Assessment of Gut Barrier Integrity in Mice Using Fluorescein-Isothiocyanate-Labeled Dextran
Summary
In the present study, fluorescein-isothiocyanate-labeled (FITC) dextran is administered to mice via oral gavage to evaluate intestinal permeability both in vivo and in plasma and fecal samples. As gut barrier function is affected in many disease processes, this direct and quantitative assay can be used in diverse research areas.
Abstract
Gut barrier integrity is a hallmark of intestinal health. While gut barrier integrity can be assessed using indirect markers such as the measurement of plasma inflammatory markers and bacterial translocation to the spleen and lymph nodes, the gold standard directly quantifies the ability of selected molecules to traverse the gut mucosal layer toward systemic circulation. This article uses a non-invasive, cost-effective, and low-burden technique to quantify and follow in real time the intestinal permeability in mice using fluorescein-isothiocyanate-labeled dextran (FITC-dextran). Prior to oral supplementation with FITC-dextran, the mice are fasted. They are then gavaged with FITC-dextran diluted in phosphate-buffered saline (PBS). One hour after the gavage, the mice are subjected to general anesthesia using isoflurane, and the in vivo fluorescence is visualized in an imaging chamber. This technique aims to assess residual fluorescence in the abdominal cavity and the hepatic uptake, which is suggestive of portal migration of the fluorescent probe. Blood and stool samples are collected 4 h after oral gavage, and the mice are sacrificed. Plasma and fecal samples diluted in PBS are then plated, and the fluorescence is recorded. The concentration of FITC-dextran is then calculated using a standard curve. In previous research, in vivo imaging has shown that fluorescence rapidly spreads to the liver in mice with a weaker gut barrier induced by a low-fiber diet, while in mice supplemented with fiber to strengthen the gut barrier, the fluorescent signal is retained mostly in the gastrointestinal tract. In addition, in this study, control mice had elevated plasma fluorescence and reduced fluorescence in the stool, while inversely, inulin-supplemented mice had higher levels of fluorescence signals in the gut and low levels in the plasma. In summary, this protocol provides qualitative and quantitative measurements of intestinal permeability as a marker for gut health.
Introduction
The gut barrier plays an important role in both health and disease. It requires a complex balance between allowing the required nutrients to permeate into the circulation from the intestinal lumen while simultaneously preventing the penetration of pro-inflammatory molecules, such as pathogens or antigens1. Increased permeability can be caused by many gastrointestinal disorders, such as liver disease or inflammatory bowel diseases (IBDs)2,3. For example, in ulcerative colitis (UC), an IBD, chronic inflammation leads to the breakdown of tight junctions, the subsequent disruption of the gut barrier, and the translocation of bacteria, potentially perpetuating mucosal and systemic inflammation4.
Gut barrier integrity is, therefore, an important marker of gut health. However, current methods for the measurement of intestinal permeability have many limitations. For instance, methods measuring plasma inflammatory markers or bacterial translocation to the spleen and lymph nodes are indirect5,6. Other methods can be invasive and time-intensive. This article describes a non-invasive and cost-effective assay that directly and quantitatively measures intestinal permeability. This assay uses fluorescein-isothiocyanate-labeled dextran (FITC-dextran) to follow intestinal permeability in real time by measuring fluorescence in vivo. In addition, measuring FITC-dextran levels in the plasma and feces quantifies the intestinal permeability (Figure 1).
The FITC-dextran permeability assay has been used previously in many different contexts, including in animal models of Parkinson's disease7, sepsis8, ischemic stroke9, and burn injury10. Additionally, this assay has been used recently to aid in understanding how the gut microbiome may be implicated in different disease processes and how it may be targeted or manipulated as a potential therapeutic. For example, it has been used to study the microbiome and microbiome-based therapeutics in aging11, IBDs12, colorectal cancer13, and autism spectrum disorder11. As gut barrier function is implicated in numerous aspects of health and disease, this assay has been used widely. Its relative simplicity and low time burden make it ideal for testing the in vivo conditions suspected to alter gut barrier integrity. Its quantitative results are useful for determining the effectiveness of a potential treatment.
