Screening Sperm for the Rapid Isolation of Germline Edits in Zebrafish
Summary
CRISPR-Cas technologies have revolutionized the field of genome editing. However, finding and isolating the desired germline edit remains a major bottleneck. Therefore, this protocol describes a robust method for quickly screening F0 CRISPR-injected zebrafish sperm for germline edits using standard PCR, restriction digest, and gel electrophoresis techniques.
Abstract
The advent of targeted CRISPR-Cas nuclease technologies has revolutionized the ability to perform precise genome editing in both established and emerging model systems. CRISPR-Cas genome editing systems use a synthetic guide RNA (sgRNA) to target a CRISPR-associated (Cas) endonuclease to specific genomic DNA loci, where the Cas endonuclease generates a double-strand break. The repair of double-strand breaks by intrinsic error-prone mechanisms leads to insertions and/or deletions, disrupting the locus. Alternatively, the inclusion of double-stranded DNA donors or single-stranded DNA oligonucleotides in this process can elicit the inclusion of precise genome edits ranging from single nucleotide polymorphisms to small immunological tags or even large fluorescent protein constructs. However, a major bottleneck in this procedure can be finding and isolating the desired edit in the germline. This protocol outlines a robust method for screening and isolating germline mutations at specific loci in Danio rerio (zebrafish); however, these principles may be adaptable in any model where in vivo sperm collection is possible.
Introduction
The CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)/Cas system is a powerful tool to perform loci-specific mutagenesis and precise genome editing in the Danio rerio (zebrafish) model system1,2,3,4. The Cas-ribonucleoprotein (RNP) is comprised of two main components: a Cas endonuclease (commonly Cas9 or Cas12a) and a locus-specific synthetic guide RNA (sgRNA)5. Together, the Cas-RNP generates a double-stranded break (DSB) in the desired locus that can be repaired by one of two intrinsic repair mechanisms. The non-homologous end joining (NHEJ) repair mechanism is error-prone and often results in a variety of insertions or deletions (indels) around the DSB. These indels can be deleterious if they introduce a frameshift mutation or premature stop in the resultant protein sequence. Alternatively, the homology-directed repair (HDR) mechanism uses a donor template with regions of homology surrounding the DSB site to repair the damage. Researchers can take advantage of the HDR system to generate precise genomic edits. Specifically, they can co-inject a double-stranded DNA donor construct that contains the desired edits as well as regions of homology flanking the DSB site in the genome. The increased economy of scale for these commercially produced CRISPR components has greatly reduced the barriers to screening multiple loci and to setting up larger-scale efforts for precise genome editing. However, in sexually reproducing animal models, a major bottleneck is the identification and isolation of germline-stable mutant animals.
The zebrafish model system exhibits several key qualities that enhance its use in reverse genetic studies. They are easy to rear in large numbers with basic aquatic housing equipment, and females exhibit high fecundity all year round6. Moreover, their external egg-laying and fertilization make them amenable to the microinjection of CRISPR/Cas components. The Cas-RNP is commonly injected into one-cell stage zebrafish embryos to generate DSBs/repair that is, in theory, inherited by all the daughter cells. However, diploid genomes require two DSB/repair events to mutagenize both homologous chromosomes. Furthermore, although Cas-RNP is injected at the one-cell stage, the DSB/repair may not occur until later points in development. Together, these factors contribute to the mosaic nature of F0-injected fish. A common practice is to outcross F0-injected fish and screen the F1 progeny for indels/specific edits. However, since not all F0-injected fish possess germline mutations, this practice results in many unproductive crosses that do not generate the desired edit. Screening the F0 germline rather than F1 somatic tissue increases the probability of isolating the desired germline edit and reduces the number of animals required in this process.
Sperm can easily be collected from F0-injected zebrafish without the need for euthanasia. This feature allows for the cryopreservation and rederivation of frozen sperm stocks7 but can also be exploited to rapidly screen, identify, and isolate the germline carriers of desired genomic mutations8,9. Brocal et al. (2016) previously described a sequencing-based method for screening germline edits in F0-injected male zebrafish10. Although useful for identifying the mutated alleles present in the germline, this approach can become costly in high throughput and may not be accessible for all labs. In contrast, the current protocol offers an approachable and cost-effective electrophoresis-based strategy for identifying germline edits. Specifically, this protocol outlines a robust method for screening and isolating germline mutations at specific loci using high-resolution agarose gel electrophoresis. In addition, this protocol describes a similar strategy for identifying the successful integration of a donor construct containing specific edits. As always, if specific edits are desired, sequencing-based strategies can be performed in tandem with the protocol described below. Although this protocol is specific to the zebrafish model system, these principles should be adaptable to any model in which the collection of sperm is a routine procedure. Together, these strategies will allow for the identification of F0-injected males with germline indels/edits that can be resolved on a gel after standard polymerase chain reaction (PCR) and/or restriction digest.
