A Triple Primary Cell Culture Model of the Human Blood-Brain Barrier for Studying Ischemic Stroke In Vitro
Summary
Here, we describe the method for establishing a triple cell culture model of the blood-brain barrier based on primary human brain microvascular endothelial cells, astrocytes, and pericytes. This multicellular model is suitable for studies of neurovascular unit dysfunction during ischemic stroke in vitro or for the screening of drug candidates.
Abstract
Ischemic stroke is a major cause of death and disability worldwide with limited therapeutic options. The neuropathology of ischemic stroke is characterized by an interruption in blood supply to the brain leading to cell death and cognitive dysfunction. During and after ischemic stroke, blood-brain barrier (BBB) dysfunction facilitates injury progression and contributes to poor patient recovery. Current BBB models primarily include endothelial monocultures and double co-cultures with either astrocytes or pericytes.
Such models lack the ability to fully imitate a dynamic brain microenvironment, which is essential for cell-to-cell communication. Additionally, commonly used BBB models often contain immortalized human endothelial cells or animal-derived (rodent, porcine, or bovine) cell cultures that pose translational limitations. This paper describes a novel well-insert-based BBB model containing only primary human cells (brain microvascular endothelial cells, astrocytes, and brain vascular pericytes) enabling the investigation of ischemic brain injury in vitro.
The effects of oxygen-glucose deprivation (OGD) on barrier integrity were assessed by passive permeability, transendothelial electrical resistance (TEER) measurements,and direct visualization of hypoxic cells. The presented protocol offers a distinct advantage inmimicking the intercellular environment of the BBB in vivo, serving as a more realistic in vitro BBB model for developing new therapeutic strategies in the setting of ischemic brain injury.
Introduction
Stroke is one of the leading causes of death and long-term disability worldwide1. The incidence of stroke rapidly increases with age, doubling every 10 years after the age of 552. Ischemic stroke occurs as a result of cerebral blood flow disruption due to thrombotic and embolic events, which encompasses more than 80% of all stroke cases3. Even now, there are relatively few treatment options available to minimize tissue death following ischemic stroke. The treatments that do exist are time-sensitive and consequentially do not always lead to good clinical outcomes. Therefore, research on complex cellular mechanisms of ischemic stroke that affect poststroke recovery is urgently needed.
The BBB is a dynamic interface for the exchange of molecules between the blood and the brain parenchyma. Structurally, the BBB is comprised of brain microvascular endothelial cells interconnected by junctional complexes surrounded by a basement membrane, pericytes, and astrocytic endfeet4. Pericytes and astrocytes play an essential role in the maintenance of BBB integrity through the secretion of various factors necessary for the formation of strong, tight junctions5,6. The breakdown of the BBB is one of the hallmarks of ischemic stroke. Acute inflammatory response and oxidative stress associated with cerebral ischemia results in the disruption of tight junction protein complexes and dysregulated crosstalk between astrocytes, pericytes, and endothelial cells, which leads to increased paracellular solute permeability across the BBB7. BBB dysfunction further promotes the formation of brain edema and increases the risk of hemorrhagic transformation8. Considering all of the above, there is great interest in understanding the molecular and cellular changes that occur at the BBB level during and after ischemic stroke.
Although many in vitro BBB models have been developed over recent decades and used in a variety of studies, none of them can fully replicate in vivo conditions9. While some models are based on endothelial cell monolayers cultured on well-insert permeable supports alone or in combination with pericytes or astrocytes, only more recent studies have introduced triple cell culture model designs. Almost all existing triple culture BBB models incorporate primary brain endothelial cells along with astrocytes and pericytes isolated from animal species or cells derived from human pluripotent stem cells10,11,12,13.
