Laparoscopic Non-Mesh Cerclage Pectopexy for Pelvic Organ Prolapse
Summary
The present pilot study describes the development of laparoscopic non-mesh cerclage pectopexy to treat pelvic organ prolapse. The procedure can be used to prevent any complications associated with the use of mesh.
Abstract
Pelvic organ prolapse (POP) is widespread among the female population and significantly impairs the patient’s quality of life. It is important to restore apical support for treating POP. Sacrocolpopexy and pectopexy are indicated for apical prolapse. Using a synthetic mesh in these techniques increases success by enhancing apical support. However, the implantation of synthetic mesh is associated with mesh-related complications. In addition, the exorbitant cost of synthetic mesh and lack of universal access limit the popularity of these procedures. The current study develops a unique technique known as laparoscopic non-mesh cerclage pectopexy (LNMCP), in which permanent cervical cerclage sutures are embedded in the round ligament until the iliopectineal ligament. The iliopectineal ligament was sutured, resulting in a firm cervical suspension. The procedure was successfully performed in 16 cases in the hospital. The surgical duration was 67.8 min ± 15.5 min, and the blood loss was 73.1 mL ± 51.1 mL. No procedural complications were seen. LNMCP is associated with an objective success rate of 100% and a subjective success rate of 93.8%. LNMCP for patients with apical prolapse obviates the need for a mesh, thereby avoiding complications associated with mesh erosion and reducing medical costs. In addition, it is easy to perform even in resource-poor areas without access to synthetic mesh.
Introduction
Pelvic organ prolapse (POP) is predominantly seen in women, especially those with multiple children. POP adversely and significantly affects the patient’s quality of life1. Apical support is critical in ensuring the overall health of the pelvic organs. However, the loss of apical support is often disregarded. Once the cervix descends to ≥−3 cm or the vaginal cuff to ≥−4 cm, an apical defect must be considered2, and an apical support procedure needs to be performed to address POP.
Sacrocolpopexy is the gold standard treatment for apical prolapse3. However, sacrocolpopexy is associated with de novo defecation disorders due to outlet obstruction4. Pectopexy, a new technique for apical repair, is similar to sacrocolpopexy in terms of clinical efficacy, with minimal defecation disorders4. The use of synthetic mesh in sacrocolpopexy and pectopexy for apical support enhanced the success rates5.
However, the implantation of synthetic mesh was associated with complications such as perforation into the bladder, urinary tract, and bowel, resulting in infection and pain6. The production of transvaginal meshes for prolapse was banned by the United States Food and Drug Administration (FDA) in April 2019, suggesting imminent restrictions against using mesh in the abdominal repair of POP6. Therefore, alternative urogynecological management options are needed for such patients.
The present study reports the development of laparoscopic non-mesh cerclage pectopexy (LNMCP) for apical prolapse therapy without using a synthetic mesh, thereby eliminating the risk of mesh-related complications.
Protocol
The protocol using LNMCP was conducted after approval by the Institutional Review Board of The University of Hong Kong-Shenzhen Hospital on 1 March, 2021 (IRB Approval Number 2021-040). All human participants provided written informed consent for publication before undergoing the procedure. All women diagnosed with symptomatic POP and cystocele or uterine descent POP-Q stage 2 or higher were included in the study. Women with a history of prolapse surgery, established malignancy of the cervix or cervical dysplasia, language issues, intention to preserve fertility, contraindications for laparoscopic surgery, such as the risk of severe adhesions, and those not available for follow-up were excluded.
1. Patient preparation
- Discuss the benefits and risks associated with the procedure as well as the risk of complications such as uncontrolled bleeding warranting blood transfusion, conversion to laparotomy, postoperative infection, and the recurrence of POP.
- Perform surgery using general anesthesia and endotracheal intubation following a previous report7. Administer prophylactic antibiotics such as intravenous cefuroxime (1.5 g, see Table of Materials) to each patient 30 min before the induction of anesthesia.
- Start perioperative thrombosis prophylaxis in women using an enoxaparin sodium injection (4,000 AxaIU, see Table of Materials) according to hospital protocols.