Protocol
This study was carried out in line with the guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University of Texas at Austin Animal Care and Use Committee (AUP-2021-00254).
1. Designing the sgRNA for CRISPR mutagenesis
- Obtain the exon sequence containing the targeted loci.
- Design a synthetic guide RNA (sgRNA) with a protospacer adjacent motif (PAM) site that is specific to the Cas endonuclease.
- If co-injecting a donor construct, design the sgRNA to have a predicted cut site as close to the desired edits as possible.
NOTE: APE is a free software with a tool to find Cas9 sgRNA sites11. Alternatively, tools such as CHOPCHOP can be used, which offers an online interface for designing sgRNAs that considers the different PAM sites of Cas9 and other Cas endonucleases12.
- If co-injecting a donor construct, design the sgRNA to have a predicted cut site as close to the desired edits as possible.
- Design forward and reverse primers to amplify the region surrounding the designed CRISPR/Cas cut site. The PCR product should be approximately 200-400 base pairs (bp) in length to be able to resolve small indels on a gel later in the protocol.
NOTE: CHOPCHOP11 automatically designs primers for every sgRNA it outputs. - Optional: Design a DNA donor construct with the desired edit(s).
- Obtain the genomic sequence of the exon containing the loci of the desired mutation.
NOTE: Intronic sequences can vary between individuals, so it is best to avoid the inclusion of intronic sequences in the donor construct as this could interfere with the homology-dependent repair pathway. - If a specific amino acid change is desired, alter the specific codon accordingly.
NOTE: When making these changes, be aware of the codon frequency. If possible, choose a codon with high usage. - To prevent CRISPR/Cas digestion of the donor construct, make additional synonymous mutations that (i) disrupt the PAM and/or (ii) disrupt the sgRNA sequence, with higher preference being placed on making non-synonymous changes closer to the PAM site.
- Use a restriction site finder (APE11 also has this feature) to find unique restriction sites (those that only occur once) in the predicted PCR product of the wild-type sequence.
- Repeat this process for the predicted PCR product that reflects the successful integration of the donor construct.
- Compare the list of restriction sites between the wild-type and donor-integrated sequences to find a restriction site that is present only in the edited sequence.
- If no unique restriction site is found, continue making synonymous changes within the donor construct until this is achieved.
NOTE: If possible, continue making synonymous mutations that would disrupt the sgRNA binding to the donor construct.
- Trim the donor sequence to a manageable size (50-100 bp) with at least 20 bp of homology surrounding the predicted CRISPR/Cas cut site and edits, if possible.
- Obtain the genomic sequence of the exon containing the loci of the desired mutation.
- Obtain the designed sgRNA and/or DNA donor oligonucleotides, as well as any primers required for genotyping.
NOTE: To prevent the digestion of the DNA donor oligonucleotides by nucleases, it is recommended to modify the first three phosphate bonds on the 5' and 3' ends of the DNA oligonucleotides with phosphorothioate. - Inject 1 nL of the injection mix into one-cell stage embryos. Typically, a standard injection mix contains the following:
1 µL of 25 µM locus-specific sgRNA
1 µL of 25 µM Cas9 endonuclease
Optional: 1 µL of 3 µM DNA donor construct- Bring the total volume to 5 µL with 0.1 M RNase-free KCl.
- Allow the F0-injected individuals to develop into sexually mature adults. The remaining protocol focuses on screening the F0-injected males for germline edits.
2. Setting up the breeding tanks
- Prime F0 males for mating by setting up breeding tanks with dividers between the F0 males and females.
- To minimize the number of breeding tanks required, set up three males across from two females in each tank.
- Set up as many F0 males as can effectively be screened and housed as individuals after sperm collection. The genotype of the females is not important since their gametes will not be collected during this procedure.
- Set up one breeding tank of wild-type males across from females. Wild-type sperm will serve as a control for the interpretation of the results later in the procedure.
- To minimize the number of breeding tanks required, set up three males across from two females in each tank.
- Incubate the prepared breeding tanks overnight.
3. Preparing the materials for the sperm collection procedure
- Prepare enough anesthetizing solution (0.016% tricaine-HCl in fish water) to fill a small glass bowl.
- Dispense 40 µL of 50 mM NaOH into 0.2 mL PCR tubes. Prepare one tube for each sperm sample that will be collected. Store on ice.
- Prepare individual 0.8 L tanks for each fish that will be sampled during the procedure. These tanks will house the sampled fish until the genotyping is complete.
NOTE: This is the primary factor that limits the number of fish that can be effectively screened in this protocol. Ensure adequate space for housing the sampled individuals until the genotyping is complete. - Moisten a 1-inch x 1-inch sponge (cut with a shallow, oval-shaped divot to hold a male zebrafish ventral side up during the procedure) in fish water and squeeze out excess liquid.
