Characterizing Epithelial Wound Healing In Vivo Using the Cnidarian Model Organism Clytia hemisphaerica
Summary
This paper describes a method to create wounds in the epithelium of a live Clytia hemisphaerica medusa and image wound healing at a high resolution in vivo. Additionally, a technique to introduce dyes and drugs to perturb signaling processes in the epithelial cells and extracellular matrix during wound healing is presented.
Abstract
All animal organs, from the skin to eyes to intestines, are covered with sheets of epithelial cells that allow them to maintain homeostasis while protecting them from infection. Therefore, it is not surprising that the ability to repair epithelial wounds is critical to all metazoans. Epithelial wound healing in vertebrates involves overlapping processes, including inflammatory responses, vascularization, and re-epithelialization. Regulation of these processes involves complex interactions between epithelial cells, neighboring cells, and the extracellular matrix (ECM); the ECM contains structural proteins, regulatory proteins, and active small molecules. This complexity, together with the fact that most animals have opaque tissues and inaccessible ECMs, makes wound healing difficult to study in live animals. Much work on epithelial wound healing is therefore performed in tissue culture systems, with a single epithelial cell-type plated as a monolayer on an artificial matrix. Clytia hemisphaerica (Clytia) provides a unique and exciting complement to these studies, allowing epithelial wound healing to be studied in an intact animal with an authentic ECM. The ectodermal epithelium of Clytia is a single layer of large squamous epithelial cells, allowing high-resolution imaging using differential interfering contrast (DIC) microscopy in living animals. The absence of migratory fibroblasts, vasculature, or inflammatory responses makes it possible to dissect the critical events in re-epithelialization in vivo. The healing of various types of wounds can be analyzed, including single-cell microwounds, small and large epithelial wounds, and wounds that damage the basement membrane. Lamellipodia formation, purse string contraction, cell stretching, and collective cell migration can all be observed in this system. Furthermore, pharmacological agents can be introduced via the ECM to modify cell:ECM interactions and cellular processes in vivo. This work shows methods for creating wounds in live Clytia, capturing movies of healing, and probing healing mechanisms by microinjecting reagents into the ECM.
Introduction
Sheets of epithelial cells cover the external surface of all metazoans, line internal organs, and divide the animal body into discrete compartments. The epithelium also separates the inner body from the external environment and protects it from damage and infection. Hence, the advent of epithelial layers was an essential part of the evolution of multicellular animals, and epithelial layers are seen in all animals from vertebrates to the most basal metazoans1. The epithelium of some organs is a single monolayer, such as in the lung air sacs, blood vessels, and gut2, as well as in the epidermis of invertebrates such as planaria and cnidarians3. In other tissues, such as the skin4 and cornea5 of vertebrates, the epithelium is stratified, meaning there are multiple epithelial cell layers2. In all cases, the most basal epithelial layer is affixed to the basement membrane, a protein sheet that forms a specialized region of the extracellular matrix (ECM)6,7,8.
Breaches in the epithelium must be rapidly repaired to recreate a continuous epithelial sheet. Damage to the epithelium occurs during natural processes, such as the shedding of epithelial cells in the gut,9,10 and as the result of inflammation or physical trauma. When a single epithelial cell is damaged, it must either repair itself or be eliminated to allow the surrounding cells to attach to each other and close the hole11,12. In wounds larger than the size of a single cell, epithelial cells must move to reach each other and repair the sheet13. This may be achieved by cell spreading if the gaps are small or may require the migration of epithelial cells from the margins of a wound to close the wound gap; this latter process is called re-epithelialization14,15. In embryonic tissues, epithelial cells spread and migrate to close wounds or are pulled across the gap by the contraction of actomyosin cables that form between the cells at the wound margin, in a mechanism resembling a purse string16. In many adult tissues, re-epithelialization involves the migration of coherent cell sheets, where cells maintain their junctions with neighboring cells14,17,18. In other tissues, cell:cell connections are dismantled and epithelial cells behave more like mesenchymal cells, moving in a coordinated but independent manner into the wound region during re-epithelialization14,19,20,21.
Epithelial cell movements are regulated by complex interactions between the migrating cells and between the cells and the ECM. While there is a tremendous amount of experimental literature addressing mechanisms of wound-activation of epithelial cells and subsequent migration, much still remains to be discovered. For example, the initial signal that activates epithelial cells to migrate in response to a wound has not been definitively identified22, nor is it completely understood how actin is redeployed to create lamellipodia on the side of epithelial cells closest to the wound22,23,24,25,26,27. Collective cell migration requires information from cells at the wound to be shared with cells distal to the wound, and the communication pathway is still unclear28. Cell:cell junctions and cell:ECM attachments must be disassembled and reformed as cells in the sheet rearrange themselves, but regulation of this process is poorly understood14,29. Making progress on these and other related questions is not only important as a fundamental biological problem but also because of the clinical significance of correct wound healing. Diseases that compromise the ability of epithelial cells to migrate correctly result in chronic wounds; an example is the genetic disease epidermolysis bullosa, where genes involved in the attachment of the epithelial cells to the ECM are mutated, resulting in fragile skin that peels and blisters. Re-epithelialization is also compromised in naturally aging tissues30,31. A better understanding is therefore essential for developing interventions to improve wound healing outcomes.