2. Pre- LNMCP preparation
- Following skin sterilization, establish the ports (see Table of Materials) using standard techniques7: a 10 mm umbilical port, a 5 mm port on the right side, and two left-sided ports measuring 5 mm each on the left side of the lower abdomen.
- During the operation, carefully monitor the patient's vital signs with the help of an anesthesiologist.
NOTE: During the anesthesia, continually evaluate the patient's oxygenation, ventilation, circulation, and temperature. - Maintain an intra-abdominal pressure of 12 mmHg and a gas flow rate of 20 L/min7.
3. LNMCP procedure
- Perform laparoscopic supracervical hysterectomy with bilateral salpingo-oophorectomy as previously described by Lyons et al.8.
- To perform cervical cerclage, suture, ligate, and attach a round ligament to the cervical stump using a permanent suture (size 2, see Table of Materials) in the uterine isthmus beginning at the 3 o'clock position on the right side in a counterclockwise direction, performing peripheral movements with the needle around the cervix to complete one round until the starting position (Figure 1). Tighten and secure the stitch upon completion of the cervical cerclage (Figure 2).
- Embed the suture (size 2) in the round ligament until the iliopectineal ligament (Figure 3).
- Expose the iliopectineal ligament near the landmarks, including the round ligament and the obliterated umbilical artery. Incise the peritoneum to a length of approximately 8 cm along the inner edge of the round ligament toward the pelvic wall until the iliopectineal ligament is reached. Locate the posterior aspect of the iliopectineal ligament immediately below the external iliac vein.
- Insert the suture (size 2) through the iliopectineal ligament to ensure tension-free anchoring (Figure 4).
- Repeat steps 3.2-3.5 on the other side of the pelvis.
- Perform re-peritonealization with an absorbable suture (size 2-0, see Table of Materials).
- Perform anterior and/or posterior colporrhaphy and/or anti-incontinence surgery before LNMCP, if indicated9.
NOTE: The postoperative pelvis and schematic shown in Figure 5 and Figure 6, respectively, illustrate the LNMCP technique.
4. Postoperative management
- Record the intraoperative and postoperative parameters, including blood loss based on vacuum drainage, the operation time of LNMCP, and perioperative complications.
- Document the primary outcomes, including objective and subjective success rates.
NOTE: An objective measure of surgical success is the absence of any prolapse extending beyond the hymen, an annoying bulge, or treatment for recurrent prolapse9. Subjective success is defined as Patient Global Impression (PGI) ≤2, based on the PGI Improvement Scale10. - Prescribe low-dose vaginal estriol for at least 6 weeks post-surgery.
- Ask the patient to perform pelvic floor exercises starting 8 weeks after the surgery.
- Perform follow-up after the surgery at intervals of 6 weeks, 6 months, and again 1 year later. Conduct a gynecological examination of the patients, who should complete the PGI Improvement Scale questionnaire10.
Representative Results
LNMCP was performed successfully in 16 patients during the past year. The calculated mean age was 58.5 years (SD 8.0). All women consented to visit the hospital for follow-up after a mean duration of 6.6 months (SD 3.8). The mean estimated blood loss was 73.1 mL (SD 51.1). The mean operation time was 67.8 min (SD 15.5). There were no complication. Only one patient complained of minor lower abdominal pain occasionally. The results revealed an objective success rate of 100% until the last follow-up, while the subjective success rate was 93.8% (Table 1).
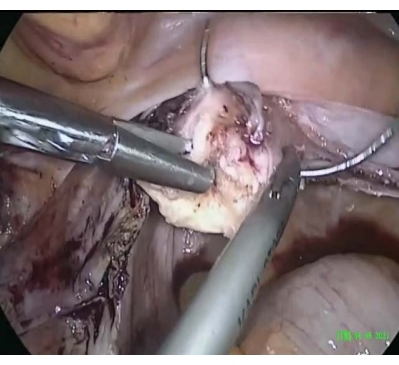
Figure 1: Cervical cerclage. Peripheral stitches were made around the cervix. Please click here to view a larger version of this figure.