- Position the sponge in the field of view of a stereomicroscope at low magnification (Figure 1A).
- Position oblique overhead lighting for optimal viewing.
4. Anesthetizing the male fish and collecting the sperm
- Prepare a clean capillary tube.
- Gently shake the tube container to release a single capillary tube.
- Place the capillary tube into the provided bulb, and set it aside.
- Transfer a male fish into the prepared anesthetizing solution (0.016% tricaine-HCl in fish water).
- Gently stir the solution with a slotted spoon to speed up the process of anesthesia.
- Once the operculum (gill) movement slows down (approximately 1-2 min), proceed with the sperm collection.
- Use the slotted spoon to transfer the fish onto a clean stack of paper towels.
- Use the slotted spoon to gently roll the fish to remove excess water.
- Gently blot the fish with clean, folded tissue paper to remove any remaining water.
- Use the slotted spoon to transfer the fish to the prepared sponge (Figure 1B).
- Position the fish ventral side up with its head nearest the dominant hand of the person performing the procedure.
- Gently blot the area around the anal fins with clean, folded tissue paper.
- Collect the sperm.
- Using the end of the capillary tube, gently move the pelvic fins laterally away from the midline to expose the cloaca (Figure 1C).
- Position the end of the capillary tube near the cloaca.
- Using the forceps, gently squeeze the sides of the fish beginning just below the gills and ending at the cloaca (Figure 1C').
- Collect sperm into the capillary tube by capillary action. Approximately 1 µL is sufficient for this procedure (Figure 1D).
NOTE: Some labs report using p10 pipet tips instead of capillary tubes for sperm collection. - If sperm is not released with gentle pressure, return the fish to the system water.
- Monitor for recovery from the anesthesia.
- Do not squeeze the fish hard to expel sperm, as this can injure the fish.
- Rest the fish for 2 weeks before attempting to collect sperm again.
- After collection, place the fish into a clean isolation tank with system water for individual housing.
- Monitor the fish for recovery from the anesthesia.
- Rest the fish for 2 weeks before attempting to collect sperm again.
- Place the capillary tube with the collected sperm into the prepared PCR tube (with 40 µL of 50 mM NaOH).
- Squeeze the rubber bulb to expel the collected sample.
- Dispose of the used capillary tube into an approved glass waste container.
- Incubate the samples on ice until all the males have been squeezed.
- Repeat steps 4.1-4.5 for each individual male.
- Label the isolation tanks and sperm samples such that the final sperm genotyping results can be traced back to the corresponding individual with putative mutations.
- Monitor all the individuals, and make sure they are upright and exploring their tanks before putting the tanks back on the system.
- Put the females used for priming back into their respective tanks on the system.
- If any fish does not recover from the anesthesia after 10 min, follow the euthanasia procedure approved by the institution associated with the lab.
- Example: Rapid chill the individual in an ice bath with fish water that is 2-4 °C.
- Dispose of the euthanized fish in an appropriate animal carcass waste container according to the procedure approved by the institution associated with the lab.
5. Extracting DNA from the sperm samples
- Briefly spin down all the sperm samples in a minicentrifuge, and place PCR tubes into a thermal cycler.
- Close the lid of the thermal cycler.
- Run the following settings in the thermal cycler:
- Heat the samples for 40 min at 95 °C.
- Cool the samples to 25 °C.
- Remove the samples from the thermal cycler.
- With a clean pipette tip for each sample, neutralize the pH by adding 10 µL of 1 M Tris-HCl (buffered to pH 8).
- Mix well by pipetting up and down.
- Briefly spin down the samples in a minicentrifuge.
- Genomic DNA samples can be stored for several days at 4 °C or placed at −20 °C for up to 6 months.
6. PCR amplification (and/or restriction digest) of the desired locus
- Amplify the desired locus using a standard PCR protocol.
- Preheat the thermal cycler to the initial denaturation temperature in the PCR reaction below.
- Prepare a 25 µL reaction mixture for each sperm sample in PCR tubes:
12.5 µL of 2x Taq Polymerase Master Mix
1.5 µL of 10 µM forward primer
1.5 µL of 10 µM reverse primer
4.5 µL of nuclease-free water
5 µL of neutralized sperm DNA sample - Mix well by pipetting up and down.
- Briefly spin down the samples in a minicentrifuge.
- Place the samples in the pre-heated thermal cycler with the following settings:
Initial denaturation: 95 °C for 3 min
35 cycles of denaturation (95 °C for 30 s), annealing (55 °C for 30 s), and extension (72 °C for 30 s)
Final extension: 72 °C for 5 min
NOTE: It may be necessary to adjust the extension temperature and/or time depending on the specific Taq polymerase and the expected length of the PCR amplification product. - Remove the PCR samples from the thermal cycler.
- Optional: Perform the donor-specific restriction digest on a small volume of the PCR product, leaving at least 5-10 µL of undigested PCR product for gel electrophoresis.