Recognizing the need to better recapitulate the human BBB in vitro, we established a triple cell culture in vitro BBB model composed of human brain microvascular endothelial cells (HBMEC), primary human astrocytes (HA), and primary human brain vascular pericytes (HBVP). This triple culture BBB model is set up on 6-well plate, polyester membrane inserts with 0.4 µm pore size. These well-inserts provide an optimal environment for cell attachment and enable easy access to both apical (blood) and basolateral (brain) compartments for medium sampling or compound application. The features of this proposed triple cell culture BBB model are assessed by measuring TEER and paracellular flux post OGD mimicking ischemic stroke in vitro, with a shortage of oxygen (<1% O2) and nutrients (by using glucose-free medium) achieved by using a humidified, sealed chamber. Additionally, induced ischemic-like conditions in this model are accurately verified by direct visualization of hypoxic cells.
Protocol
NOTE: See the Table of Materials for details related to all cells, materials, equipment, and solutions used in this protocol.
1. Triple cell culture BBB model setting
- Seeding pericytes
- Cultivate HBVP in T75 culture flasks with an activated surface for cell adhesion within a 5% CO2 incubator at 37 °C until confluent. Once confluence is reached, aspirate the old pericyte medium and wash the cells with 5 mL of warm Dulbecco's phosphate-buffered saline (DPBS). Aspirate the DPBS and detach the cells from the flask using a combination of 4 mL of warm trypsin-EDTA solution and 1 mL of DPBS.
NOTE: Avoid using passages later than P7. - Incubate the flask for 5 min at 37 °C in a CO2 incubator. View under a microscope to confirm whether the cells are detached from the flask. Add 5 mL of warm pericyte medium (containing 2% fetal bovine serum [FBS]) to the flask and transfer the detached cells to a 50 mL centrifuge tube.
- Centrifuge the cell suspension for 3 min at 200 × g, allowing the cells to form a pellet in the bottom of the tube. Aspirate the medium from the tube, ensuring the cell pellet remains intact.
- Resuspend the cell pellet in pericyte medium; calculate the amount of medium depending on the confluence of the cells and the number of well-inserts needed. Take 10 µL of the resuspended cells, place them into a cell counting slide, and count the number of cells.
- Determine the cell density and seed 300,000 cells/insert in 1 mL of pericyte medium onto the abluminal side of the well-inserts (6-well format).
NOTE: When seeding HBVP on the underside of inserts, it is very important to first flip the well-insert plates upside down. While the plate is laid flat on a surface, remove the bottom section to expose the abluminal side of the well-inserts. After adding pericyte cell suspension onto the abluminal side, cover the well-inserts with the flipped plate to prevent evaporation. Keep all plates turned upside down in a 5% CO2 incubator at 37 °C overnight.
- Cultivate HBVP in T75 culture flasks with an activated surface for cell adhesion within a 5% CO2 incubator at 37 °C until confluent. Once confluence is reached, aspirate the old pericyte medium and wash the cells with 5 mL of warm Dulbecco's phosphate-buffered saline (DPBS). Aspirate the DPBS and detach the cells from the flask using a combination of 4 mL of warm trypsin-EDTA solution and 1 mL of DPBS.
- Seeding astrocytes
- Cultivate HA in T75 flasks within a 5% CO2 incubator at 37 °C until confluence is reached. Follow the above steps 1.1.1-1.1.4 using astrocyte medium (also containing 2% FBS) instead of pericyte medium.
NOTE: Avoid using passages later than P9. - Determine the cell density and seed 300,000 cells/well onto the bottom of the tissue culture 6-well plates. Cover the plate to prevent evaporation and keep all plates in a 5% CO2 incubator at 37 °C overnight.
- Cultivate HA in T75 flasks within a 5% CO2 incubator at 37 °C until confluence is reached. Follow the above steps 1.1.1-1.1.4 using astrocyte medium (also containing 2% FBS) instead of pericyte medium.
- Seeding endothelial cells
- Cultivate HBMEC in tissue culture dishes within a 5% CO2 incubator at 37 °C until confluent. Follow the above steps 1.1.1-1.1.4 using complete classic medium (containing 10% FBS) instead of pericyte medium.
NOTE: Avoid using passages later than P12. - Take out the tissue culture 6-well plates containing astrocytes and the well-inserts (6-well format) containing pericytes from the 5% CO2 incubator at 37 °C. Aspirate the astrocyte medium from the tissue culture 6-well plates. Add 1 mL of pericyte medium and 1 mL of astrocyte medium to each well.
