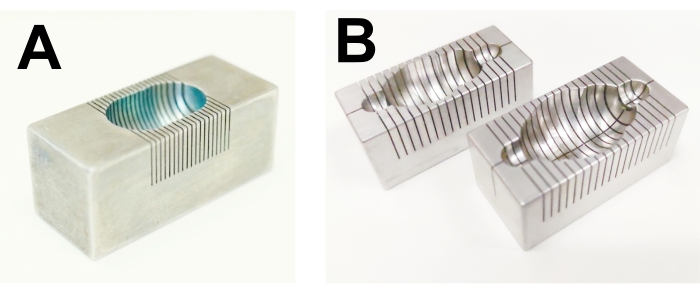
Figure 1: Matrices for the rat heart and brain slicing. (A) Rat heart, (B) rat brain. Please click here to view a larger version of this figure.
- After cutting, transfer the slices to a 15 mL plastic tube. Add 5 mL of 1% triphenyltetrazolium chloride (TTC) dissolved in phosphate-buffered saline (PBS) to the tube with the heart slices, and incubate for 10 min in a water bath at 37 °C.
- After incubation in TTC solution, wash the heart slices at least 2-3 times with PBS and prepare for image capture.
2. Brain staining and slicing
- After the middle cerebral artery occlusion experiment3,12, remove the brain, including the brainstem, from the skull, and wash it in ice-cold PBS.
- Choose the correct size of the brain stainless-steel matrix (see the Table of Materials) depending on the weight of the animals (Figure 1B). Place the brain with its ventral side up in the brain matrix.
NOTE: When seated in the matrix, the brain's ventral surface must be parallel to the top surface of the mold. - Using blades, restrict the frontal and caudal parts (2 blades from both sides) of the brain.
NOTE: Slicing matrix-compatible razor blades must be used. In general, a compatible, single-edge razor blade (thickness of up to 0.01 inch (0.254 mm)) can be used for rat brain slicing. - Put the blades partially (not fully cutting the brain) into the channels between the first and the last blades. When all the blades are inserted and arranged in parallel, press all the blades down with the palm at the same time to cut the brain into 2 mm coronal slices.
- Grasp the blades firmly along the sides with two fingers and remove them together with the sliced brain from the matrix.
- Arrange the brain slices one by one in a tray (70 mL, 72 x 72 mm). When arranging the slices, ensure that the anterior surface of each slice is always facing up.
- Pour warm (+37 °C) 1% TTC solution in PBS onto the brain slices, and incubate them for 8 min at 37 °C in the dark.
NOTE: The brain slices must be fully immersed in TTC solution during the incubation. - After incubation in 1% TTC solution, transfer the brain slices to the blue plastic tray to capture images. Arrange the brain slices in sequential order from the frontal to the caudal part, and use a scalpel to separate the hemispheres in the sagittal plane.
NOTE: The surface of the tray should be washable, matte, and of a color that contrasts brain slices (i.e., not red, white, or pale pink).
3. Macro photography
- Photograph the tissue slices immediately after staining.
NOTE: Heart slices can be stored in cold PBS (at +4 °C) or formalin solution for up to 30 min. Brain slices can be stored in formalin for a prolonged period (1-2 weeks). - Set up the camera of choice with a charged battery, memory card, and attached lens on a stand (Figure 2)
NOTE: Turn the lights on at least 5-10 min before image acquisition to warm up the equipment. LED lights reach full brightness in microseconds.
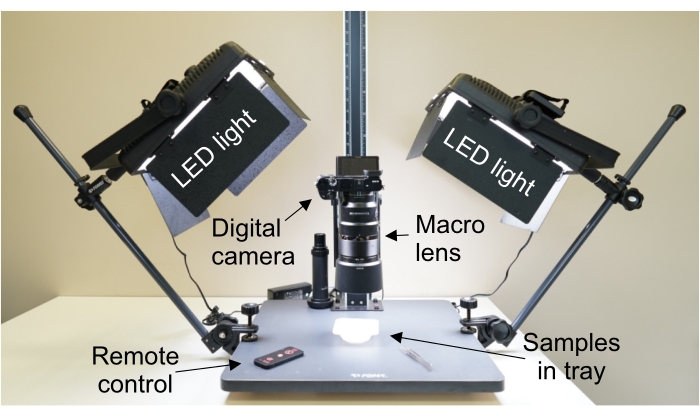
Figure 2: Camera and lights set up for macro photography. Camera is perpendicular to the imaging surface to ensure that the focal plane of the camera is parallel to the samples. Abbreviation: LED = light-emitting diode. Please click here to view a larger version of this figure.