Epithelial cell migration in wound healing has been studied using both in vitro approaches and model organisms. The majority of studies of wound healing and mechanisms of cell migration have been carried out in tissue culture, where monolayers of a single epithelial cell type are grown on a substrate that substitutes for the ECM. Cell monolayers are either scratched or grown with stencils to create gaps of specific shapes and sizes and then observed32,33,34. The in vitro model allows an ideal visualization of cell behavior, as well as the opportunity to change qualities of the substrate, to expose cells to drugs and abiotic and biotic factors, and to transfect cells with constructs that express or suppress various genes of interest. However, this reductionist approach may fail to capture some of the important parameters involved in epithelial cell behavior in an in vivo context, including communication between various cell types and signaling events that occur in the ECM11. In vivo models provide the authentic context of a wound, with multiple cell types, overlapping signaling pathways, and a complex ECM35. One such model for wound healing studies is the mouse19, in which recent advances have allowed researchers to observe epidermal cells during healing of full thickness wounds in live animals36. The mouse and other in vivo systems present challenges to study re-epithelialization, however. First, the great advantage of observing cell behavior in a natural context is balanced by the complexity of the temporally overlapping events that occur during vertebrate wound healing, including blood clotting, recruitment of immune cells and inflammation, recruitment of fibroblasts, and cell de-differentiation, re-vascularization, and remodeling of the ECM. Further, opaque tissues make imaging difficult. The Drosophila larva and Zebrafish epidermis systems37,38 have overcome some of these difficulties because of their relative simplicity39.
Our lab recently introduced a new model for studying epithelial wound healing: the medusa (jellyfish) form of the hydrozoan cnidarian Clytia hemisphaerica (Clytia)40. Clytia is an emerging model organism with a fully sequenced and annotated genome41, single cell RNAseq transcriptome42, and protocols in place for genome modification (mutagenesis and transgenesis)43,44,45. Cnidarians are one of the oldest extant lineages to have epithelial layers, so understanding cnidarian wound healing provides insights into the ancestral pathways that ensured epithelial integrity. For those pathways that have been conserved throughout the tree of life, Clytia offers an exciting new system to study epithelial cell dynamics and the functional regulation of wound healing in vivo.
The epithelium covering the upper surface of the Clytia medusa (exumbrella) is a monolayer of transparent, squamous epithelial cells that are approximately 50 µm wide by 1-2 µm thick (Figure 1). They are attached to an ECM called the mesoglea — the "jelly" of the jellyfish. The mesoglea is compositionally similar to the ECM found in other animals46,47,48 including vertebrates, has a basement membrane40, and is completely transparent. The epithelial layer in the Clytia medusa can be easily scratched or wounded (see below). The simplicity and transparency of the epithelium and ECM allows high resolution imaging of the cells and their movements during healing. Recently, Kamran et al. characterized the healing of small wounds in the Clytia epithelium in detail40. It was demonstrated that healing in Clytia occurs through lamellipodia-based cell-crawling, cell spreading, and collective cell migration, as well as purse string closure that is more typical of embryonic systems (although seen previously in adult animal structures such as the cornea49). Clytia wound healing is extremely fast, as has been seen in other systems that lack an inflammatory response40,50. Healing in the Clytia exumbrella is completely dependent on movements of the existing epithelial cells — no cells proliferate or migrate through the ECM to the wound site (Supplemental Movie 1). All of these findings suggest that Clytia is a useful model system to study epithelial wound healing. Indeed, the ease of imaging epithelial cells in Clytia during wound healing led to the discovery that epithelial cell lamellipodia extend and spread over areas of exposed ECM as long as there is an intact basement membrane; if the basement membrane is damaged, epithelial healing switches to a purse string mechanism40. This was the first demonstration of a mechanism underlying the decision to close by lamellipodia-based crawling versus purse string closure, highlighting the importance of specific cell:ECM interactions in healing and of observing cells in their natural context.
Below, protocols are described for creating and imaging single-cell microwounds, small wounds that close primarily by cell spreading, and large wounds that require collective cell migration to close. Furthermore, a protocol is described for the introduction of small molecules into the ECM and epithelial cells, allowing experimental perturbations of putative regulatory pathways of wound healing.
Protocol
1. Animal culture
- Maintain Clytia polyp colonies on microscope slides and medusae in artificial sea water (ASW) at 18 °C in a zebrafish system, with 2 L zebrafish tanks for polyp colonies and custom-made 5 L pseudo-kreisel tanks for medusae (Supplemental Figure 1)51. ASW consists of 4% Instant Ocean in deionized (DI) H2O.
- Feed the animals daily with 2-3-day-old artemia as described51.
NOTE: Wound healing imaging is easier if the animals have not been recently fed, as there is less debris released from the gut into the field of view. - Collect baby medusae from the established polyp colonies as needed by placing colonies in a 2 L beaker filled with 1 L of ASW overnight. Use 2-3-week-old female medusae for all wound healing experiments. Propagation of Clytia has been described in detail elsewhere51.
2. Wounding
- Creating microwounds within and between cells (20-500 µm2)
- Create a modified transfer pipette by cutting the tip with scissors to make a larger opening (0.5-0.7 cm diameter).
NOTE: The opening in the pipette should be wide enough to avoid any damage to the animal. - Using the modified transfer pipette, place the medusa on a depression slide with the medusa exumbrella facing up, with just enough ASW to cover the animal.
- Place a coverslip over the animal and image immediately (see below for description of imaging). The coverslip compresses the mesoglea, and the rebound of the compressed tissue creates a force that pushes the cells slightly apart52. This immediately appears as gaps between each cell and damage within some cells (Figure 1B,B', Figure 2, and Figure 3A-C).
- Create a modified transfer pipette by cutting the tip with scissors to make a larger opening (0.5-0.7 cm diameter).
- Creating small epithelial wounds (0.02-0.125 mm2)
- Using a modified transfer pipette (as above), place the medusa on a depression slide with the medusa exumbrella facing up.
- Using a 200 µL pipette tip, gently scratch the surface of the medusa. Gentle scratching can also create rips in the basement membrane, which are readily apparent22. Cover the animal with a coverslip for imaging. Alternatively, placement of the coverslip is sometimes sufficient to create small epithelial wounds even without scratching (Figure 1C,C', Figure 2, and Figure 3A-C).
NOTE: Do not press down when scratching the surface of the medusa, as this damages the ECM and creates an irregular surface — epithelial cells migrating on an irregular surface are more difficult to keep in focus.
- Creating large epithelial wounds (0.5-0.9 mm2)
- Make a microinjection needle using a micropipette puller and glass capillary tube (step 5.2). Place the empty microinjection needle into a microinjector holder affixed to a micromanipulator. Cut the tip of the needle so that the opening is approximately 20-40 µm.