- Aspirate the pericyte medium from the well-inserts and place them into the tissue culture 6-well plates containing the seeded astrocytes. Seed HBMEC at a density of 300,000 cells/well in 2 mL of complete classic medium onto the apical side of the same well-inserts.
NOTE: The endothelial cells should be seeded on the apical side of the well-inserts on the next day after seeding pericytes on the abluminal side of the well-inserts and astrocytes on tissue culture 6-well plates. Cells should be maintained in triple culture for 6 days to induce BBB-like properties. The cell culture medium should be changed in both well-insert compartments 24 h prior to experiments.
- Cultivate HBMEC in tissue culture dishes within a 5% CO2 incubator at 37 °C until confluent. Follow the above steps 1.1.1-1.1.4 using complete classic medium (containing 10% FBS) instead of pericyte medium.
2. Induction of the oxygen-glucose deprivation
- Wash the cells 3x with DPBS. For triple cell cultures subjected to OGD, add glucose-free medium (without L-glutamine and phenol red) to both the apical and basolateral compartments. Replace the culture medium with fresh medium in normoxic control cell cultures. Place control triple cultures in the 5% CO2 incubator at 37 °C.
NOTE: Medium changes prior to the induction of OGD or immediately after OGD might create mechanical stress that would further impact the endothelial cell monolayers. Thus, the steps with medium replacement are included for normoxic control cell cultures. - Place a Petri dish containing 20 mL of sterile water in the hypoxia incubator chamber to provide adequate humidification of the cultures. Open the chamber by releasing the ring clamp. Arrange the cell cultures on the shelves. Seal the chamber by securing the ring clamp.
- Open both the inlet and outlet ports of the chamber. Attach the tubing coming from the top of the flow meter to the chamber. Attach the tubing coming from the bottom of the flow meter to the gas tank containing the gas mixture of 95% N2/5% CO2 via an air filter.
- Open the tank flow control valve by turning it counterclockwise to allow minimum gas flow. Slowly open the pressure regulator valve by turning clockwise.
- Flush the chamber with the gas mixture at a flow rate of 20 L/min for 5 min. Disconnect the chamber from the gas source and firmly close both white plastic clamps.
- Turn off the tank flow control valve by turning clockwise. Close the pressure regulator valve by turning counterclockwise.
- Place the hypoxia chamber in a conventional incubator at 37 °C for 4 h.
NOTE: For adding subsequent reoxygenation period, wash the cells 3x with DPBS, add fresh medium in all triple cultures, and keep for an additional 24 h in the 5% CO2 incubator at 37°C. According to the manufacturer's instructions, the oxygen concentration remaining in the chamber is 0% after no less than 4 min of purging with anaerobic gas mixture at 20 L/min.
3. TEER measurements
- Place the sterilized TEER instrument into the biosafety cabinet and plug the electrodes into the epithelial voltohmmeter. Sterilize the electrodes in 30 mL of 70% isopropyl alcohol solution for a minimum of 30 min.
- Turn on the TEER instrument and set the function to ohms.
- Remove the electrodes from the 70% isopropyl alcohol solution and place them in 20 mL of DPBS for a minimum of 30 min until the digital readout on the TEER device reads 0 ohms.
- Insert the long prong of the electrode through one of the three openings in the well-insert hanger of the blank well-insert control, lowering it until it touches the bottom of the well. Ensure that the short prong is resting above the apical culture on the bottom of the well-insert.
NOTE: The blank well-insert control is comprised of 2 mL of complete classic medium in the apical compartment and a combination of 1 mL of pericyte medium and 1 mL of astrocyte medium in the basolateral compartment. Ascertain that the electrodes are held at a 90° to the well-insert while taking the TEER measurement. Using the average of two or three readings obtained in the same well (per opening) can help to decrease the variability. - Wait until the digital readout values on the TEER instrument level off before recording the value. Place the electrodes back into DPBS to wash them between measurements. Continue to collect all the TEER measurements for two more blank well-insert controls.