- Depending on the available light sources, select the appropriate white balance settings or perform color temperature calibration according to the instructions in the camera manual.
NOTE: White LED light (color temperature 6,500 K) is the preferred light source to avoid light flickering by fluorescent light bulbs. - Switch the camera to fully manual mode, set the ISO 100 and aperture to f/10, and adjust the shutter speed for optimal image exposure. Ensure that the camera focal plane is parallel to the surface where the sample will be placed.
NOTE: Histogram function is useful to ensure that tissue slices are not overexposed. - Attach or enable a wired or wireless remote trigger to prevent camera shake when the shutter is released.
NOTE: An alternative is to enable a delayed shutter function, which will delay the trigger for 2 or 10 s after pressing the trigger button. - Immerse the heart slices completely in a container with PBS.
NOTE: Immersed slides tend to float away from their position. To minimize floating of the slices, use the smallest possible tray into which all slices can fit and the minimal amount of immersion solution, ensuring that the specimen is fully immersed. Alternative methods include placing the slices between glass slides or using a polarizing filter on the lens. A circular polarizing filter is attached to the lens and rotated until reflections in a live-view display of the camera disappear. - Arrange the brain slices in a dry tray without PBS or other liquids.
NOTE: A polarizing filter is very convenient to capture images of brain slices. - Place the container with slices under the camera with the macro lens and ensure that all slices fully fit in the field of view. Ensure that the slices are on the same plane, i.e., not curved or rolled.
- Check the exposure and adjust the camera settings if needed.
NOTE: Once set, do not change the exposure and other settings during the entire experiment. - Capture the number (or other identification) of the sample and image the tissue slice using a remote trigger.
NOTE: A size marker, such as a mm ruler, should be included in the field of view when absolute quantification of specimen size is necessary. - Rotate the slices and capture their images from the other side.
Representative Results
Figure 3A is a photograph of a methylene blue- and TTC-stained heart slice after myocardial infarction, which contains enough detail and color information for further planimetric analysis of infarct size (Figure 3B). We tested how freezing of the heart for 24 h affects the integrity of heart tissues (Figure 3C). Freezing for a prolonged period (>1 h, Figure 3C) reduces mitochondrial function; thus, TTC staining of the heart is not red but pale pink, and the border between necrotic and viable tissues is blurred (Figure 3C).
Further, two methods were compared for the reduction of reflections in the specimens. Immersion is the most efficient method and produces detailed images with good contrast (Figure 4A). The second method is the use of a polarizing filter attached to the lens. The polarizing filter also is effective; however, the filter slightly reduces the resolution and microcontrast of the image (Figure 4B). An example image of a heart slice without immersion or filter (Figure 4C) contains many reflections and is not suitable for further analysis.
Brain slices are not immersed because of slice management (floating) problems. In the planimetric analysis, it is important to compare the unaffected (healthy) side of the brain (Figure 5A) with the stroke-affected side (Figure 5B). Brain slices are easier to manage on a dry plate or tray, and a polarizing filter is used to remove reflections. A tray with blue background is used for brain slice photography (background selection described previously5).
Manual camera settings were used to ensure full control of exposure and white balance. Camera settings should be adjusted before or at the beginning of the experiment according to the available light source. This ensures optimal exposure and white balance of all images to allow uniform analysis (Figure 6A). The automatic settings of the camera are not perfect and can result in varying camera parameters, causing inappropriate results and the introduction of image-to-image variability.
Figure 6 shows examples of overexposed (Figure 6B) and underexposed images (Figure 6C) of heart slices. Sufficient attention should be paid to the correct white balance settings of the camera to match a particular light source used in the camera-light setup. Incorrect white balance settings may result in a shift to blue or yellow (Figure 6D) and magenta or green (Figure 6E) cast in the image.