- Prepare a 30 µL reaction in 0.2 mL PCR tubes:
10 µL of unpurified PCR product
15 µL of nuclease-free water
3 µL of 10x restriction enzyme buffer
2 µL of restriction enzyme (BstNI is used in the representative results for analyzing the dnah10 knock-in allele)
NOTE: Many restriction enzymes are still active in a typical PCR reaction buffer; however, the addition of restriction digest buffer can help improve the results. Refer to specific manufacturer instructions for troubleshooting if any problems occur. - Mix well by pipetting up and down.
- Briefly spin down the samples in a minicentrifuge.
- Place the samples in a thermal cycler with the following settings:
- Cut the PCR amplicon at 60 °C for 1 h.
NOTE: The cut temperature and incubation time may need to be adjusted for the specific restriction enzyme being used. - Inactivate the restriction enzyme with the required temperature and incubation time, if indicated by the manufacturer.
NOTE: The BstNI enzyme does not require heat inactivation.
- Cut the PCR amplicon at 60 °C for 1 h.
- Prepare a 30 µL reaction in 0.2 mL PCR tubes:
7. Performing gel electrophoresis to separate PCR amplicons of varying sizes
- Prepare 500 mL of 0.5x TBE running buffer: add 50 mL of 10x TBE buffer concentrate to 450 mL of deionized water.
- Prepare 100 mL of 4% agarose gel in 0.5x TBE running buffer: add 4 g of agarose with 10 µL of 10,000x gel stain to 100 mL of 0.5x TBE running buffer. Microwave until the agarose powder is fully dissolved.
- Pour the gel solution into an appropriately sized gel casting frame, and insert the comb on one side of the gel.
NOTE: A larger gel size (recommended: 15 cm x 15 cm) is advantageous for this procedure, as it provides the amplicons with more space to resolve on the gel.- If a restriction digest product is to be analyzed, it is best to run it on the same gel as the PCR product so that the results can be compared. Adjust the comb well size as necessary to fit all the samples on the gel.
- Leave the gel at room temperature until solidified. Carefully remove the comb. Carefully remove the gel from the casting frame.
- Pour the gel solution into an appropriately sized gel casting frame, and insert the comb on one side of the gel.
- Set up the gel electrophoresis rig.
- Position the gel in the electrophoresis rig such that the wells are nearest the negative electrode. Pour 0.5x TBE running buffer into the electrophoresis rig until the wells are fully submerged.
- Load the gel.
- Load 5 µL of DNA ladder into the first well (choose a DNA ladder that contains DNA fragments that are similar in size to the PCR amplicon).
- Using a new pipette tip for each sample (including the wild-type control sample), load 5-10 µL of the PCR product into the remaining wells.
- Optional: If a restriction digest product is to be analyzed, load these samples onto the gel as well.
NOTE: It is best to load equal amounts of DNA between the PCR and the restriction digest product. For example, in the 30 µL restriction digest reaction mixture, 10 µL of PCR product was added. Therefore, the DNA concentration of the 15 µL of digest product was comparable to the 5 µL of unpurified PCR product.
- Run the gel.
- Connect the electrodes to the gel electrophoresis rig, and apply 150 V for at least 1 h to ensure adequate separation of the amplicons.
- Image the gel to view the DNA bands.
NOTE: Strongly mutagenized CRISPR-targeted loci will run as multiple band sizes representing a combination of insertion/deletion alleles with the wild-type amplicons.- If the gel bands are not resolved after 1 h, apply 150 V to the gel for 15 min intervals until the bands are sufficiently separated on the gel.
- Optional: If screening for the insertion of a precise edit such as an SNP, epitope tag, or fluorescent tag, screen the sperm DNA with PCR methods that are specific to the desired edit. For example, when identifying a donor-specific restriction site, an additional band will be present in the restriction digest product compared to the PCR product of the sperm sample.
8. Isolating germline stable alleles
- After 2 weeks of rest from the sperm squeezing procedure, outcross the F0 individuals whose sperm genomic DNA contained the desired edits.
NOTE: Sperm generation from individual spermatogonia clones may be cyclical in zebrafish; thus, the desired allele might not be producing sperm at the time of the outcross. For this reason, additional crossing may have to be performed more than once to isolate the allele implicated by the initial screening. - Once the F1 outcrossed fish are sexually mature, proceed with standard genotyping methods (for example, fin-clip DNA) using the same PCR and gel electrophoresis protocol performed during the screening of the sperm genomic DNA.
- Sanger sequence the PCR products to identify the F1 individuals with the same desired edit.
- Optional: If the desired edit introduces or disrupts a restriction site, screen with an appropriate restriction digest protocol.
- In-cross F1 heterozygotes with the same desired edit.
- Screen the F2 progeny for the expected phenotype and/or genomic edits.