- Collect the TEER measurements of the sample plates using steps 3.4-3.5 taken for the control measurements. Once all measurements have been taken, place the electrode back into the 70% isopropyl alcohol solution for 30 min. Disconnect the electrodes from the TEER instrument and allow them to air-dry.
- Calculate the TEER values. Use equation (1) to subtract the mean ohm value of the blank well-insert control from the ohm value of the sample, and then multiply this resistance value by the area of the membrane insert (cm2) to give the reported TEER value in Ω∙cm2.
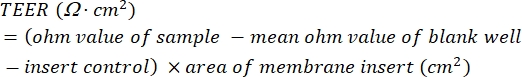 (1)
(1)
4. Assessment of the BBB paracellular permeability
NOTE: Perform all steps involving FITC-dextran in a cell culture biosafety cabinet with the lights turned off. Cover the FITC-dextran solutions with aluminum foil to minimize photobleaching.
- Prepare solutions containing FITC-dextrans of 20 and 70 kDa (0.1 mg/mL) using phenol red-free endothelial cell growth medium and allow to stir on a rocking platform shaker for 1 h. Filter the solutions using a 0.22 µm syringe filter.
- Aspirate the medium from the basolateral compartment and replace it with 2 mL of phenol red-free endothelial cell growth medium in the triple cell culture BBB model. Wash the cells in the apical compartment twice with Hanks' balanced salt solution (HBSS).
- Add 1 mL of the FITC-dextran solution in the apical compartment and cover the plate with aluminum foil. Place the plate into a 5% CO2 incubator at 37 °C for 1 h.
- Take 100 µL of medium from the basolateral compartments and transfer it into a black 96-well plate. Measure the fluorescence using a microplate reader with the excitation and emission wavelengths set to 480 nm and 530 nm, respectively.
5. Detection of hypoxia in live cells
- Seed 200 µL of HBMEC, HA, and HBVP in the center of poly-d–lysine-coated, 35 mm glass bottom dishes at a density of 150,000 cells/dish. Before seeding HBMEC, coat the bottom of the dishes with the attachment factor. Allow the cells to attach to the glass surface by leaving them overnight at 37 °C in a CO2 incubator.
NOTE: It is important to place dishes with human primary cells at the same time with a triple well-insert BBB model in a hypoxia chamber for OGD. - Once confluence is reached, discard the culture medium and add 2 mL of prewarmed glucose-free medium (for OGD) or normal medium (for controls) containing 2 µL of 1 mM Hoechst 33342 (final concentration 0.2 µM) and 0.5 µL of 5 mM stock solution of the referenced Image-iT green hypoxia reagent (final concentration 1 µM). After OGD treatment, replace the medium with imaging optimized medium in all dishes.
- Perform fluorescence live-cell imaging with the GFP filter and top-stage confocal microscope incubator as described previously14,15.
Representative Results
To examine the effects of astrocytes and pericytes on the barrier function of HBMEC, we constructed the triple cell culture BBB model on cell culture inserts (Figure 1A) along with HBMEC monoculture and two double co-culture models as controls (Figure 1B). Double co-culture controls included a non-contact co-culture of HBMEC with HA and contact co-culture of HBMEC with HBVP. After 6 days in co-culture, all experimental setups were subjected to OGD for 4 h. The barrier integrity of the endothelial monolayer in the indicated BBB configurations was assessed by determining TEER before and after OGD, as well as after 24 h of reoxygenation (Figure 1C). For 4 h, the OGD caused a significant decrease in TEER values only in HBMEC monoculture and the co-culture model with HBMEC and HBVP. These decreased levels reached the baseline levels following 24 h of reoxygenation. No effect of OGD on the co-culture model with HBMEC and HA pointed tothe protective role of astrocytes against ischemic conditions in vitro.