NOTE: Cut needles for large epithelial wounds can be stored and reused to increase consistency between experiments. - Set the hold pressure on the microinjector to zero, and set the eject pressure to approximately 20 PSI. Set the microinjector to deliver a 2 s pulse of air.
NOTE: The eject pressure may need to be adjusted based on the diameter of the needle opening (i.e., smaller tips will use higher pressure, while larger tips will use lower pressure). - Place the medusa with the exumbrella facing up on a depression slide on the stage of a dissecting scope, with just enough ASW to cover the animal. Using the micromanipulator, adjust the microinjection needle tip so that it is just above the water. To do this, carefully dip the tip into water (water may enter the pipette tip), then retract it so that it is close to the medusa's epithelial surface.
NOTE: The tip should be positioned over one quadrant of the medusa. The medusa's radial canals divide the medusa bell into four distinct quadrants. Targeting a quadrant will result in cleaner imaging, as the gonads and the radial canals are excluded from the wound area. - Pulse air by pressing start on the injector. Repeat the pulse in the same spot two to four times, depending on the width of the tip. Larger tips require fewer pulses.
NOTE: An indent in the water/medusa caused by the pulse of air should be visible. - Cover the wounded animal with a coverslip for imaging large wounds (Figure 1D,D').
- Follow the steps below (section 3) for imaging epithelial wound healing.
- Make a microinjection needle using a micropipette puller and glass capillary tube (step 5.2). Place the empty microinjection needle into a microinjector holder affixed to a micromanipulator. Cut the tip of the needle so that the opening is approximately 20-40 µm.
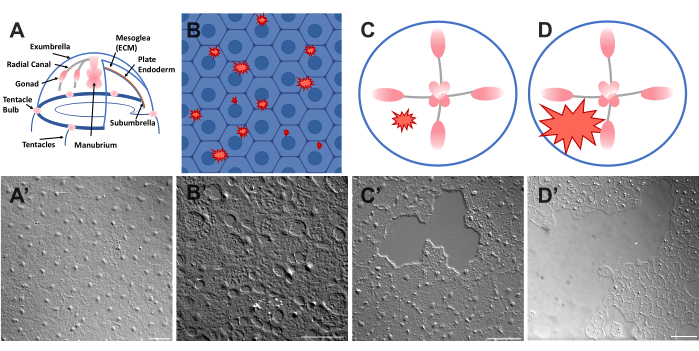
Figure 1: Intact and wounded exumbrella epithelial layer in Clytia medusa. (A) Cartoon graphic of the Clytia medusa body. (A') Intact medusa exumbrella epithelium viewed from above. (B) Cartoon of single-cell microwounds (red jagged shapes) with epithelial cells in blue. (B') Single cell microwounds. (C) Cartoon of a small epithelial wound (red jagged shape). (C') Small epithelial wound. (D) Cartoon of a large epithelial wound (red jagged shape). (D') Large epithelial wound. Images were all obtained using DIC microscopy. Scale bars in (A'–C'): 50 µm. Scale bar in (D'): 100 µm. Please click here to view a larger version of this figure.
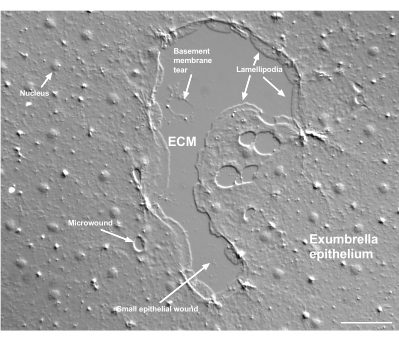
Figure 2: Multiple size wounds and a damaged basement membrane. A typical small exumbrella epithelial wound is shown, with labels indicating lamellipodia that form from marginal cells. In addition, microwounds within and between epithelial cells are seen. Note the small basement membrane tear in the upper portion of the wound. Movie 4 shows healing of this wound. Scale bar: 50 µm. Please click here to view a larger version of this figure.
3. Imaging epithelial wound healing
- Make sure that the microscope has been aligned for Köhler illumination53 and that it has been correctly set up for differential interfering contrast (DIC) microscopy54. Epithelial cells are almost invisible with standard optics (Figure 3D,E).
- Adjust the focus to the exumbrella. Although this is a thin layer, hexagonal cells should be clear.
NOTE: The exumbrella and subumbrella are separated by a thick mesoglea that is supported by vertical fibers. The subumbrellar cells are in the same focal plane as the radial canals. If initially focused on the subumbrellar layer, then adjust the focus slowly through the mesoglea and vertical fibers until finding the exumbrella. - Manually identify a wound to image. For large wounds, use a 10x objective. For smaller wounds and single-cell wounds, use a 20x objective.
- Start a program that collects images as a movie in real time or that collects a series of images at regular intervals. Monitor the progress to make sure that the wound area does not drift out of the field of view and that the cells of interest remain in focus.
- Single-cell wounds close within a minute; therefore, image their closure with a movie.
- To capture details of cell dynamics for small wounds, collect images approximately every 10 s. Closure of small wounds takes 20-50 min depending on size.
- Do not image the unsealed slides for more than 45 min, as evaporation of water from the slide over time leads to animal death and rupture of the cells.
- For longer observation, seal around the coverslip with petroleum jelly to reduce evaporation.
NOTE: Some medusa may pulse on the slide, which interferes with imaging. In this case, mounting animals in a 1:10 dilution of 1% Ethyl 3-aminobenzoate methanesulfonate (Tricaine), adjusted to pH 7.5, in ASW serves as an effective anesthetic and has no apparent effect on healing in a 1 h time frame. However, the animals will die if left for several hours in Tricaine.