Representative Results
The experimental approaches described in this protocol allow for the more rapid identification of genome edits or putative deleterious alleles by focusing on the analysis of thousands of genomes derived from the collection of F0-injected male sperm. Figure 2 highlights how to interpret the results obtained using this protocol.
To generate mutations in the p2ry12 locus, one-cell stage zebrafish embryos were injected with Cas9 endonuclease and a p2ry12-specific sgRNA (Figure 2B, gray highlight). F0-injected fish were put in the system until sexual maturity, and sperm was collected from six individual males. DNA was extracted from the sperm samples using high heat and basic conditions (HotSHOT DNA extraction) and neutralized prior to the PCR amplification of the p2ry12 locus. The PCR products were separated on a high-percentage (4%) agarose gel for 1.5 h at a high voltage (150 V). In this example, the wild-type amplicon ran as a single bright band of around 250 base pairs in length (Figure 2C; WT). In contrast, the F0-injected male amplicons containing indels ran on the gel as multiple bands (Figure 2C; lanes #1-2, #5-8). Alternatively, the mutated F0-injected male amplicons could run as a single band with altered gel mobility (not shown in the p2ry12 example, but evident in the PCR product of the dnah10 F0-male #2; Figure 2E). It was less clear on the p2ry12 example gel whether sample #3 and sample #4 contained indels in comparison to the wild-type band, so these individuals may not be the best founder candidates. The isolation of alleles from F0 males with more distinctive amplicon alterations is best for propagating stable germline carriers as they are easily scored on 4% agarose gel. For example, the F0-male #6 sperm sample appeared to contain a large deletion in one of the alleles (Figure 2C; lane 6; bright band with increased gel mobility). If this large deletion allele was selected for in the F1 generation, it would be easily distinguishable from the WT allele on a 4% agarose gel. Alternatively, if a collection of different alleles is desired, the F0-male #7 could be an effective founder candidate since its PCR product appeared to contain several discrete alleles of various mobilities. Once a founder male is selected, the desired allele can be isolated by outcrossing the individual back to the original wild-type strain used for injections.
To generate a specific knock-in mutation in the dnah10 gene, one-cell stage zebrafish embryos were injected with Cas9 endonuclease, a dnah10-specific sgRNA (Figure 2D; gray highlight), and a DNA donor oligonucleotide containing the desired edit (Figure 2D; red) and a donor-specific BstNI restriction site (Figure 2D, brackets). This design allows for the easy identification of donor integration with a restriction digest after PCR. Furthermore, by altering base pairs within the sgRNA recognition site (Figure 2D; gray highlight), this design prevents the Cas9 digestion of the donor sequence. Once the F0-injected fish reached sexual maturity, sperm was collected, and DNA was extracted using the hot shot method. From these samples, the dnah10 locus was amplified using PCR, and the products were separated on a high-percentage (4%) agarose gel for 1 h at a high voltage (150 V). In this example, the wild-type amplicon ran as a single bright band of around 400 base pairs in length (Figure 2E; top panel: WT). In contrast, the F0-injected male amplicons containing indels ran as multiple bands (Figure 2E; top panel: lanes #1, #4, and #5) or as a single band with decreased gel mobility (Figure 2E; top panel: lane #2). To determine if any of the sperm samples contained donor-integrated alleles, the PCR products were digested with the BstNI restriction enzyme for 1 h, and the product was run on a 4% agarose gel for 1 h at high voltage (150 V). Comparing the undigested PCR product (Figure 2D; top panel) to the digested product (Figure 2D; bottom panels) reveals samples with likely integration of the donor construct. In this example, the F0-injected male #1 had an additional band in the digested product that was not present in the PCR product (Figure 2D; bottom panel: lane #1, circled). Therefore, this male represents the best founder candidate for establishing a mutant line with the desired knock-in.
Once the putative F0 carrier male is outcrossed, the allele must be sequence-verified in the F1 progeny. It is suggested to directly Sanger sequence the amplicons derived from the F1 heterozygous fish followed by performing heterozygous allele analysis methods such as Poly Peak Parser13 or using a variety of next-generation sequencing-based methods such as MiSeq14 or Hi-Tom15 sequencing. This is a necessary and complementary approach to ensure that the precise edit or deleterious indel mutation is actually going germline and is not just an artifact of the gel electrophoresis analysis. Indeed, the use of next-generation sequencing approaches to sequence sperm DNA from F0 carriers can easily be used in place of the gel electrophoresis analysis described in this protocol. However, having an easily scorable agarose gel genotyping method is a more economical and egalitarian approach for the global community of zebrafish researchers.