Although there was no difference in baseline TEER between triple and HBMEC/HBVP double co-culture models, the TEER values in the triple co-culture model were significantly higher than those in the double co-culture controls or monoculture control immediately after OGD, suggesting that integration of HA and HBVP into the triple BBB model plays a critical role in maintaining the BBB functional integrity under pathological conditions (Figure 1C). We also investigated the permeability of small (20 kDa) and large (70 kDa) molecular mass FITC-dextrans across the endothelial monolayer in the developed triple culture model. The paracellular permeability of endothelial monolayers to 20 kDa molecular mass FITC-dextran was drastically increased in HBMEC monoculture and to a lesser extent in the co-culture model with HBMEC and HBVP as compared to normoxic controls (Figure 1D). Furthermore, 20 kDa FITC-dextran permeability levels were the lowest in the triple BBB model among all models under normoxic and OGD conditions. No changes were observed in 70 kDa FITC-dextran flux across endothelial monolayers in any of the models (Figure 1E). These data suggest that ischemic-hypoxic damage was not so severe to cause changes in paracellular permeability to large molecules such as plasma proteins.
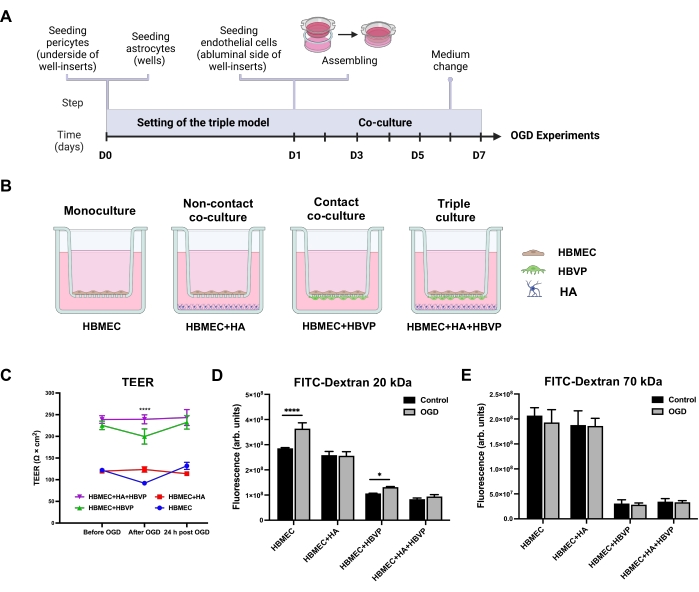
Figure 1: Effects of oxygen-glucose deprivation on transendothelial electrical resistance and endothelial permeability in human primary mono-, co-, and triple culture BBB models. (A) Schematic timeline for the setting of the triple primary cell culture BBB model. (B) Schematic representations of the configurations for in vitro static human primary cell-based BBB models. A monoculture model contains HBMEC seeded on the apical side of the well-insert porous membrane. A non-contact co-culture contains HBMEC seeded on the upper surface of the well-insert support and HA seeded at the bottom of the culture well. A contact co-culture model includes HBVP seeded on the lower surface of the well-insert support with HBMEC on the upper surface. For the triple culture model, HBMEC are seeded on the upper surface of the support with HBVP seeded on the lower surface and HA seeded on the bottom of the culture wells. (C) Changes in TEER monitored immediately before OGD, after 4 h of OGD, and after 24 h of reoxygenation. Data represented as mean ± SEM (n = 5-6). ****P < 0.0001 compared with mono- and co-culture models (two-way ANOVA). (D) Paracellular permeability to 20 kDa FITC-dextran across endothelial monolayers was measured after 4 h of OGD. (E) Paracellular permeability to 70 kDa FITC-dextran across endothelial monolayers was measured after 4 h of OGD. Data represented as mean ± SD (n = 5-6). *P < 0.05. ****P < 0.0001 (two-way ANOVA). Abbreviations: OGD = oxygen-glucose deprivation; TEER = transendothelial electrical resistance; HBMEC = human brain microvascular endothelial cells; HA = human astrocytes; HBVP = human brain vascular pericytes; FITC-dextran = dextran conjugated to fluorescein isothiocyanate; arb. units = arbitrary units. Please click here to view a larger version of this figure.