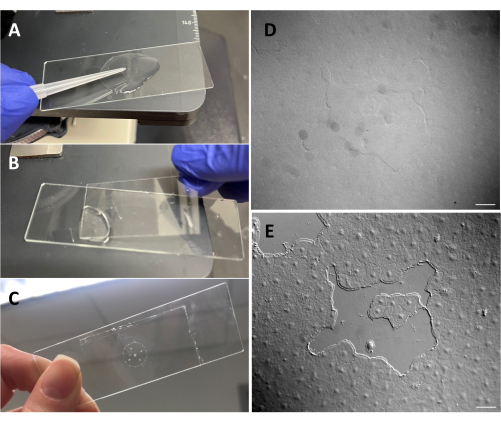
Figure 3: Creating a small wound in the exumbrellar epithelium. (A) Gentle scratching of the exumbrella with a 200 µL pipette tip to create a small epithelial wound. (B) Placing the coverslip is sometimes sufficient to create small epithelial wounds. (C) Medusa mounted on a depression slide. (D) Small epithelial wound image without DIC optics and (E) with DIC optics. Scale bars: 50 µm Please click here to view a larger version of this figure.
4. Analysis
- Preparing image files
NOTE: To process the image files, use FIJI/ImageJ with updated BioFormat plugins.- Set the scale to the correct pixel per micron ratio before registering the image stack; Analyze > Set Scale. This is necessary for extracting actual size measurements in downstream analyses.
- Often, the animal drifts slightly on the microscope slide; therefore, to eliminate drift in movies, register the images using the FIJI plugin linear stack alignment with SIFT. Plugins > Registration > Linear Stack Alignment with SIFT.
- Save the registered stack as a .avi file. File > Save As > AVI… In the pop-up, set the frame rate (animated figures herein are set to 10 fps) and click OK. Use this output to perform wound healing analysis.
- Analysis of wound area
- Using the lasso tool in FIJI/ImageJ, outline the wound by tracing the cell edges. Measure the wound area that was just outlined with Command+M or CTRL+M.
- Repeat wound area measurement every 10 frames. The measurements from FIJI/ImageJ can then be plotted using Prism 9 (Figure 4).
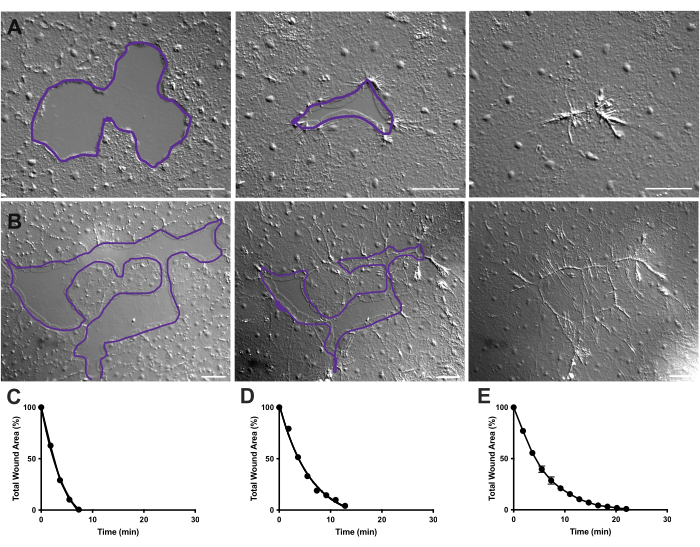
Figure 4: Analysis of wound area in small epithelial wounds. (A) Example of a small epithelial wound healing over 10 min. (B) Example of a different epithelial wound healing over 21 min. The purple outlines in A,B are comparable to the measurements of wound areas using the lasso tool in FIJI/ImageJ. (C) Normalized reduction of the wound area over time in A. (D) Normalized reduction of the wound area over time in B. (E) Average reduction of the wound area over time for 14 small wounds. n = 14. Error bars centered around mean ± SEM. Scale bars: 50 µm Please click here to view a larger version of this figure.
5. Mesogleal injections
- Creating injection dish
- Prepare polydimethylsiloxane (PDMS) by combining a PDMS base and curing agent, in a ratio of 10 parts base to 1 part curing agent by weight. Stir vigorously to fully mix the base and the curing agent.
- To remove bubbles, put the mixture in a vacuum chamber for 15 min. Pour the mixture into a 60 mm Petri dish with microcentrifuge tube caps to hold the mold in place. Immediately place the mold on tube caps at a 45° slant and tape in place. The mold is three stacked, offset glass slides glued together to create ridges in the final injection dish.
- Place the entire dish, mold, and mixture into an oven at 60 °C for 2 h to cure the elastomer. Remove the mold for a completed injection dish.
- Micropipette pulling
- Using a microelectrode puller, design a pulling program. Use a one-step program with high velocity. The heat is approximately the glass RAMP test result55,56. Check resulting micropipettes for long consistent tapers.
NOTE: Use thin wall glass borosilicate capillaries with a 1.0 mm outer diameter, 0.75 mm inner diameter, and 10 cm length.
- Using a microelectrode puller, design a pulling program. Use a one-step program with high velocity. The heat is approximately the glass RAMP test result55,56. Check resulting micropipettes for long consistent tapers.
- Injection of dyes and drugs
- Make a microinjection needle (as above).
- Backfill the microinjection needle using a long pipette tip with an excess volume of dye or drug for injection into the medusa.
NOTE: For Clytia, dimethyl sulfoxide (DMSO) should be kept at a <1:100 dilution with ASW, as higher DMSO concentrations impede wound healing. If injecting a clear solution, Fast Green FCF solution (1:100 dilution of 0.1% Fast Green FCF in ASW) can be added to visualize the injected liquid. - Using a modified transfer pipette as above, place a medusa with the subumbrella facing up into a PDMS injection dish with just enough ASW to cover the animal (Figure 5C). Place the dish on the stage of a dissecting scope.
NOTE: Limiting excess ASW prevents the medusa from swimming in the dish and allows more successful injections. - Focus on the microinjection needle tip and advance it into the water near the medusa. With the micromanipulator, press the needle into the dish until it bends and breaks. This tip opening is approximately 10-20 µm.