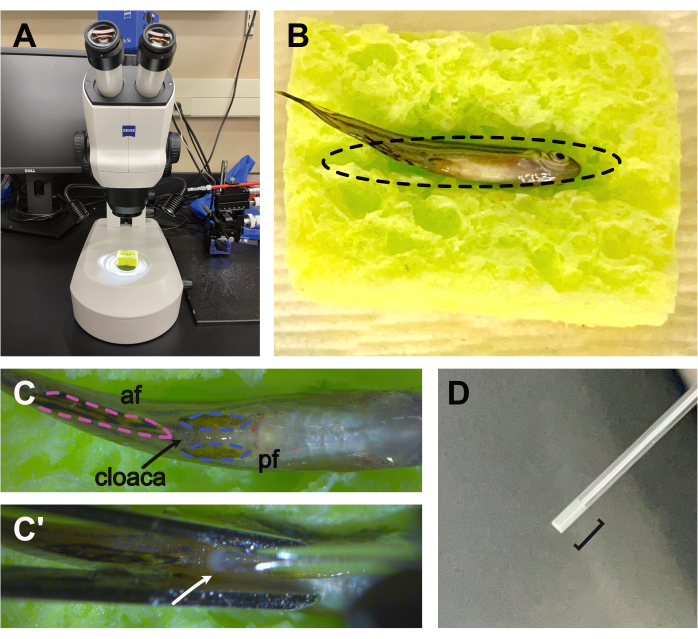
Figure 1: Setup for the sperm squeezing procedure. (A) The sponge is positioned underneath a stereomicroscope at low magnification with overhead lighting. (B) The moistened 1 in x 1 in sponge, cut with an oval divot (dashed line), is used to hold the anesthetized male fish ventral side up during the procedure. (C) Anatomy of the ventral side of the anesthetized male fish depicting the anal fins (af, pink), pelvic fins (pf, blue), and cloaca (arrow), where sperm will be expelled during the procedure. (C') Filter forceps are used to gently squeeze the anesthetized male fish from the gills to the cloaca, while the expelled sperm is drawn into a glass pipette by capillary action (white arrow). (D) A capillary tube containing sufficient expelled sperm (opaque liquid; black bracket) for downstream DNA extraction and analysis. Please click here to view a larger version of this figure.
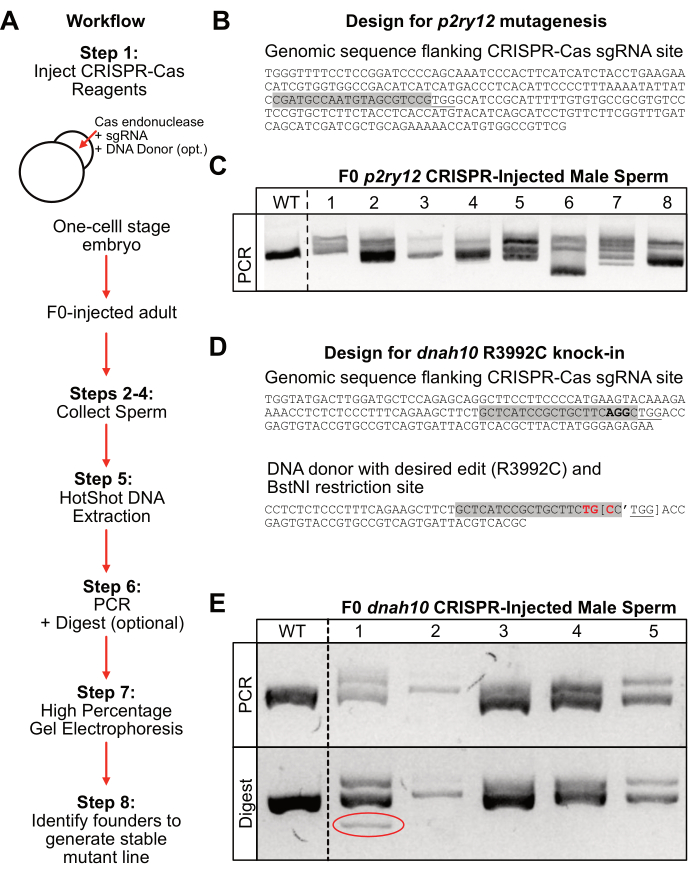
Figure 2: Workflow and representative results. (A) General workflow of the protocol. (B) Representative design of the p2ry12 sgRNA site (grey highlight) with a Cas9-specific PAM site (underlined). (C) Representative results of the 4% gel electrophoresis after 1.5 h at 150 V. Wild-type (WT) control and F0-p2ry12 CRISPR-injected male sperm samples (1-8) after PCR amplification. (D) Representative design of the dnah10 sgRNA site (grey highlight) with a Cas9-specific PAM site (underlined) near the targeted codon (bold). DNA donor sequence with the desired codon edit (red bold) and BstNI restriction site (brackets with apostrophe marking the cut site). (E) Representative results of the 4% gel electrophoresis after 1 h at 150 V. Top: PCR product of the wild-type (WT) and F0 dnah10 CRISPR-injected male sperm samples (1-5). Bottom: BstNI restriction digest product of the above samples. Sample 1 demonstrates the successful integration of the donor construct based on the additional band after digestion (red circle). Please click here to view a larger version of this figure.