To verify OGD-induced hypoxic injury, HBMEC, HA, and HBVP were cultured in the wells of 35mm glass bottom dishes and loaded with the hypoxia reagent, whose fluorescent signal increased with reduced oxygen levels. All primary human types exposed to OGD exhibited strong green fluorescence, proving that the imaged live cells were hypoxic (Figure 2). After estimating the effects of having different perivascular cell types (astrocytes and pericytes), or their combination in the BBB model on the barrier properties following an OGD protocol, we concluded that the triple model was superior to the other BBB models tested.
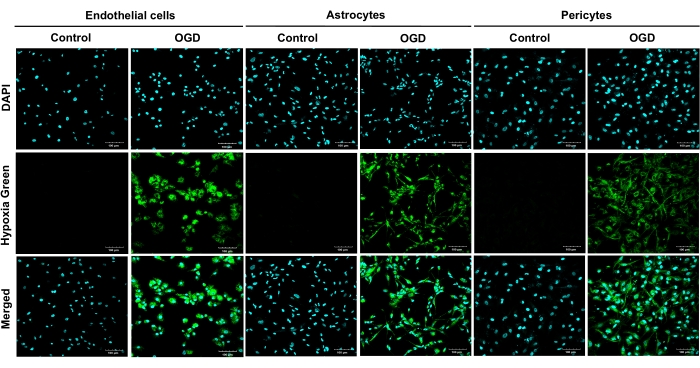
Figure 2: Live staining of hypoxic primary human brain microvascular endothelial cells, human astrocytes, and human brain vascular pericytes following 4 h of oxygen-glucose deprivation. OGD exposures were carried out on all primary cells (HBMEC, HA, and HBVP) using a hypoxia reagent and glucose-free medium for 4 h. Results from four independent experiments are shown. Cultures were imaged at 20x magnification. Scale bars = 100 μm. Abbreviations: OGD = oxygen-glucose deprivation; HBMEC = human brain microvascular endothelial cells; HA = human astrocytes; HBVP = human brain vascular pericytes; DAPI = 4',6-diamidino-2-phenylindole. Please click here to view a larger version of this figure.
Discussion
In this protocol, we describe a method to set up a reliable triple endothelial cell-pericyte-astrocyte culture BBB model for studying BBB dysfunction in the setting of ischemic stroke in vitro. Considering that pericytes are the nearest neighbors of endothelial cells in vivo, HBVP are plated on the underside of the well-inserts in this model16. Although this configuration lacks the direct cell-to-cell communication between astrocytes and endothelial cells, this arrangement allows for indirect cell-to-cell communication between cell types via secreted soluble factors. The presence of astrocytes and pericytes in this system greatly improves HBMEC integrity, limiting paracellular permeability of the monolayer to small size tracers. Additionally, astrocytes and pericytes have a protective effect on HBMEC in OGD conditions.
In the developed protocol, we optimized the cell ratio (1:1:1) and culture medium mixture for the basolateral compartment. The triple BBB model displayed almost 2.5x higher TEER values (239 ± 9 Ω·cm2) than the control HBMEC monoculture (112 ± 11 Ω·cm2) after 6 days of co-culturing, the time necessary for the proper HBMEC monolayer formation17,18. The TEER values achieved in this triple model are comparable to those in the other previously described triple model, in which HA were in contact with HBMEC and HBVP were seeded on the bottom of the plate wells19. In the other human primary cell triple culture model with the same cell configuration and OGD exposure time, the baseline TEER values were substantially lower than those in this work and did not reach the standard levels (100 Ω·cm2)20. The use of 6-well well-insert plates with larger compartments allowed us to decrease the medium change frequency to avoid the disruption of substance exchange between the cell populations.
OGD experiments were performed using a sealed hypoxia incubator chamber containing an anaerobic gas mixture (95% nitrogen and 5% carbon dioxide). One of the main advantages of this protocol is that the severity of the ischemic injury can be easily adjusted to specific needs by varying the length of the OGD period. After evaluating the TEER and dextran permeability, 4 h of OGD did not compromise BBB integrity in the constructed triple model. Therefore, to study ischemic stroke in vitro, longer exposure to OGD needs to be applied to induce BBB breakdown. Live-cell imaging, provided in the protocol, allows real-time monitoring of different cell types and verification of OGD injury, which represents an advantage in many studies.