In this study, the effect of diet on gut barrier function was evaluated using the FITC-dextran assay. The intestinal permeability of mice receiving a control diet and the intestinal permeability of mice receiving an inulin-supplemented diet were compared. Inulin is a beneficial oligosaccharide that has been shown to improve gut barrier function12,13. For in vivo fluorescence measurements (background), one additional untreated mouse was used as a negative control and received PBS instead of FITC-dextran. This experiment demonstrates that the FITC-dextran assay is a valuable tool for evaluating intestinal permeability.
Protocol
All procedures were performed following the Canadian Council on Animal Care guidelines after approval by the Institutional Animal Care Committee of the CRCHUM. Eight-week-old female BALB/c mice, obtained from a commercial source (see Table of Materials), were used for the present study. The animals received dietary supplementation with 10% inulin wt/wt for 2 weeks. A control group received a similar diet lacking the inulin supplement. Mice had ad libitum access to the diet. An overview of the assay is shown in Figure 1.
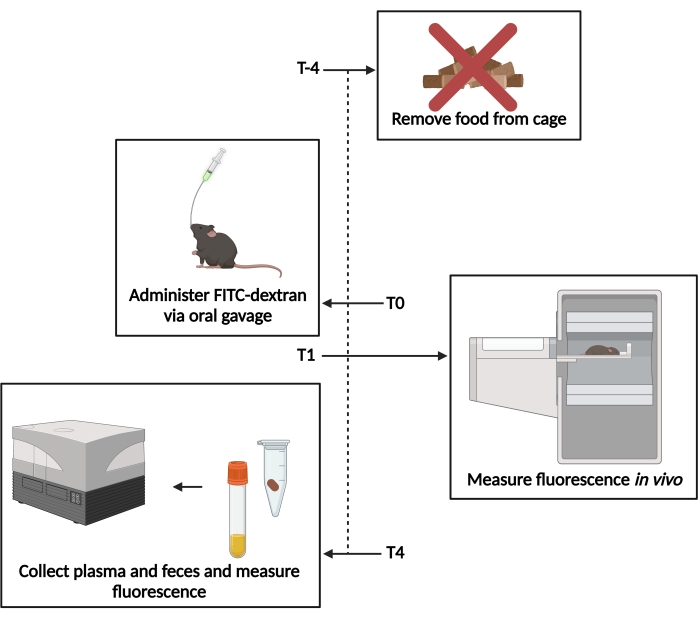
Figure 1: Schematic of the FITC-dextran assay. T−4– 4 h prior to gavage, food access was removed. T0– FITC-dextran was administered via oral gavage. T1 – 1 h post gavage, the in vivo fluorescence was evaluated. T4– 4 h post gavage, the fecal and plasma samples were collected, and the fluorescence was measured. Please click here to view a larger version of this figure.
1. Administration of FITC-dextran
- Prior to administering FITC-dextran, fast the mice for 4 h while maintaining ad libitum access to water.
NOTE: The fasting must preferably be initiated at the beginning of the light cycle (in the morning). The mice may be transferred to a new cage without bedding during fasting to limit coprophagy. - Prepare 200 µL of 80 mg·mL−1 4 kDa FITC-dextran (see Table of Materials) diluted in sterile 1x phosphate-buffered saline (PBS) (per mouse). Freshly prepare the samples immediately prior to administration, and keep them protected from light.
- Administer 200 µL of the FITC-dextran suspension via oral gavage to each mouse using a 38 mm 22 G sterilized, curved gavage needle with a ball- or pear-shaped tip (see Table of Materials). Start a timer after the first gavage, and wait for 5-10 min before gavaging the next mouse to allow for in vivo measurements (step 2), always maintaining 1 h post gavage. Keep the remaining FITC-dextran suspension for the standard curve.
NOTE: Immediately following the gavage, food may be replaced to ensure the formation of feces.
2. In vivo fluorescence measurement
- Anesthetize the mice 1 h after the gavage using 2.5% isoflurane or an alternative anesthetic. Confirm that the animal is appropriately anesthetized by pinching the toe or the tail and ensuring that the animal does not react.
- Remove the fur from the abdominal area using an electric shaver, and generously apply ophthalmic lubricating ointment to the eyes to prevent drying. Then, place the mice individually in the imaging chamber lying dorsally.
NOTE: One control mouse must be included that receives PBS or saline instead of FITC-dextran to account for background during in vivo imaging.
- Remove the fur from the abdominal area using an electric shaver, and generously apply ophthalmic lubricating ointment to the eyes to prevent drying. Then, place the mice individually in the imaging chamber lying dorsally.