NOTE: This needle can be used repeatedly for the same dye/drug injections that day. It is recommended to use a fresh tip each day and for separate dyes/drugs. - Using the micromanipulator, insert the tip of the needle through the subumbrella into the mesoglea without puncturing the exumbrella.
NOTE: A creasing/folding of the epithelium will be noticeable. Once the needle is inserted into the medusa, the creasing/folding ceases. - On the microinjector, set the hold pressure to zero and ejection pressure to ≤20 PSI. Inject into one or two quadrants, filling each with a spot of dye or drug roughly 1/4 of the area of that quadrant.
NOTE: Depending on the size of the medusa, larger or smaller volumes are appropriate in single injection spots. Overfilling the medusa results in extreme damage to the epithelium and even death of the animal. - Depending on what dye or drug is being injected, animals are placed into a beaker of fresh ASW to allow for dye or drug diffusion and incubation.
- For imaging, mount the medusa onto a depression slide using a modified transfer pipette, positioning the animal so the exumbrella is facing up (Figure 5). Animals can be wounded at this stage to test the effect of an injected reagent.
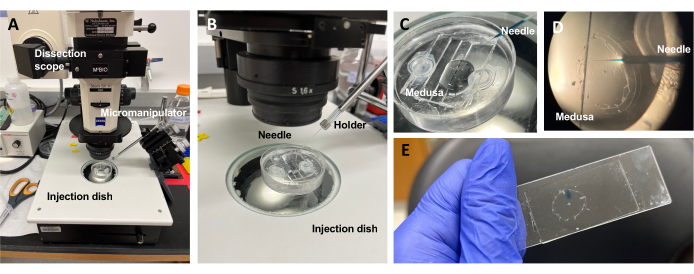
Figure 5: Injection setup for introducing dyes or drugs to the ECM. (A) Injection setup. (B) Close-up of the injection setup showing microinjection needle orientation (approximately 45° angle relative to the animal in the dish). (C) Close-up of the silicone injection dish with the medusa in a small amount of ASW for injection. (D) A microinjection needle loaded with Fast Green FCF entering the mesoglea of the medusa through the subumbrella. (E) Post-injection of Fast Green FCF in a mounted medusa. Please click here to view a larger version of this figure.
Representative Results
Following the protocols above, single-cell microwounds, small wounds, and large wounds were imaged. Registered stacks of image files were saved as .avi files.
In Movie 1, microwounds can be seen to close between and within cells (Figure 1 and Figure 2). Small lamellipodia are observed during closure, followed by contraction and healing. Debris is excluded and released into the water. Healing is completed in a minute or less.
In Movie 2 and 3, small wounds of different shapes heal through the formation of lamellipodia, extension of lamellipodial contacts, and spreading of cells at the wound margin, as previously described40 (Figure 1 and Figure 2). Cells in tiers behind the marginal cells do not participate in healing of wounds of this size nor is there collective cell migration. Rapid and progressive closure of epithelial gaps is followed by tissue contraction along the newly formed wound seam40. The normalized rate of healing of these two wounds, expressed as a percentage of the original area over time, is shown (Figure 4C,D). While there is some variability in the dynamics of wound closure, averaging the percent area closure over time for 14 wounds of various shapes ranging from 0.02-0.125 mm2 allows the establishment of an average curve for wound healing in untreated animals (Figure 4E).
Damage to the basement membrane can be clearly seen when it occurs (Figure 2). In Movie 4, cells at the margin of a small wound in which there is basement membrane damage spread around the damaged area, and gap closure is completed with a purse string contraction.
If the tissue is dehydrated or too damaged to repair, cell movements can stop, or the entire sheet of cells can burst (Movie 5 and Movie 6). This usually happens after long periods of imaging (45 minutes or longer). If cell bursting occurs early in imaging, the sample is discarded.
As shown in Movie 7, large wounds heal in several stages. First, the edge of the wound becomes smooth and regular due to contractions at the margin, as previously reported57. Then, lamellipodia are seen to form from the cells at the wound margin, with lamellipodia moving forward to maximize contact with adjacent lamellipodia. Tracking of the nuclei in cells at the wound margin and several tiers behind the marginal cells shows that large gaps close by collective cell migration40. Cells never detach but move together as a sheet.
The introduction of dyes and pharmacological agents can be a powerful tool for dissecting biological mechanisms. Many substances are excluded from Clytia (not shown), likely because of the mucus layer that coats the surface of the animal. However, microinjection can be used to directly introduce molecules into the ECM, disrupting ECM structure or perturbing regulatory activities in the ECM. In addition, dyes and other molecules are able to enter epithelial cells from the basal side. For example, Figure 6 shows nuclear staining with Hoechst, membrane staining with FM1-43, and inhibition of lamellipodia formation by cytochalasin B after these reagents are microinjected into the ECM. The introduction of these molecules to the ECM and epithelial cells before wounding allows experiments that test the effect of pharmacological tools on the healing process.
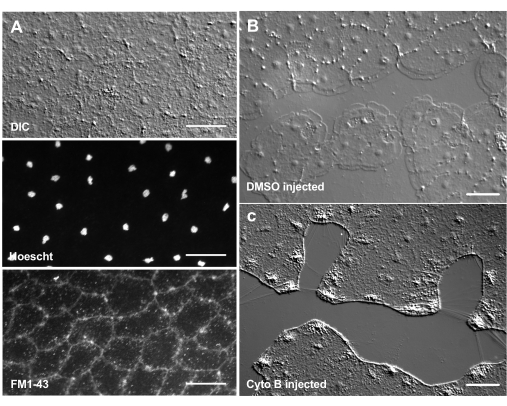
Figure 6: Epithelial cells of the medusa after microinjection of dyes or pharmacological agents. (A) Epithelial cells shown in top panel 5 min after injection with 20 µM Hoechst (nuclei) and 50 µM FM1-43 (membranes).(B,C) Wound healing after injection with 1:1,000 DMSO control (B) or 100 µM Cytochalasin B (C). Wounds were made 15 min after injection. Images were taken 5 min post-wounding. The formation of lamellipodia is inhibited by cytochalasin B. The apparent "fibers" often seen between cells in the wound area are believed to be the result of tension stretching the basement membrane — they do not stain with phalloidin (not shown). Scale bars: 50 µm. Please click here to view a larger version of this figure.