Discussion
This protocol describes a method for rapidly characterizing putative genome edits or targeted mutations using CRISPR-Cas technology by focused analysis on F0 male sperm genomes. This protocol should be amenable to other animal models where sperm is readily available for sampling without euthanasia. This method will increase the throughput of screening for desired edits and is especially useful for identifying rare HDR-mediated knock-in events. This approach also serves to reduce the number of experimental animals used to find a stable germline edit by facilitating the rapid screening of potentially thousands of genomes in one sperm sample from a putative F0 carrier, which is in contrast with more traditional approaches that may require the screening of hundreds of embryos derived from crosses of putative F0 carriers.
This protocol builds on established protocols for sperm collection in zebrafish7,10,14,16,17,18 by including a reproducible technique for identifying germline edits using high-resolution gel electrophoresis. This approach can easily be incorporated into any standard CRISPR/Cas workflow to increase the throughput for the screening and isolation of target genome edits. Additionally, this protocol is appropriate for labs staffed with personnel with a range of training and experience, as well as teaching labs. However, our method of sperm collection is not sufficient for cryopreservation, which has been expertly described in previous publications10,17.
This protocol uses a high-resolution agarose gel electrophoresis to identify putative male carriers of desired indel and precise genomic mutations. However, sperm genomic DNA is amenable to a myriad of other approaches, including fluorescent fragment analysis or barcoded sequencing14, high-resolution melt analysis18, or simple amplicon detection of fluorescent tags or epitopes using standard gel electrophoresis approaches. The downstream validation of all alleles using approaches such as Sanger-based or next-generation sequencing must be done before starting experimental work on putative alleles. Indeed, existing methods for screening for mutations in embryos using the next-generation sequencing approach14 could circumvent the need for analysis on agarose gels, which is described in this protocol. However, having a gel-scorable genotyping method is a more cost-effective and egalitarian approach to consider given that not all labs have easy access to cheap next-generation sequencing methods during the isolation and experimental phases of working with each mutant strain.
In summary, this protocol provides step-by-step directions for reproducibly screening sperm genomes from CRISPR/Cas-edited males such that fewer crosses and less PCR screening of embryos are needed to screen for desired edits. The application of this method will decrease the number of fish that need to be created and analyzed to successfully identify the edited alleles of interest, which also reduces the time and cost of personnel for generating stable lines.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Anna Hindes at Washington University School of Medicine for her initial efforts in obtaining good-quality sperm genomic DNA using the hot shot method. This work was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award (R01AR072009 to R.S.G.).
Materials
| Agarose powder | Fisher BioReagents | BP1356-100 | |
| Breeding tanks | Carolina Biological | 161937 | |
| BstNI Restriction Enzyme | NEB | R0168S | |
| Cas9 Endonuclease | IDT | 1081060 | |
| DNA Ladder, 100 bp | Thermo Scientific | FERSM0241 | |
| dnah10 donor construct | Sigma-Aldrich | DNA Oligo in Tube; 0.025 nM, standard desalt purification, dry. Phosphorothioate bond on the donor at the first three phosphate bonds on both the 5’ and 3’ ends (5'-CCTCTCTCCCTTTCAGAAGCTTC TGCTCATCCGCTGCTTCTGCCT GGACCGAGTGTACCGTGCCGTC AGTGATTACGTCACGC-3') |
|
| dnah10 forward primer | Sigma-Aldrich | DNA Oligo in Tube; 0.025 nM, standard desalt purification, dry (5'-CATGGAACTCTTTCCTAATGAGT TTGGC-3') |
|
| dnah10 reverse primer | Sigma-Aldrich | DNA Oligo in Tube; 0.025 nM, standard desalt purification, dry ('5-AGTAGAGATCACACATCAACAGA ATACAGC-3') |
|
| dnah10 synthetic sgRNA | Synthego | Synthetic sgRNA, target sequence: 5'-GCTCATCCGCTGCTTCAGGC-3' | |
| Electrophoresis power supply | Thermo Scientific | 105ECA-115 | |
| Filter forceps | Millipore | XX6200006P | |
| Fish (system) water | Generic | n/a | |
| Gel electrophoresis system (including casting frame, comb, and electrophoresis chamber) | Thermo Scientific | B2 | |