- Image the mice using a fluorescence imaging chamber (see Table of Materials). Acquire images of the abdominal region, setting the laser length at 470 nm and the resolution at 2.0 mm.
- Start the machine and software by clicking and holding the start button. Allow the system to warm up.
NOTE: The system may need 20 min or more to warm up, so the machine must be started early so as not to interfere with imaging the mice at 1 h after gavage. - Click on Device status and ensure all the configured devices show "OK" before proceeding.
- If needed, warm up the appropriate laser by clicking on Laser control and then clicking on the Laser name button of the desired laser.
- Start a new study by clicking on New study. Save under the appropriate file with the desired name.
- Click on Study options, then enter the Specimen ID, and choose the correct laser and experiment.
- Open the imaging chamber and place the animal dorsally on the scanning plate. Secure the limbs and tail with tape, and ensure that the nose and mouth fit snugly in the anesthesia tube, maintaining 2.5% isoflurane.
- Adjust the height of the scanning plate so that the scanning region is slightly ventral from the animal's midline. Adjust the plate by turning the adjustment knob inside the imaging chamber. Close and lock the imaging chamber door.
- Select the area to be scanned by using the Draw tool. Include the whole width of the abdomen from just above the liver to the rectum. Click on the Modify tool to adjust the area once drawn.
- Set the scan resolution to 2.0 mm, and then click on Next. Once power automation is completed, ensure that the settings are correct, and make any adjustments needed. Click on Start to begin the scan.
- Once the scan is complete, remove the animal from the imaging chamber, and place it in an incubator to maintain body temperature while recovering from anesthesia.
- Click on Continue study to maintain the settings, and then repeat steps 2.2.5-2.2.10 until all the mice have been scanned.
- Click on the power button and hold it for 3 s to power down the imaging chamber.
- Start the machine and software by clicking and holding the start button. Allow the system to warm up.
- Evaluate the fluorescence by comparing the abdominal fluorescence of each animal and the control mouse on uniformly scaled images using a software associated with the imaging system used (see Table of Materials).
- Open the image files by finding them under the chosen file name. Open all the files that have synchronized settings at the same time.
- Using the image settings toolbar, use the Sync image and Sync scale buttons to sync the settings for the images, allowing for an accurate comparison.
- Save the images with their adjusted scales.
3. Fluorescence measurement in fecal samples and plasma
- Collect a fecal pellet from each mouse in a sterile tube 4 h after gavage. Keep the tubes in the dark on ice.
- Anesthetize the mice via intraperitoneal injection of 240 mg/mL pentobarbital sodium (dilution, 1:100; see Table of Materials). Administer at a dose of 0.03 mL/g body weight.
- Collect blood samples of at least 700 µL in a tube made for plasma collection containing heparin or EDTA to prevent clotting (see Table of Materials) by inserting a glass capillary tube into the retro-orbital plexus14.
NOTE: Alternative methods for blood collection include cardiac puncture or withdrawal from the tail vein. As this is a terminal procedure, the mice need to be euthanized via cervical dislocation or an alternative humane method. Follow local animal ethics committee recommendations for euthanasia.
- Collect blood samples of at least 700 µL in a tube made for plasma collection containing heparin or EDTA to prevent clotting (see Table of Materials) by inserting a glass capillary tube into the retro-orbital plexus14.
- Centrifuge the blood samples at 9,390 x g for 10 min at room temperature. Transfer the plasma to a new sterile tube, and keep it in the dark on ice.
- Dilute 50 mg of fecal samples in 200 µL of 1x PBS and dilute the plasma 1:2 with 1x PBS. The dilution ratio can be modified based on the intensity of the fluorescence signal.
- Generate a standard curve using serial dilutions of FITC-dextran in 1x PBS. Starting with the highest concentration of 20 mg·mL−1 FITC dextran, dilute by a factor of 1:1 serially 7-10 times.
NOTE: The concentrations, thus, must read 20 mg·mL−1, 10 mg·mL−1, 5 mg·mL−1, 2.5 mg·mL−1, 1.25 mg·mL−1, 0.625 mg·mL−1, 0.3125 mg·mL−1, etc. - Plate 100 µL samples and standards in an opaque black 96-well plate. Include a PBS blank. Read the fluorescence on a fluorescent plate reader (see Table of Materials) with the absorption at 530 nm and the excitation at 485 nm.