In this protocol, 0.4 µm polyester well-inserts were chosen as a basis for this triple BBB model. A polyester membrane is considered the best insert type for cell visualization in fluorescence-imaging applications, which provides an excellent opportunity to visualize modifications of tight junction proteins and transporters if needed. While the selected small pore diameter creates a low permeability to small hydrophilic molecules across endothelial monolayers, it also limits the potential application of the present triple BBB model for transendothelial migration assays, in which 3 µm or 8 µm pore sizes are required.
Finally, another limitation of this model can be related to the lack of shear stress, which has been shown to promote the formation of tight junctions in dynamic triple BBB in vitro systems21. In conclusion, the established robust and physiologically relevant triple culture BBB model, composed of primary human cells, represents a valuable tool for drug screening and studying BBB dysfunction associated with ischemic stroke.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grants MH128022, MH122235, MH072567, MH122235, HL126559, DA044579, DA039576, DA040537, DA050528, and DA047157.
Materials
| 24 mm Transwell with 0.4 µm Pore Polyester Membrane Insert | Corning | 3450 | |
| 35 mm Glass Bottom Dishes | MatTek Life Sciences (FISHERSCI) | P35GC-1.5-14-C | |
| Astrocyte Medium | Science Cell | 1801 | |
| Attachment Factor | Cell Systems (Fisher Scientific) | 4Z0-201 | |
| BD 60 mL Syringe | BD | 309653 | |
| BrainPhys Imaging Optimized Medium | STEMCELL Technologies | 5791 | |
| Complete Classic Medium With Serum and CultureBoost | 4Z0-500 | Cell Systems | |
| Corning 50 mL PP Centrifuge Tubes (Conical Bottom with CentriStar Cap | VWR | 430829 | |
| Corning 75cm² U-Shaped Canted Neck Not Treated Cell Culture Flask | Corning | 431464U | |
| Corning CellBIND 96-well Flat Clear Bottom Black Polystyrene Microplates | Corning | 3340 | |
| Countes Cell Counting Chamber Slides | Thermo Fisher Scientific | C10228 | |
| Countess II FL Automated Cell Counter | Thermo Fisher Scientific | ZGEXSCCOUNTESS2FL | |
| Decon CiDehol 70 Isopropyl Alcohol Solution | Fisher Scientific | 04-355-71 | |
| Disposable Petri Dishes | VWR | 25384-088 | |
| DMEM Medium (No glucose, No glutamine, No phenol red) | ThermoFisher | A14430-01 | Glucose-free medium |
| DPBS (No Calcium, No Magnesium) | ThermoFisher | 14190250 | |
| EBM Endothelial Cell Growth Basal Medium, Phenol Red Free, 500 mL | Lonza | CC-3129 | |
| EVOM2 Epithelial Volt/Ohm (TEER) Meter with STX2 electrodes | World Precison Instruments | NC9792051 | Epithelial voltohmmeter |
| Fluorescein isothiocyanate–dextran (wt 20,000) | Millipore Sigma | FD20-250MG | |
| Fluorescein isothiocyanate–dextran (wt 70,000) | Millipore Sigma | FD70S-250MG | |
| Fluorview FV3000 Confocal Microscope | Olympus | FV3000 | |
| Gas Tank (95% N2, 5% CO2) | Airgas | X02NI95C2003071 | |
| HBSS (No calcium, No magnesium, no phenol red) | Thermofisher | 14025092 | |
| Hoechst 33342, Trihydrochloride, Trihydrate – 10 mg/mL Solution in Water | ThermoFisher | H3570 | |
| Human Astrocytes | Science Cell | 1800 | |
| Human Brain Vascular Pericytes | Science Cell | 1200 | |
| Hypoxia Incubator Chamber | STEMCELL Technologies | 27310 | |
| Image-iT Green Hypoxia Reagent | ThermoFisher | I14834 | |
| Pericyte Medium | Science Cell | 1201 | |
| Primary Human Brain Microvascular Endothelial Cells | ACBRI 376 | Cell Systems | |
| Rocking Platform Shaker, Double | VWR | 10860-658 | |
| Single Flow Meter | STEMCELL Technologies | 27311 | |
| SpectraMax iD3 Microplate Reader | Molecular Devices | 75886-128 | |
| Syringe Filter, 25 mm, 0.22 μm, PVDF, Sterile | NEST Scientific | 380121 | |
| TPP Mutli-well Plates (6 wells) | MidSci | TP92406 | |
| TPP Tissue Culture Flasks T-75 Flasks | MidSci | TP90075 | Flasks with activated surface for cell adhesion |
| Trypsin-EDTA (0.25%), phenol red | ThermoFisher | 25200056 | |
| UltraPure Distilled Water | Invitrogen (Life Technologies) | 10977-015 | |
| Uno Stage Top Incubator- | Oko Lab | UNO-T-H-CO2-TTL |
References
- Mozaffarian, D., et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 133 (94), 38 (2016).