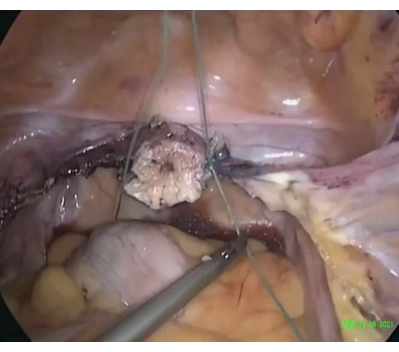
Figure 2: Completion of the cervical cerclage. The stitch was tightened and secured around the cervix, similar to the cerclage. Please click here to view a larger version of this figure.
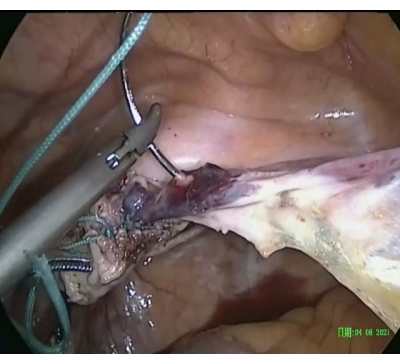
Figure 3: Suture embedded in the round ligament. The suture was passed through and embedded in the round ligament until the iliopectineal ligament. Please click here to view a larger version of this figure.
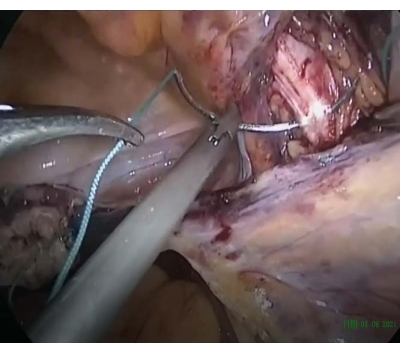
Figure 4: Suture passed through the iliopectineal ligament. The suture was passed through the iliopectineal ligament to keep a tension-free suture anchoring. Please click here to view a larger version of this figure.
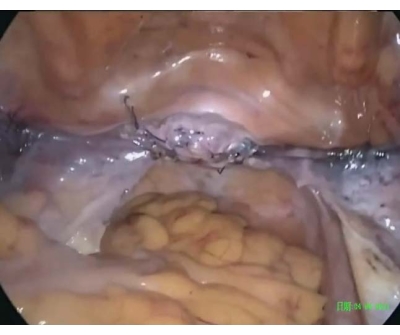
Figure 5: Postoperative pelvis. The cervix was raised to POP-Q stage 0 to avoid overcorrection and ensure tension-free anchoring. Please click here to view a larger version of this figure.
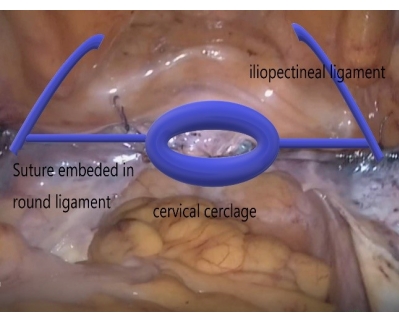
Figure 6: Schematic diagram describing the LNMCP technique. With cervical cerclage, embedding the suture in the round ligament, and fixing the suture on the iliopectineal ligament, LNMCP used only suture to treat apical prolapse and eliminated mesh-erosion complications. Please click here to view a larger version of this figure.
| Baseline demographic features of participants and the outcomes of LNMCP involving 16 cases | |
| Age | 58.5±8.0 years |
| BMI | 24.0±3.3 |
| Parity | 2.1±0.9 |
| POP-Q stage, range | 2–4 |
| Ba | 2.0±2.1 cm |
| Bp | -1.5±1.3 cm |
| C | 2.1±1.8 cm |
| Operation time | 67.8±15.5 min |
| Estimated blood loss | 73.1±51.1 ml |
| Duration of follow-up | 6.6±3.8 months |
| complications | None |
| Objective success rate | 100% |
| Subjective success rate (PGI≤ 2) | 93.80% |
Table 1: Baseline demographic features of the participants and the outcomes of LNMCP involving 16 cases. Parameters include operation time, estimated blood loss, duration of follow-up, complications, and objective and subjective success rates. BMI = body mass index. POP-Q, Ba, Bp, and C refer to scores associated with pelvic organ prolapse measurement. PGI = Patient Global Impression.