NOTE: The samples and standards can be plated in duplicate or triplicate, and then their fluorescence values are averaged. - Determine the concentration of FITC-dextran per sample by comparing the fluorescence to the known concentrations of the standard curve. In the samples, multiply the concentration by the dilution factor (step 3.5).
Representative Results
The analysis of the in vivo fluorescence showed that mice who received only the control diet had a higher hepatic intake of FITC-dextran and higher levels of residual fluorescence in the abdominal cavity compared to the mice who received the inulin-supplemented diet (Figure 2A). Some fluorescence was visible in the caecum of the mice that received the inulin diet, but there was little to no hepatic intake, indicating that these diets protected against increased intestinal permeability.
The fluorescence levels in plasma and fecal samples work to reinforce and quantify their in vivo counterparts. The mice that received the inulin-supplemented diet had significantly lower levels of FITC-dextran in their plasma compared to the mice that received only the control diet (Figure 2B). This indicates that they had improved gut barrier function because less FITC-dextran could permeate the intestinal barrier into the circulation. Concordantly, the mice that received the inulin diet had significantly higher levels of FITC-dextran in their feces than the mice that received only the control diet (Figure 2C). This reinforces that they had intact gut barrier function as the FITC-dextran remained in the colon until excretion, as is considered normal. The lower levels of FITC-dextran in the feces of the control mice indicate that it permeated through the intestinal barrier into the circulation rather than being appropriately excreted. The high levels of FITC-dextran in the plasma reinforce this finding.
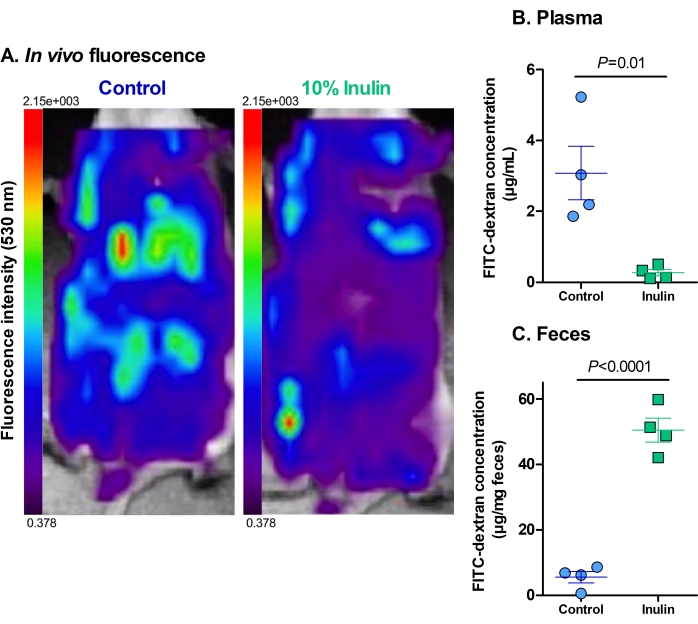
Figure 2: Dietary supplementation with inulin decreases the translocation of FITC-dextran through the gut barrier. (A) The residual fluorescence and hepatic uptake of FITC-dextran. Red = highest intensity; dark purple = lowest intensity. Maximum fluorescence of 2.15 x 103, minimum fluorescence of 0.378. (B) The plasmatic concentration of FITC-dextran. P = 0.010. (C) The fecal concentration of FITC-dextran. P = 0.00003. N = 4 per group. Data are represented as mean ± SEM. Each dot represents one mouse. Unpaired Student's t-test. Please click here to view a larger version of this figure.
Discussion
The gut barrier function is an integral part of many different disease processes. Thus, assessing intestinal permeability in a non-invasive, cost-effective, and quantifiable way is essential for accurately representing these diseases in animal models. The FITC-dextran assay provides the possibility for this representation. However, this protocol involves several critical steps that must be completed accurately to obtain reliable results. Firstly, ensuring the use of appropriately sized FITC-dextran is essential. For examining in vivo permeability, 4 kDa FITC-dextran is the optimal molecular weight, and as the molecular weight increases, the permeability decreases15. Thus, using FITC-dextran of a different molecular weight may provide confusing or unreliable results. Additionally, it is important to note the time of each gavage and to adjust the time points for in vivo data collection and the collection of plasma and feces accordingly. For example, if two mice are gavaged 10 min apart, the in vivo fluorescence readings and the collection of feces and plasma must also occur 10 min apart. Comparing the fluorescence at the same time points allows for a more accurate representation of the differences in permeability. Furthermore, the order in which the animals from different groups are tested should be alternated to prevent a clustering effect due to timing. Instead of testing all the animals in Group A first, then all the animals in Group B second (AAABBB), it is recommended for the group to be switched after each animal (ABABAB).