Movie 1: Time-lapse movie of single cell microwound healing. Time elapsed: 20 s. Frame rate: 10 fps. Scale bar: 50 µm. Please click here to download this Movie.
Movie 2: Time-lapse movie of a small epithelial wound healing. Time elapsed: 9 min 54 s. Frame rate: 10 fps. Scale bar: 50 µm. Please click here to download this Movie.
Movie 3: Time-lapse movie of a small epithelial wound healing. This wound is larger and more irregularly shaped than the wound in Movie 2. Time elapsed: 20 min 54 s. Frame rate: 10 fps. Scale bar: 50 µm. Please click here to download this Movie.
Movie 4: Time-lapse movie of a small wound and a microwound healing with a basement membrane tear. Lamellipodia spread around the basement membrane tear, although they can advance over the rest of the ECM. Once the region of the wound with the basement membrane damage is surrounded, a purse string contraction pulls cells over the region. Time elapsed: 19 min 4 s. Frame rate: 10 fps. Scale bar: 50 µm. Please click here to download this Movie.
Movie 5: Cells dying in a small epithelial wound. Cell death is likely due to dehydration of the animal. Time elapsed: 4 min 24 s. Frame rate: 10 fps. Scale bar: 100 µm. Please click here to download this Movie.
Movie 6: A small epithelial wound fails to complete healing. Time elapsed: 42 min 32 s. Frame rate: 10 fps. Scale bar: 50 µm. Please click here to download this Movie.
Movie 7: Large epithelial wound healing. Time elapsed: 25 min 29 s. Frame rate: 10 fps. Scale bar: 100 µm. Please click here to download this Movie.
Supplemental Figure 1: Clytia tank dimension schematics. 3D visualization of the custom-made Clytia tanks. (A) Front and back view. (B) Side view. The cut-out in the piece shown in green is covered with nylon mesh. Water enters the tank directly over the mesh, sweeps over the mesh and creates a circular current. Water exits the system through the hole in the end piece shown in blue.Please click here to download this File.
Supplemental Movie 1: Acellular extracellular matrix in Clytia. Z-stack of Clytia taken using confocal microscopy. The stack initially focuses on the exumbrella and then scans every 10 µm through the ECM to the plate endoderm and subumbrella. Images using DIC (left) and Hoechst nuclear staining (right) demonstrate the lack of cells in the ECM. Scale bar: 100 µm. Please click here to download this File.
Discussion
Here, the methodology is presented for imaging wounds in vivo in Clytia, a relatively new invertebrate model organism40,43,58. There are several factors that make this system a unique and powerful research tool, distinct from other models used to study wound healing and re-epithelialization. First, the monolayer epithelium is attached to a transparent ECM, hence resembling in vitro tissue culture assays (Figure 1, Figure 2, Figure 3, Figure 4). As in in vitro assays, cells can be imaged at high resolution. However, unlike in tissue culture, there is an authentic cellular environment and ECM, so that wound healing can be viewed in the context of the complex signaling events that occur in a live injured animal. Second, Clytia lacks inflammatory responses, migratory fibroblasts, vasculature, and blood. This allows the re-epithelialization process to be studied in vivo in the absence of the overlapping events that occur in more complex adult animals during wound healing59. Third, the ECM is acellular (Supplemental Movie 1) and large, allowing easy access with a microinjection needle (Figure 5 and Figure 6). Using this approach, researchers can test the effect of pharmacological reagents that perturb ECM structure or signaling on wound healing in vivo. Reagents can also be introduced into epithelial cells, and their effects on in vivo wound healing can be assessed. Fourth, there are protocols that exist for creating mutants and transgenic animals in the Clytia system42,43,44,45. In vivo wound healing can therefore be observed in animals with increased/decreased expression of genes of interest.
There are several critical steps in this technique. First, as shown in Figure 3, it is necessary to use a microscope that is correctly configured for DIC microscopy as the flat, transparent epithelial cells are nearly invisible with standard light microscopy. It is also important to develop the skill to wound animals gently so that the epithelium is damaged without gouging the ECM. A similarly gentle touch is necessary for microinjecting materials into the ECM, as extensive damage to the animal during injection might compromise a subsequent analysis of wound healing. While there is a learning curve to these techniques, even beginner students have mastered them quickly in the Malamy lab. Indeed, these protocols have been used to demonstrate cell migration in undergraduate lab courses at The University of Chicago.
For optimal imaging, it is important that the animal does not move and the chosen wound area does not drift out of the field of view. If animals are pulsing, treatment with Tricaine as described is very effective. For drifting, it is often necessary to manually reposition the sample. These movements can be eliminated from the final movie using the registration function in FIJI/ImageJ.
A limitation with this system is that it is not possible to create identical wounds, as wounds vary in both shape and size using the methods described here. Therefore, it can be difficult to quantitate the exact rate of wound closure or cell migration. Positional markers such as carbon grains stick to the exposed ECM in a wounded animal and can be used to measure the rate of collective cell migration in large wounds (not shown). For small wound closure analysis, even with variable wound size and shape, there is a limited range of rates of closure among wounds of this size (Figure 4). It is therefore possible to quantitatively detect the effects of promotive or repressive pharmacological reagents.
While this work describes the characterization of wound healing using only DIC microscopy, the same approaches can be used to image healing using fluorescence or confocal microscopy. To aid in this, protocols are in place to generate transgenic animals in which various cellular and extracellular proteins are fluorescently labeled. Concurrent imaging with DIC and fluorescence, combined with perturbation of wound healing using pharmacological agents or mutant lines, will be a powerful approach to understanding mechanisms that underlie the wound healing process in the epithelium.