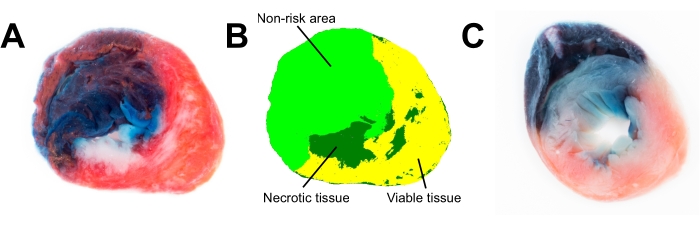
Figure 3: Images of rat cardiac slices. (A) Fresh heart slice was analyzed in ImageProPlus 6.3 software using color segmentation (B). (C) TTC staining poorly discriminates between viable and necrotic tissue in the frozen heart slice (frozen for 24 h). Abbreviation: TTC = 2,3,5-triphenyl-2H-tetrazolium chloride. Please click here to view a larger version of this figure.
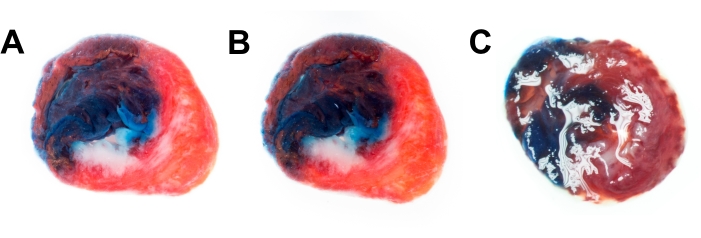
Figure 4: Techniques for the reduction of reflections. Rat heart slice image captured immersed in PBS (A) and using polarizing filter (B). (C) Heart slice with reflections when neither immersion nor filter is used. Abbreviation = PBS = phosphate-buffered saline. Please click here to view a larger version of this figure.
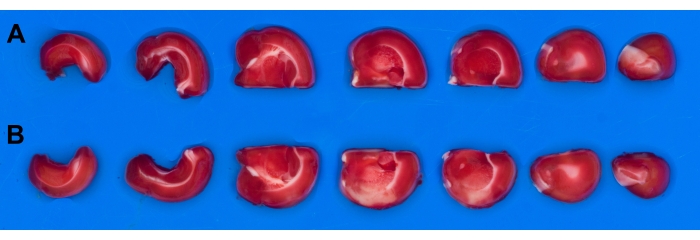
Figure 5: Images of rat brain slices. Rat brain was cut into seven slices and stained with TTC after ischemia-reperfusion. Using polarizing filter results in acquisition of reflection-free image. (A) Slices from the undamaged hemisphere ; (B) Slices from the stroke-affected hemisphere. Abbreviation: TTC = 2,3,5-triphenyl-2H-tetrazolium chloride. Please click here to view a larger version of this figure.
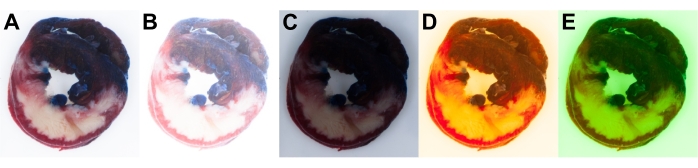
Figure 6: Rat heart slice images. Correctly (A) and incorrectly (B–E) captured heart slice images. Incorrect exposure settings result in overexposed (B) and underexposed images (C). Incorrect white balance settings result in yellow (D) or green cast in the image (E). Please click here to view a larger version of this figure.
Discussion
Preparation of the heart after IR starts with the reocclusion of blood cardiac arteries and the perfusion of blue dye for the discrimination of at-risk areas from non-risk areas. Methylene blue or Evans blue dyes are most frequently used for this purpose2. As an excessively high pressure might damage heart valves and, thus, partially or completely stain at-risk areas, it is better to perfuse the heart with a pressure-controlled system, such as the Langendorff apparatus or a simplified version of a hydrostatic pressure system-equipped syringe or pump. Controlled perfusion will ensure physiological pressure, and the dye will usually not enter the occluded region of the heart. Both flow speed- and pressure-controlled techniques are safeguards against overstaining.
One of the most serious mistakes in viable tissue processing is keeping tissues in a freezer for a prolonged time before staining. Freezing is mainly used because researchers want to perform heart staining the day after the IR experiment or later. Moreover, freezing is used to make the cutting of the heart easier. We found that short-term freezing of the heart for up to 5-10 min negligibly affects the integrity of heart tissues and facilitates cutting the tissues (particularly for mouse hearts) into thin slices. However, freezing for prolonged periods damages membranes and decreases cell viability and mitochondrial function13. As a result, TTC staining of functioning mitochondria is affected, and the border between necrotic and viable tissues is poorly delineated (blurry). Overall, freezing of rat harts should be avoided, and only short-term freezing of mouse hearts can be used for easier cutting.