- Yousufuddin, M., Young, N. Aging and ischemic stroke. Aging. 11 (9), 2542-2544 (2019).
- Donkor, E. S. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Research and Treatment. , 3238165 (2018).
- Kadry, H., Noorani, B., Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS. 17 (1), 69 (2020).
- Brown, L. S., et al. Pericytes and neurovascular function in the healthy and diseased brain. Frontiers in Cellular Neuroscience. 13, 282 (2019).
- Cabezas, R., et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Frontiers in Cellular Neuroscience. 8, 211 (2014).
- Abdullahi, W., Tripathi, D., Ronaldson, P. T. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. American Journal of Physiology-Cell Physiology. 315 (3), 343-356 (2018).
- Candelario-Jalil, E., Dijkhuizen, R. M., Magnus, T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 53 (5), 1473-1486 (2022).
- He, Y., Yao, Y., Tsirka, S. E., Cao, Y. Cell-culture models of the blood-brain barrier. Stroke. 45 (8), 2514-2526 (2014).
- Thomsen, L. B., Burkhart, A., Moos, T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS One. 10 (8), 0134765 (2015).
- Song, Y., Cai, X., Du, D., Dutta, P., Lin, Y. Comparison of blood-brain barrier models for in vitro biological analysis: one cell type vs three cell types. ACS Applied Bio Materials. 2 (3), 1050-1055 (2019).
- Xu, L., et al. Silver nanoparticles induce tight junction disruption and astrocyte neurotoxicity in a rat blood-brain barrier primary triple coculture model. International Journal of Nanomedicine. 10, 6105-6118 (2015).
- Appelt-Menzel, A. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Reports. 8 (4), 894-906 (2017).
- Zhang, Y., et al. Rational construction of a reversible arylazo-based NIR probe for cycling hypoxia imaging in vivo. Nature Communications. 12 (1), 2772 (2021).
- Palacio-Castañeda, V., Kooijman, L., Venzac, B., Verdurmen, W. P. R., Le Gac, S. Metabolic switching of tumor cells under hypoxic conditions in a tumor-on-a-chip model. Micromachines. 11 (4), 382 (2020).
- Ramsauer, M., Krause, D., Dermietzel, R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB Journal. 16 (10), 1274-1276 (2002).
- Lyck, R., et al. ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood-brain barrier. Journal of Cerebral Blood Flow & Metabolism. 37 (8), 2894-2909 (2017).
- Rizzi, E., et al. A triple culture cell system modeling the human blood-brain barrier. Journal of Visualized Experiments. (177), (2021).
- Kumar, S., Shaw, L., Lawrence, C., Lea, R., Alder, J. P50: Developing a physiologically relevant blood brain barrier model for the study of drug disposition in glioma. Neuro-Oncology. 16 (6), (2014).
- Stone, N. L., England, T. J., O’Sullivan, S. E. A novel transwell blood brain barrier model using primary human cells. Frontiers in Cellular Neuroscience. 13, 230 (2019).
- Al Ahmad, A., Taboada, C. B., Gassmann, M., Ogunshola, O. O. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. Journal of Cerebral Blood Flow & Metabolism. 31 (2), 693-705 (2011).