Discussion
POP is highly prevalent among women, adversely affecting the quality of patients' lives. The increase in the elderly population has contributed substantially to the rise in the number of women diagnosed with POP and individuals interested in a prophylactic intervention over the last 40 years11. Prolapse may involve cystocele associated with the anterior vaginal wall and rectocele affecting the posterior vaginal wall or the apex. POP mostly involves cystocele, and women who suffer from cystocele at or beyond the hymen mostly have apical defect concomitantly12. Therefore, interventions to restore apical support are critical in most surgical interventions for POP.
Several procedures are available to restore apical support. Laparoscopic sacrocolpopexy represents the mainstay of therapy for apical prolapse, with high success rates and long-term durability3. However, the mesh bridges the pelvic space carrying the sacrum and vagina, reducing and restricting the colon's movement, resulting in pain during defecation13. Laparoscopic pectopexy can replace sacrocolpopexy for the treatment of apical prolapse. The technique entails using the lateral components of the iliopectineal ligament to repair the prolapsed tissues. Pectopexy reduced the frequency of defecation disorders by ensuring that the pelvic space of the rectum is not decreased13.
However, both procedures use synthetic mesh for support, resulting in complications such as mesh perforation into the bladder, urinary tract, and bowel, infection, and pelvic pain5. In addition, synthetic mesh is expensive, which limits its application in areas with limited resources. Therefore, we have developed a non-mesh suspension procedure known as LNMCP.
LNMCP is based on the following key features:
Preservation of the cervix
The cervix is a key structure in the pelvic suspensory system. It provides attachment for the cardinal and uterosacral ligaments. Cervical preservation is preferred to increase the stability of the fixation in the apical region14. During a supracervical hysterectomy, the preservation of a portion of the lower uterine segment results in the formation of a mushroom-shaped cervical stump and fixation of the cervical cerclage firmly on the cervix.
Identification of iliopectineal ligament
The iliopectineal ligament is prepared starting with a surgical incision superficially adjacent to the round ligament, located next to the external iliac vessels and the obturator nerve anatomically15. The soft tissue in this area is removed to uncover the iliopectineal ligament without lacerating the corona mortis, an anastomosis between the external iliac and the obturator veins16.
Cervical cerclage
The ring suture around the cervical isthmus ensures firm fixation of the cervical cerclage to the cervix and prevents tearing of the cervical stump.
Suture embedded in the round ligament
The suspension creates significant tension between the cervical stump and the iliopectineal ligament. A naked suture with high tensile strength may damage the adjacent organs, such as the bowels and bladder. Therefore, a suture embedded in the surrounding ligament can prevent such complications.
A tension-free suspension
The cervix is raised to POP-Q stage 0 to avoid overcorrection and ensure tension-free anchoring.
This pilot study presents a new procedure known as LNMCP using only sutures to treat apical prolapse. LNMCP showed excellent safety, efficacy, and feasibility. The current data indicate favorable outcomes, with the estimated blood loss, operative time, and objective and subjective parameters of success comparable to those obtained during laparoscopic pectopexy13. Further, LNMCP avoids using a synthetic mesh, thereby eliminating any risk of related complications and reducing the medical expenses for the patients. Thus, it is easy to perform even in areas with limited resources and without access to synthetic mesh.
However, this pilot study involves a small number of cases and reports short-term results, which is a limitation. Additional studies involving large case series and extensive follow-up are required to corroborate the encouraging results.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank all the patients who consented to participate in the pilot study and the staff who assisted us with our research.