The next step is tissue staining in 1% TTC solution at 37 °C14. The staining solution should be prewarmed—particularly important for staining of brain slices. When using the prewarmed solution, the optimal staining time for heart slices is 10 min. A longer incubation or a temperature higher than 37 °C results in brown colorization of the heart tissues. Proper staining of specimens and consistent red color intensity are important for further image analysis. In the final steps before photography, the tissue slices are rinsed 2-3 times with cold PBS or a similar buffer to remove TTC and excess methylene blue from the solution to avoid blue casting in the photograph. Heart slices should be photographed shortly after staining to obtain the best image quality. Heart staining remains of good quality if stored for up to 60 min in the cold (+4 °C) PBS. Stained brain slices and aortic tissues are usually stored in a 4% neutral formaldehyde solution and retain good quality for a week. Overnight storage of brain tissues in formalin (+4 °C) does not impair the color intensity of normal tissue and is acceptable for image acquisition. However, formalin induces swelling and destaining of heart slices. Therefore, the storage of heart tissues in formalin is not recommended.
The next step is image acquisition. Many laboratories use flatbed scanners as an image acquisition tool that is expected to replace a digital camera and lighting setup. We determined that the scanning of slices does not provide sufficient image resolution and color separation and therefore is not suitable for imaging heart slices. In particular, scanner resolution is insufficient for mouse hearts, and we noticed poor rendering of methylene blue. In contrast, a scanner might be an alternative to a photo camera for imaging brain slices stained only with TTC or other single dyes. For the scanning of tissue slices, scanning software that ensures constant exposure settings is essential. Overall, a flatbed scanner is less capable and cannot replace a digital camera for most imaging applications.
The background behind specimens is also important. Ideally, the bottom of the tray should be of a color not present in the stained specimen. For example, to quantify the area of methylene blue and TTC (red) staining in an automated or semiautomated manner, white, red, blue, yellow, and brown backgrounds should be avoided. Thus, a green background would be preferable. Nevertheless, color selection depends on the preferences of the operator, who postprocesses the image. Many scientists prefer a white background because a white background can be deleted in image postprocessing and converted into completely white (RGB white code 255,255,255). Then, one should exclude completely white from the list of selected colors used for semiautomated analysis and count only pale necrotic areas, which are not completely white if not overexposed. Blue and green backgrounds are suitable for the photography of brain slices and aortas.
The optimal imaging tool for tissue photography is a single-lens reflex or mirrorless interchangeable lens digital camera with a compatible macro lens. Capturing very small objects might require a combination of a camera and a microscope; nevertheless, a macro lens usually has sufficient (at least 1:2) magnification to obtain detailed images of a mouse heart. Many manufacturers offer affordable digital cameras and macro lenses to obtain high-resolution and high-magnification photographs. All up-to-date digital cameras have characteristics and functions necessary for macro photography, including the possibility to mount on a stand, a high number of pixels (usually >20 Mpx), live view, mirror lock-up, time-lapse features, remote shutter, and the ability to manually set camera parameters, thus ensuring a constant shutter speed, aperture, white balance, and ISO setting. Compact cameras with the above-mentioned features and lens magnification of at least 1:2 can also be used for macro photography. Because of lens characteristics, some compact cameras should be placed in close proximity to the object, and the experimenter must ensure that the camera body does not affect the illumination of the specimen.
For macro photography with any type of interchangeable lens camera, a high magnification (1:1-1:2) macro lens is required. We suggest using macro lenses with a focal length ranging from 50 mm to 100 (120) mm or equivalent on the full-frame (24 mm x 36 mm) sensor. Smaller sensor cameras have different sensor sizes, and magnification should be recalculated accordingly. For the photography of heart slices, an ergonomic distance of the 100 mm macro lens front element to the subject is approximately 150 mm. This setting allows operators to keep all the equipment on a table, with easy access to the camera controls. A 50 mm macro lens might be considered for photography of larger objects, such as brain slices, because a wider field of view is necessary to obtain all slices in a single photograph.