Disclosures
The authors have nothing to disclose.
Acknowledgements
E.E.L.L. is supported by a grant from the National Science Foundation PRFB 2011010. We would like to thank Tsuyoshi Momose and Evelyn Houliston for helping us establish our Clytia colonies, Jean-Baptiste Reynier for collection of the microwound healing images, Harry Kyriazes for construction of the pseudo-kreisel tanks, and Elizabeth Baldo for maintaining the Clytia habitat. Figure 1B was created with BioRender.com.
Materials
| 20500 ACE EKE Microscope Fiber Optic Light Source | Kramer Scientific Corporation | ||
| AxioCam 506 mono | ZEISS | 426557-0000-000-MA285 | |
| Capillary tubes | World Precision Instruments | TW1004 | |
| Cytochalasin B | Abcam | ab143482 | |
| Depression slides | Amscope | BS-C12 | |
| DMR with DIC options and fluorescence halogen lamp | Leica | ||
| Ethyl 3-aminobenzoate methanesulfonate | Sigma Aldrich | E10521-10G | |
| Fast Green FCF | Thermo Scientific | A16520-06 | |
| FM1-43 | Biotium | 70022 | Excitation/Emission: 480/598 nm |
| Hoechst 33342 | Thermo Scientific | 62249 | Excitation/Emission: 361/497 nm |
| imageJ | NIH | ||
| Microloader tips (0.5-10 μL /2-20 μL) | Eppendorf | 930001007 | |
| Micromanipulator | World Precision Instruments | 3301R / M3301L | |
| Microscope Cover Glass (22X40-1.5) | Fisherbrand | 12-544-BP | |
| Petri Dish (60 mm x 15 mm) | Fisherbrand | FB085713A | |
| PicoNozzle v2 | World Precision Instruments | 5430-ALL | |
| Pipette puller | Sutter Instrument Co | P-97 | |
| Pneumatic PicoPump | World Precision Instruments | PV820 | |
| Polycarbonate vacuum, desiccator | Bel-art | F42025-0000 | |
| Prism 9 | GraphPad | ||
| STEMI Sv11 Dissection scope | ZEISS | STEMI SV11 | |
| SYLGARD 184 | Dow Silicones | 1024001 | |
| Transfer pipettes | Fisherbrand | 13-711-7M | |
| Z-Hab mini system | Pentair | ||
| ZEN Microscopy software | Zeiss |
References
- Tyler, S. Epithelium-the primary building block for metazoan complexity. Integrative and Comparative Biology. 43 (1), 55-63 (2003).
- Kurn, H., Daly, D. T. Histology, Epithelial Cell. StatPearls. , (2022).
- Schempp, C., Emde, M., Wölfle, U. Dermatology in the Darwin anniversary. Part 1: Evolution of the integument. Journal of the German Society of Dermatology. 7 (9), 750-757 (2009).
- Lopez-Ojeda, W., Pandey, A., Alhajj, M., Oakley, A. M. Anatomy, Skin (Integument). StatPearls. , (2022).
- Bukowiecki, A., Hos, D., Cursiefen, C., Eming, S. A. Wound-healing studies in cornea and skin: parallels, differences and opportunities. International Journal of Molecular Sciences. 18 (6), 1257 (2017).
- Frantz, C., Stewart, K. M., Weaver, V. M. The extracellular matrix at a glance. Journal of Cell Science. 123 (24), 4195-4200 (2010).
- Hynes, R. O. The evolution of metazoan extracellular matrix. The Journal of Cell Biology. 196 (6), 671-679 (2012).
- Fidler, A. L., et al. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. eLife. 6, 24176 (2017).
- Bullen, T. F., et al. Characterization of epithelial cell shedding from human small intestine. Laboratory Investigation; a Journal of Technical Methods and Pathology. 86 (10), 1052-1063 (2006).
- Watson, A. J. M., et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 129 (3), 902-912 (2005).
- Sonnemann, K. J., Bement, W. M. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annual Review of Cell and Developmental Biology. 27, 237-263 (2011).
- Abreu-Blanco, M. T., Verboon, J. M., Parkhurst, S. M. Single cell wound repair. BioArchitecture. 1 (3), 114-121 (2011).
- Fenteany, G., Janmey, P. A., Stossel, T. P. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Current Biology. 10 (14), 831-838 (2000).
- Pastar, I., et al. Epithelialization in wound healing: a comprehensive review. Advances in Wound Care. 3 (7), 445-464 (2014).
- Rousselle, P., Braye, F., Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Advanced Drug Delivery Reviews. 146, 344-365 (2019).
- Bement, W. M., Forscher, P., Mooseker, M. S. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. The Journal of Cell Biology. 121 (3), 565-578 (1993).
- Vedula, S. R. K., Ravasio, A., Lim, C. T., Ladoux, B. Collective Cell migration: a mechanistic perspective. Physiology. 28 (6), 370-379 (2013).
- Li, L., He, Y., Zhao, M., Jiang, J. Collective cell migration: Implications for wound healing and cancer invasion. Burns & Trauma. 1 (1), 21-26 (2015).
- Bornes, L., Windoffer, R., Leube, R. E., Morgner, J., van Rheenen, J. Scratch-induced partial skin wounds re-epithelialize by sheets of independently migrating keratinocytes. Life Science Alliance. 4 (1), 202000765 (2021).
- Theveneau, E., Mayor, R. Collective cell migration of epithelial and mesenchymal cells. Cellular and Molecular Life Sciences. 70 (19), 3481-3492 (2013).
- Haensel, D., Dai, X. Epithelial-to-mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Developmental Dynamics. 247 (3), 473-480 (2018).
- Cordeiro, J. V., Jacinto, A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nature Reviews. Molecular Cell Biology. 14 (4), 249-262 (2013).