Materials
| Cefuroxime Sodium for Injection (1.5 g) | Guanzhou Baiyunshan Tianxin Pharmaceutical CO.LTD. | H20000015 | |
| ENDOPATH XCEL Trocars | Johnson & Johnson MedTech | B12LP | |
| Enoxaparin Sodium Injection (0.4 mL:4000AxaIU) | Sanofi Winthrop Industrie | H20170269 | |
| ETHIBOND EXCEL Polyester Suture | Johnson & Johnson MedTech | W4843 | ETHIBOND EXCEL GRN 4X75CM M5 |
| Laparoscopy | Stryker corporation | X 800 | |
| VICRYL (polyglactin 910) Suture | Johnson & Johnson MedTech | JV323 | VICRYL VIO 75CM M3 |
References
- Brown, H. W., et al. International urogynecology consultation chapter 1 committee 2: Epidemiology of pelvic organ prolapse: prevalence, incidence, natural history, and service needs. International Urogynecology Journal. 33 (2), 173-187 (2022).
- Karmakar, D. Commentary to "Defining normal apical vaginal support: a relook at the POSST study". International Urogynecology Journal. 30 (1), 53-54 (2019).
- Nygaard, I. E., et al. Abdominal sacrocolpopexy: A comprehensive review. Obstetrics & Gynecology. 104 (4), 805-823 (2004).
- Noé, K. G., Schiermeier, S., Alkatout, I., Anapolski, M. Laparoscopic pectopexy: A prospective, randomized, comparative clinical trial of standard laparoscopic sacral colpocervicopexy with the new laparoscopic pectopexy-postoperative results and intermediate-term follow-up in a pilot study. Journal of Endourology. 29 (2), 210-215 (2015).
- Szymczak, P., Grzybowska, M. E., Wydra, D. G. Comparison of laparoscopic techniques for apical organ prolapse repair – A systematic review of the literature. Neurourology and Urodynamics. 38 (8), 2031-2050 (2019).
- Rovner, E., de Tayrac, R., Kirschner-Hermanns, R., Veit-Rubin, N., Anding, R. Is polypropylene mesh material fundamentally safe for use as a reconstructive material in vaginal surgery: ICI-RS 2019. Neurourology and Urodynamics. 39, 132-139 (2020).
- Zeng, L., et al. Introduction of intracapsular rotary-cut procedures (IRCP): A modified hysteromyomectomy procedures facilitating fertility preservation. Journal of Visualized Experiments. (143), e58410 (2019).
- Lyons, T. L. Laparoscopic supracervical hysterectomy. Obstetrics and Gynecology Clinics of North America. 27 (2), 441-450 (2000).
- IJsselmuiden, M. v. Hysteropexy in the treatment of uterine prolapse stage 2 or higher: laparoscopic sacrohysteropexy versus sacrospinous hysteropexy-A multicentre randomised controlled trial (LAVA trial). BJOG. 127 (10), 1294 (2020).
- Chatziioannidou, K., Veit-Rubin, N., Dallenbach, P. Laparoscopic lateral suspension for anterior and apical prolapse: a prospective cohort with standardized technique. International Urogynecology Journal. 33 (2), 319-325 (2022).
- Wu, J. M., Hundley, A. F., Fulton, R. G., Myers, E. R. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetrics & Gynecology. 114 (6), 1278-1283 (2009).
- Elliott, C. S., Yeh, J., Comiter, C. V., Chen, B., Sokol, E. R. The predictive value of a cystocele for concomitant vaginal apical prolapse. The Journal of Urology. 189 (1), 200-203 (2013).
- Obut, M., Oglak, S. C., Akgol, S. Comparison of the quality of life and female sexual function following laparoscopic pectopexy and laparoscopic sacrohysteropexy in apical prolapse patients. Gynecology and Minimally Invasive Therapy. 10 (2), 96-103 (2021).
- Petros, P. The integral system. Central European Journal of Urology. 64 (3), 110-119 (2011).
- Noe, K. G., Spuntrup, C., Anapolski, M. Laparoscopic pectopexy: A randomised comparative clinical trial of standard laparoscopic sacral colpo-cervicopexy to the new laparoscopic pectopexy. Short-term postoperative results. Archives of Gynecology and Obstetrics. 287 (2), 275-280 (2013).
- Kostov, S., et al. Corona mortis, aberrant obturator vessels, accessory obturator vessels: Clinical applications in gynaecology. Folia Morphologica. 80 (4), 776-785 (2021).