To obtain sharp images with high resolution, a camera should be mounted onto a sturdy stand, which, together with a light setup, is called a photography copy stand. Mounting the camera on a stand and a remote (wired or wireless) trigger eliminates camera shake and ensures a constant distance from the target. A camera-lighting setup with two constant-light sources from both sides, angled approximately 30-60° relative to the subject plane, ensures sufficient illumination of specimens and helps avoid reflections at the same time. The camera should be mounted precisely so that the sensor is parallel to the subject plane. To evenly illuminate the image field, both lamps should be equally oriented and placed at the same distance from the subject. Light sources placed at various distances from the subject cause uneven illumination. Additionally, blinking light sources are a reason for variations in image exposure. Overall, it is important to accurately place the camera and light sources to precisely acquire images of well-illuminated specimens.
Tissue samples reflect light (glisten), which appear as white spots in the images. These light reflection spots do not contain useful color information, and accordingly, these parts of the images cannot be used for accurate quantitative analysis of images. Light reflections from tissue slices can be removed by various methods. The most efficient is the full immersion of tissue samples in a container with a saline or PBS solution. A similar approach is the insertion of tissue slices below (or between) glass plates. This method is efficient against reflections; however, the image resolution can be lower than that of photographs of immersed tissues.
One can also use a polarizing filter mounted on a lens to eliminate light reflections. Circular polarizing filters are widely available but vary considerably in quality depending on price, and cheap filters can significantly reduce image resolution. Reflected light can be filtered off by turning the moving part of the polarizing filter at an angle. The efficacy of the polarizing filter might be affected by some light sources (e.g., strong LED light). Overall, after removal of extra liquid, a polarizing filter can eliminate all reflections from the brain slices; however, sample immersion in buffer solution is the easiest and most cost-efficient approach for heart slices.
Manual settings of the shutter speed, aperture, ISO, and white balance are important to maintain full control of the imaging process. The sample, background, and characteristics of the light source influence the camera exposure metering system in automatic settings; therefore, manual settings are necessary to maintain constant exposure and white balance between multiple photographs during the experiment. For macro photography, the suggested aperture setting is between f/8 and f/16. By decreasing the aperture, the depth of field increases, which is helpful if the object is not in a single plane. However, diffraction limits the total resolution of photography in the case of smaller apertures. The optimal aperture for most lenses is usually f/10 because in this setting, resolution drop is negligible, and depth of field is sufficient. ISO values that range from 50 to 400 (lower is better) are usually optimal to minimize image artifacts (noise). The shutter speed then remains to be changed to obtain correct exposure using the mentioned aperture and ISO settings in existing light conditions. Manual settings are important for consistent image analysis. Standardized imaging ensures the use of the same color thresholding settings throughout any study, which requires segmentation analysis. For example, semiautomated analysis by ImagePro software based on a segmentation file with predefined colors of blue, red, and white (+pale pink) can be used over the years if specimen images have consistent colors, white balance, and exposure.
The white balance setting should be adjusted depending on the color temperature of the light source that is used to illuminate a sample. White balance can be selected from camera built-in presets or using the manual calibration of a grey target. The benefit of image capturing in RAW format is that white balance can be adjusted during software postprocessing of the image. As RAW files contain much more information than JPEG files, RAW file postprocessing provides an excellent opportunity for the correction of color balance and exposure, as well as to obtain better image resolution. Because most cameras can capture JPEG and RAW files simultaneously, we suggest capturing the RAW file and saving it as a backup.
Overall, this protocol describes a methodology for rat heart and brain tissue slicing and staining and provides guidelines for establishing lighting and camera setups and photography techniques for high-resolution image acquisition for further analysis. This method is applicable to all experimental small-animal organ photography.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors were supported by European Union's Horizon 2020 research and innovation program under grant agreement No 857394, Project FAT4BRAIN.