| Gel imaging light box | Azure Biosystems | AZI200-01 | |
| Gel stain, 10000X | Invitrogen | S33102 | |
| Glass bowl, 250 mL | Generic | n/a | |
| Isolation tanks, 0.8 L | Aquaneering | ZT080 | |
| Microcap capillary tube with bulb, 20 µL | Drummond | 1-000-0020/CA | |
| Minicentrifuge | Bio-Rad | 12011919EDU | |
| Micropipettes, various with appropriate tips | Generic | n/a | |
| Microwave | Generic | n/a | |
| Nuclease free water | Promega | P119-C | |
| Paper towels | Generic | n/a | |
| PCR tubes, 0.2 mL | Bioexpress | T-3196-1 | |
| Plastic spoon, with drilled holes/slots | Generic | n/a | |
| KCl solution, 0.2 M RNAse Free | Sigma-Aldrich | P9333 | |
| p2ry12 forward primer | Sigma-Aldrich | DNA Oligo in Tube; 0.025 nM, standard desalt purification, dry (5'-CCCAAATGTAATCCTGACCAGT -3') |
|
| p2ry12 reverse primer | Sigma-Aldrich | DNA Oligo in Tube; 0.025 nM, standard desalt purification, dry (5'-CCAGGAACACATTAACCTGGAT -3') |
|
| p2ry12 synthetic sgRNA | Synthego | Synthetic sgRNA, target sequence: 5'-GGCCGCACGAGGTCTCCGCG-3' | |
| Restriction Enzyme 10X Buffer | NEB | B6003SVIAL | |
| NaOH solution, 50 mM | Thermo Scientific | S318; 424330010 | |
| Sponge, 1-inch x 1-inch cut with small oval divot | Generic | n/a | |
| Stereomicroscope | Zeiss | Stemi 508 | |
| Taq polymerase master mix, 2X | Promega | M7122 | |
| TBE Buffer Concentrate, 10X | VWR | E442 | |
| Thermal Cycler | Bio-Rad | 1861096 | |
| Tissue paper | Fisher Scientific | 06-666 | |
| Tricaine-methanesulfonate solution (Syncaine, MS-222), 0.016% in fish water (pH 7.0±0.2) | Syndel | 200-266 | |
| Tris Base, 1M (Buffered with HCl to ph 8.0) | Promega | H5131 |
References
- Auer, T. O., Duroure, K., De Cian, A., Concordet, J. P., Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Research. 24 (1), 142-153 (2014).
- Hwang, W. Y., et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature Biotechnology. 31 (3), 227-229 (2013).
- Jao, L. E., Wente, S. R., Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proceedings of the National Academy of Sciences of the United States of America. 110 (34), 13904-13909 (2013).
- Troutwine, B. R., et al. The Reissner fiber is highly dynamic in vivo and controls morphogenesis of the spine. Current Biology. 30 (12), 2353-2362 (2020).
- Xu, Y., Li, Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Computational and Structural Biotechnology Journal. 18, 2401-2415 (2020).
- Creaser, C. W. The technic of handling the zebrafish (Brachydanio rerio) for the production of eggs which are favorable for embryological research and are available at any specified time throughout the year. Copeia. 1930 (4), 159-161 (1934).
- Carmichael, C., Westerfiel, M., Varga, Z. M. Cryopreservatin and in vitro fertilization at the zebrafish international resource center. Methods in Molecular Biology. 546, 45-65 (2009).
- Wang, Y., Troutwine, B. R., Zhang, H., Gray, R. S. The axonemal dynein heavy chain 10 gene is essential for monocilia motility and spine alignment in zebrafish. Developmental Biology. 482, 82-90 (2021).
- Gray, R. S., et al. Postembryonic screen for mutations affecting spine development in zebrafish. Developmental Biology. 471, 18-33 (2021).
- Brocal, I., et al. Efficient identification of CRISPR/Cas9-induced insertions/deletions by direct germline screening in zebrafish. BMC Genomics. 17, 259 (2016).
- Davis, M. W., Jorgensen, E. M. ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program. Frontiers in Bioinformatics. 2, 816619 (2022).
- Labun, K., et al. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Research. 47, 171-174 (2019).
- Hill, J. T., et al. Poly Peak Parser: Method and software for identification of unknown indels using sanger sequencing of polymerase chain reaction products. Developmental Dynamics. 243 (12), 1632-1636 (2014).
- Varshney, G. K., et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Research. 25 (7), 1030-1042 (2015).
- Liu, Q., et al. Hi-TOM: A platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Science China. Life Sciences. 62 (1), 1-7 (2019).
- Sorlien, E. L., Witucki, M. A., Ogas, J. Efficient production and identification of CRISPR/Cas9-generated gene knockouts in the model system Danio rerio. Journal of Visualized Experiments. (138), e56969 (2018).
- Draper, B. W., Moens, C. B. A high-throughput method for zebrafish sperm cryopreservation and in vitro fertilization. Journal of Visualized Experiments. (29), e1395 (2009).
- Parant, J. M., George, S. A., Pryor, R., Wittwer, C. T., Yost, H. J. A rapid and efficient method of genotyping zebrafish mutants. Developmental Dynamics. 238 (12), 3168-3174 (2009).