This assay can be modified to include only the evaluation of plasma and fecal samples if there is a lack of access to an imaging machine. Though direct fluorescence imaging in vivo allows for the visualization of hepatic intake and residual abdominal fluorescence, evaluating fluorescence in the plasma and fecal samples still provides a quantitative measurement of intestinal permeability. Furthermore, as demonstrated by the described experiment, the fluorescence levels in the plasma and feces correlate well with the in vivo imaging. Additionally, this assay can be modified to include only the in vivo imaging. This allows the animals to be kept alive to continue testing other parameters or monitor how intestinal permeability changes over time. The ability to modify this assay, therefore, makes it accessible, yet still quantitative. Finally, the dosage of 200 µL of 80 mg·mL−1 FITC-dextran given to each mouse has been used previously and was shown to be effective in mice with small differences in body weight16. Furthermore, it is important to note that all the mice used in the representative results section weighed approximately 20 g, allowing the same dosage to be used for each mouse. To account for differences in body weight, however, FITC-dextran can be administered at a dosage of 0.6-0.8 mg/g body weight, for example17. Crucially, regardless of the dosage used, it is important to limit the amount gavaged to each mouse to less than 10 mL·kg−1 to prevent complications or discomfort18.
Though the FITC-dextran assay provides an effective method for evaluating gut barrier function, it still has some limitations. One limitation of this model is that it requires fasting the mice for several hours, meaning it is unreliable to compare these results to those from mice that have not been fasted. Additionally, fasting may affect the outcomes in certain models that require strict feeding schedules, such as when measuring blood glucose in animal models for diabetes.
Despite these limitations, the FITC-dextran assay remains an effective method for analyzing intestinal permeability as it is quantitative, versatile, cost-effective, and less invasive than many classical methods. For example, common probes used for measuring intestinal permeability are small saccharide probes or Cr-EDTA, which have some advantages19. However, some saccharide probes have only region-specific permeability. As they are hydrolyzed in the distal portion of the small intestine, they provide no insight into colonic permeability19. On the other hand, Cr-EDTA can provide information about colonic permeability but requires measurements for 24 h, making the time burden of this method much higher than that of the FITC-dextran assay20. Furthermore, neither of these methods provides the direct in vivo imaging of this assay. Therefore, the FITC-dextran assay provides a relatively simple, direct, and effective option as compared to alternative methods for measuring intestinal permeability.
Finally, in disease processes such as IBDs4, Alzheimer's disease21, and liver disease2, intestinal permeability is an important parameter that could be measured using the FITC-dextran assay to improve studies. For example, in developing novel treatments, such as immunotherapies for IBDs, this assay can be used to test the efficacy of the therapeutic for maintaining gut barrier integrity. Considering that impaired gut barrier function may be implicated in perpetuating the chronic inflammation in UC, for example, examining how well a therapeutic protects against increased permeability is important4. This is only one example, but the FITC-dextran assay is an accessible and quantifiable way to measure intestinal permeability in many different areas and aspects of research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (grant RGPIN-2018-06442 to MMS). We thank the animal facility at the CRCHUM and Dr. Junzheng Peng from the Cardiovascular Phenotyping Platform.