Materials
| 1 mL syringe | Sagimed | N/A | |
| 2,3,5-Triphenyltetrazolium chloride (TTC) | Sigma-Aldrich | 298-96-4 | |
| 5 mL syringe | Sagimed | N/A | |
| 50 mL syringe | Terumo | N/A | |
| Adult Rat Brain Slicer Matrix | Zivic Instruments | BSRAS001-1 | |
| Aortic cannula for mouse heart | ADInstruments | SP3787 | |
| Aortic cannula for rat heart | ADInstruments | SP3786 | |
| Calcium chloride dihydrate, ≥99% | Acros Organics | 207780010 | |
| Cover Glass Forceps, Angled | Fine Science Tools | 11073-10 | |
| Hemostatic forceps | Agnthos | 13008-12 | |
| Hoya 62 mm alpha Circular Polarizer Filter | Hoya | HOCPA62 | |
| Magnesium chloride hexahydrate | Penta | 16330-31000 | |
| Methylene Blue | SigmaAldrich | M9140 | |
| Mouse Heart Slicer Matrix | Zivic Instruments | HSMS005-1 | |
| Polyethylene plastic tubing | BD Intramedic | N/A | |
| Potassium chloride for biochemistry | Acros Organics | 418205000 | |
| Potassium phosphate, monobasic, ≥99% | Acros Organics | 205920025 | |
| Rat Heart Slicer Matrix | Zivic Instruments | HSRS001-1 | |
| Scissors curved with blunt ends | Agnthos | 14013-15 | |
| Scissors for cleaning heart | Agnthos | 14058-11 | |
| Single Edge Razor Blades | Zivic Instruments | BLADE012.1 | |
| Sodium bicarbonate for biochemistry, 99.5% | Acros Organics | 447100010 | |
| Sodium chloride | Fisher bioreagents | BP358-10 | |
| Sony Alpha a6000 Mirrorless Digital Camera | Sony | ILCE6000 | Can be repalaced by any up-to-date digiatal camera |
| Sony FE 90 mm F/ 2.8 Macro G OSS | Sony | SEL90M28G | Important, lens should be compatible with camera |
| Sony SF32UZ SDHC 32 GB Class 10 UHS | Sony | 2190246141 | |
| Surgical blade | Heinz Herenz Hamburg Germany | BS2982 | |
| Thermo-Shaker | BioSan | PST-60HL-4 | |
| Toothed tissue forceps | Agnthos | 11021-12 | |
| Toothed tissue forceps for cleaning heart | Agnthos | 11023-10 | |
| Weigh tray, 70 mL | Sarsted | 71,99,23,212 |
References
- Bell, R. M., Mocanu, M. M., Yellon, D. M. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. Journal of Molecular and Cellular Cardiology. 50 (6), 940-950 (2011).
- Botker, H. E., et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Research in Cardiology. 113 (5), 39 (2018).
- Uluc, K., Miranpuri, A., Kujoth, G. C., Akture, E., Baskaya, M. K. Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. Journal of Visualized Experiments: JoVE. (48), e1978 (2011).
- Zvejniece, L., Svalbe, B., Liepinsh, E., Pulks, E., Dambrova, M. The sensorimotor and cognitive deficits in rats following 90- and 120-min transient occlusion of the middle cerebral artery. Journal of Neuroscience Methods. 208 (2), 197-204 (2012).
- Liepinsh, E., Kuka, J., Dambrova, M. Troubleshooting digital macro photography for image acquisition and the analysis of biological samples. Journal of Pharmacological and Toxicological Methods. 67 (2), 98-106 (2013).
- Kolwicz, S. C., Tian, R. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. Journal of Visualized Experiments: JoVE. (42), e2069 (2010).
- Herr, D. J., Aune, S. E., Menick, D. R. Induction and assessment of ischemia-reperfusion injury in Langendorff-perfused rat hearts. Journal of Visualized Experiments: JoVE. (101), e52908 (2015).
- Liepinsh, E., et al. Inhibition of L-carnitine biosynthesis and transport by methyl-gamma-butyrobetaine decreases fatty acid oxidation and protects against myocardial infarction. British Journal of Pharmacology. 172 (5), 1319-1332 (2015).
- Nakamura, K., Al-Ruzzeh, S., Ilsley, C., Yacoub, M. H., Amrani, M. Acute effect of cerivastatin on cardiac regional ischemia in a rat model mimicking off-pump coronary surgery. Journal of Cardiac Surgery. 20 (6), 507-511 (2005).
- Li, Q., Morrison, M. S., Lim, H. W. Using a cardiac anchor to refine myocardial infarction surgery in the rat. Lab Animal. 39 (10), 313-317 (2010).
- Wu, Y., Yin, X., Wijaya, C., Huang, M. H., McConnell, B. K. Acute myocardial infarction in rats. Journal of Visualized Experiments: JoVE. (48), e2464 (2011).
- Vavers, E., et al. The neuroprotective effects of R-phenibut after focal cerebral ischemia. Pharmacological Research. 113, 796-801 (2016).
- Acin-Perez, R., et al. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO Journal. 39 (13), 104073 (2020).
- Kloner, R. A., Darsee, J. R., DeBoer, L. W., Carlson, N. Early pathologic detection of acute myocardial infarction. Archives of Pathology & Laboratory Medicine. 105 (8), 403-406 (1981).