- Abreu-Blanco, M. T., Watts, J. J., Verboon, J. M., Parkhurst, S. M. Cytoskeleton responses in wound repair. Cellular and Molecular Life Sciences. 69 (15), 2469-2483 (2012).
- Klarlund, J. K., Block, E. R. Free edges in epithelia as cues for motility. Cell Adhesion & Migration. 5 (2), 106-110 (2011).
- Enyedi, B., Niethammer, P. Mechanisms of epithelial wound detection. Trends in Cell Biology. 25 (7), 398-407 (2015).
- Niethammer, P. The early wound signals. Current Opinion in Genetics & Development. 40, 17-22 (2016).
- Jacinto, A., Martinez-Arias, A., Martin, P. Mechanisms of epithelial fusion and repair. Nature Cell Biology. 3 (5), 117-123 (2001).
- Mayor, R., Etienne-Manneville, S. The front and rear of collective cell migration. Nature reviews. Molecular Cell Biology. 17 (2), 97-109 (2016).
- Gupta, S., Yap, A. S. How adherens junctions move cells during collective migration. Faculty Reviews. 10, 56 (2021).
- Blair, M. J., Jones, J. D., Woessner, A. E., Quinn, K. P. Skin structure-function relationships and the wound healing response to intrinsic aging. Advances in Wound Care. 9 (3), 127-143 (2020).
- Falanga, V., et al. Chronic wounds. Nature Reviews. Disease Primers. 8 (1), 50 (2022).
- Liang, C. -. C., Park, A. Y., Guan, J. -. L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols. 2 (2), 329-333 (2007).
- Jonkman, J. E. N., et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adhesion & Migration. 8 (5), 440-451 (2014).
- Poujade, M., et al. Collective migration of an epithelial monolayer in response to a model wound. Proceedings of the National Academy of Sciences. 104 (41), 15988-15993 (2007).
- Masson-Meyers, D. S., et al. Experimental models and methods for cutaneous wound healing assessment. International Journal of Experimental Pathology. 101 (1-2), 21-37 (2020).
- Park, S., et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nature Cell Biology. 19 (2), 155-163 (2017).
- Tsai, C. -. R., Wang, Y., Galko, M. J. Crawling wounded: molecular genetic insights into wound healing from Drosophila larvae. The International Journal of Developmental Biology. 62 (6-7-8), 479-489 (2018).
- Richardson, R., et al. Adult zebrafish as a model system for cutaneous wound-healing research. The Journal of Investigative Dermatology. 133 (6), 1655-1665 (2013).
- Erickson, J. R., Echeverri, K. Learning from regeneration research organisms: The circuitous road to scar free wound healing. Developmental Biology. 433 (2), 144-154 (2018).
- Kamran, Z., et al. In vivo imaging of epithelial wound healing in the cnidarian Clytia hemisphaerica demonstrates early evolution of purse string and cell crawling closure mechanisms. BMC Developmental Biology. 17 (1), 17 (2017).
- Chari, T., et al. Whole-animal multiplexed single-cell RNA-seq reveals transcriptional shifts across Clytia medusa cell types. Science Advances. 7 (48), (2021).
- Weissbourd, B., et al. A genetically tractable jellyfish model for systems and evolutionary neuroscience. Cell. 184 (24), 5854-5868 (2021).
- Momose, T., et al. High doses of CRISPR/Cas9 ribonucleoprotein efficiently induce gene knockout with low mosaicism in the hydrozoan Clytia hemisphaerica through microhomology-mediated deletion. Scientific Reports. 8 (1), 11734 (2018).
- Houliston, E., Leclère, L., Munro, C., Copley, R. R., Momose, T. Past, present and future of Clytia hemisphaerica as a laboratory jellyfish. Current Topics in Developmental Biology. 147, 121-151 (2022).
- Schmid, V., et al. The extracellular matrix (mesoglea) of hydrozoan jellyfish and its ability to support cell adhesion and spreading. Hydrobiologia. 216 (1), 3-10 (1991).
- Day, R. M., Lenhoff, H. M. Hydra mesoglea: a model for investigating epithelial cell-basement membrane interactions. Science. 211 (4479), 291-294 (1981).
- Zhang, X., et al. The collagens of hydra provide insight into the evolution of metazoan extracellular matrices. The Journal of Biological Chemistry. 282 (9), 6792-6802 (2007).
- Danjo, Y., Gipson, I. K. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. Journal of Cell Science. 111 (22), 3323-3332 (1998).
- Arenas Gómez, C. M., Sabin, K. Z., Echeverri, K. Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Developmental Dynamics. 249 (7), 834-846 (2020).
- Lechable, M., et al. An improved whole life cycle culture protocol for the hydrozoan genetic model Clytia hemisphaerica. Biology Open. 9 (11), (2020).
- Casares, L., et al. Hydraulic fracture during epithelial stretching. Nature Materials. 14 (3), 343-351 (2015).
- Wayne, R. Chapter 4 – Bright-Field Microscopy. Light and Video Microscopy (Third Edition). , 95-116 (2019).
- Murphy, D. B., Davidson, M. W. . Fundamentals of Light Microscopy and Electronic Imaging: Second Edition. , (2012).
- . Micropipette Techniques for Electrophysiology Available from: https://www.sutter.com/micropipette/cookbook.html (2022)
- Brown, A. L., Johnson, B. E., Goodman, M. B. Making patch-pipettes and sharp electrodes with a programmable puller). Journal of Visualized Experiments. (20), e939 (2008).
- Klarlund, J. K. Dual modes of motility at the leading edge of migrating epithelial cell sheets. Proceedings of the National Academy of Sciences. 109 (39), 15799-15804 (2012).
- Houliston, E., Momose, T., Manuel, M. Clytia hemisphaerica: a jellyfish cousin joins the laboratory. Trends in Genetics. 26 (4), 159-167 (2010).
- Rodrigues, M., Kosaric, N., Bonham, C. A., Gurtner, G. C. Wound healing: a cellular perspective. Physiological Reviews. 99 (1), 665-706 (2019).