Materials
| 50 ppm Fe Diet (10% Inulin) | Envigo Teklad | TD.190651 | Representative Results |
| 50 ppm Fe Diet (FeSO4) | Envigo Teklad | TD.190723 | Representative Results |
| BALB/c Mice 49-55 Days, Female | Charles River | 028BALB/C | Representative Results |
| BD 1 mL Syringe Tuberculin Slip Tip | Becton, Dickinson and Company | 309659 | For gavage |
| BD Microtainer Tubes – With LH (Lithium Heparin) | Becton, Dickinson and Company | 365965 | For plasma collection |
| Centrifuge 5420 | Eppendorf | S420KN605698 | |
| Curved Gavage Needle (Gavage Cannula) 7.7.0 38 mm x 22 G | Harvard Apparatus Canada | 34-024 | No longer available – A potential alternative is available at Instech Labs (FTP-22-38) |
| Euthanyl (Pentobarbital Sodium) 240 mg/mL | Bimeda-MTC Animal Health Inc. | 141704 | 1/100 dilution; Administered via intraperitoneal injection at 0.03 mL/g body weight |
| FITC-dextran 4 | TdB Labs | 20550 | |
| Heparinized Capillary Tubes | Kimble Chase Life Science and Research | 2501 | For retro-orbital blood collection |
| Microplate, PS, 96-well, Flat-bottom (Chimney Well), Black, Flutrac, Med. Binding | Greiner Bio-one | 655076 | |
| MiniARCO Clipper Kit | Kent Scientific | CL8787-KIT | For hair removal |
| Optix MX2 and Optix Optiview | Advanced Research Technologies | 2.02.00.6 | Fluorescence imaging machine and software |
| Phosphate Buffered Saline 1x (PBS) | Wisent Inc | 311-010-LL | |
| Puralube Vet Ointment | Dechra | 12920060 | Ophthalmic ointement to prevent eye damage during anesthesia |
| Spark Multiplate Reader | Tecan | 30086376 |
References
- König, J., et al. Human intestinal barrier function in health and disease. Clinical and Translational Gastroenterology. 7 (10), 196 (2016).
- Lorenzo-Zuniga, V., et al. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 55 (9), 1306-1312 (2006).
- Schwarz, B. T., et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 132 (7), 2383-2394 (2007).
- Schmitz, H., et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 116 (2), 301-309 (1999).
- Fouts, D. E., Torralba, M., Nelson, K. E., Brenner, D. A., Schnabl, B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. Journal of Hepatology. 56 (6), 1283-1292 (2012).
- Galipeau, H. J., Verdu, E. F. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterology and Motility. 28 (7), 957-965 (2016).
- Bordoni, L., et al. Positive effect of an electrolyzed reduced water on gut permeability, fecal microbiota and liver in an animal model of Parkinson’s disease. PLoS One. 14 (10), 0223238 (2019).
- Wang, Q., Fang, C. H., Hasselgren, P. -. O. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 281 (3), 1013-1023 (2001).
- Crapser, J., et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging. 8 (5), 1049-1063 (2016).
- Mal Earley, Z., et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 10 (7), 0129996 (2015).
- Sharon, G., et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 177 (6), 1600-1618 (2019).
- Schroeder, B. O., et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host & Microbe. 23 (1), 27-40 (2018).
- Hajjar, R., et al. Improvement of colonic healing and surgical recovery with perioperative supplementation of inulin and galacto-oligosaccharides. Clinical Nutrition. 40 (6), 3842-3851 (2021).
- JoVE. Lab Animal Research. Blood Withdrawal I. JoVE Science Education Database. , (2022).
- Costantini, T. W., et al. Quantitative assessment of intestinal injury using a novel in vivo, near-infrared imaging technique. Molecular Imaging. 9 (1), 30-39 (2010).
- Thevaranjan, N., et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe. 21 (4), 455-466 (2017).
- Chassaing, B., Aitken, J. D., Malleshappa, M., Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Current Protocols in Immunology. 104, 1-14 (2014).
- Turner, P. V., Brabb, T., Pekow, C., Vasbinder, M. A. Administration of substances to laboratory animals: Routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science. 50 (5), 600-613 (2011).
- Arrieta, M. C., Bistritz, L., Meddings, J. B. Alterations in intestinal permeability. Gut. 55 (10), 1512-1520 (2006).
- von Martels, J. Z. H., Bourgonje, A. R., Harmsen, H. J. M., Faber, K. N., Dijkstra, G. Assessing intestinal permeability in Crohn’s disease patients using orally administered 52Cr-EDTA. PLoS One. 14 (2), 0211973 (2019).
- Gonzalez-Escamilla, G., Atienza, M., Garcia-Solis, D., Cantero, J. L. Cerebral and blood correlates of reduced functional connectivity in mild cognitive impairment. Brain Structure and Function. 221 (1), 631-645 (2016).

