Measuring O2 Consumption in Drosophila melanogaster Using Coulometric Microrespirometry
Summary
Coulometric respirometry is ideal for measuring the metabolic rate of small organisms. When adapted for Drosophila melanogaster in the present study, measured O2 consumption was within the range reported for wildtype D. melanogaster by previous studies. Per-fly O2 consumption by CASK mutants, which are smaller and less active, was significantly lower than the wildtype.
Abstract
Coulometric microrespirometry is a straightforward, inexpensive method for measuring the O2 consumption of small organisms while maintaining a stable environment. A coulometric microrespirometer consists of an airtight chamber in which O2 is consumed and the CO2 produced by the organism is removed by an absorbent medium. The resulting pressure decrease triggers electrolytic O2 production, and the amount of O2 produced is measured by recording the amount of charge used to generate it. In the present study, the method has been adapted to Drosophila melanogaster tested in small groups, with the sensitivity of the apparatus and the environmental conditions optimized for high stability. The amount of O2 consumed by wildtype flies in this apparatus is consistent with that measured by previous studies. Mass-specific O2 consumption by CASK mutants, which are smaller and known to be less active, was not different from congenic controls. However, the small size of CASK mutants resulted in a significant reduction in O2 consumption on a per-fly basis. Therefore, the microrespirometer is capable of measuring O2 consumption in D. melanogaster, can distinguish modest differences between genotypes, and adds a versatile tool for measuring metabolic rates.
Introduction
The ability to measure metabolic rate is crucial for a complete understanding of an organism in its environmental context. For example, it is necessary to measure metabolic rate in order to understand its role in lifespan1, the role of diet in metabolism2, or the threshold for hypoxic stress3.
There are two general approaches to measuring the metabolic rate4. Direct calorimetry measures energy expenditure directly by measuring heat production. Indirect calorimetry measures energy production through other means, often via respirometric measurement of O2 consumption (VO2), CO2 production or both. Although direct calorimetry has been applied to small ectotherms, including Drosophila melanogaster5, respirometry is technically simpler and more commonly used.
Several forms of respirometry have been used successfully to measure metabolic rate in wildtype and mutant D. melanogaster and have provided insight into the metabolic effects of temperature6, social environment3, diet3,7,and neurodevelopmental disorders8. These fall into two classes, which vary considerably in cost and complexity. Manometry is the simplest and least expensive9,10, in which flies are placed into a sealed chamber that contains a CO2 absorbent and which is connected via a thin capillary to a fluid reservoir. As O2 is consumed and CO2 absorbed, pressure in the chamber decreases and fluid is drawn into the capillary. The fluid-filled volume of the capillary is therefore proportional to VO2. More elaborate versions, which compensate for the force exerted by the fluid in the capillary, have also been used on D. melanogaster1. Manometry has the advantages of being simple and inexpensive, but, because it is sensitive to pressure, requires constant environmental conditions. Further, because consumed O2 is not replaced, the partial pressure of O2 (PO2) gradually decreases inside the chambers.
Respirometry using gas analysis is also regularly used for D. melanogaster. In this case, gases are sampled at regular intervals from sealed chambers containing flies and sent to an infrared analyzer2,6,11. This type of apparatus has the advantages that it is available commercially, is less sensitive to environmental conditions, and gases are refreshed during sampling so that PO2 remains stable. However, the equipment can be expensive and complex to operate.
A recently developed coulometric microrespirometer12 provides an inexpensive, sensitive, and stable alternative to existing systems. In practice, an organism is placed into an airtight chamber where it consumes O2 and the exhaled CO2 is removed by an absorbent material, resulting in a net decrease in chamber pressure. When the internal pressure decreases to a pre-set threshold (ON-threshold), current is passed through an electrolytic O2 generator, returning pressure to a second threshold (OFF-threshold) stopping electrolysis. Charge transfer across the O2 generator is directly proportional to the amount of O2 required to re-pressurize the chamber and can therefore be used to measure the O2 consumed by the organism4. The method is highly sensitive, measures VO2 precisely, and the regular replacement of O2 can maintain PO2 at a nearly constant level for hours or days.
The coulometric microrespirometer used in this study employs a multi-modal (pressure, temperature, and humidity) electronic sensor. The sensor is operated by a microcontroller that detects small changes in pressure and activates O2 generation when a low pressure threshold is reached12. This apparatus is assembled from off the shelf parts, can be used with a wide variety of chambers and experimental environments, and has been employed successfully to examine the effects of body mass and temperature on the beetle Tenebrio molitor. In the present study, the microrespirometer has been adapted to measure O2 consumption in D. melanogaster, which has approximately 1% of the mass of T. molitor. Sensitivity of the apparatus has been increased by reducing the threshold for activating O2 generation, and environmental stability has been enhanced by conducting experiments in a temperature-controlled water bath and by maintaining humidity inside the chambers at or near 100%.
The CASK (Calmodulin-dependent Serine Protein Kinase) protein, part of the family of membrane-associated guanylate kinases (MAGUK), is a molecular scaffold in different multi-protein complexes, and mutations in CASK are associated with neurodevelopmental disorders in humans and in D. melanogaster13,14. A viable D. melanogaster mutant, CASKΔ18, disrupts activity of dopaminergic neurons15 and reduces activity levels by more than 50% compared to congenic controls14,16. Because of the reduced activity levels of CASK mutants and the role of catecholamines in regulating metabolism17 we hypothesized that their standard metabolic rate, and therefore O2 consumption, would be dramatically reduced compared to controls.
O2 consumption was measured in CASKΔ18 and their wildtype congeners, w(ex33). Groups of flies were placed into respirometry chambers, O2 consumption was measured, O2 consumption was calculated and expressed on both a mass-specific and per-fly basis. The apparatus recorded VO2 in wildtype flies that was consistent with previous studies, and it could differentiate between the per-fly O2 consumption of wildtype and CASK mutant flies.
Protocol
1. Fly rearing and collection
- Maintain flies at 25 °C in narrow vials containing standard Drosophila food.
NOTE: The sample size for each genotype should comprise at least nine replicates, each consisting of a single respirometer chamber containing 15-25 flies, set up as described below. - Transfer the flies every 2-3 days.
- Anesthetize flies with CO2, collect groups of 15-25 males of each genotype, and place each group into fresh, unyeasted food vials.
NOTE: Males were used to reduce variability due to reproductive status. The method applies to both sexes. - Allow the flies to recover at 25 °C for at least 24 h.
NOTE: By the time of the experiment, flies should be 1-4 days old. The frequency of collections described in step 1.3 can be set to narrow the age range of the flies.
2. Setup and assembly of respirometer chamber
- Turn on the water bath and set it to the desired temperature for the experiment.
NOTE: The experiments below were conducted at 25 °C using 50 mL Schlenk tubes as chambers. Components are to be assembled as shown in Figures 1A, 1B, and 1C. - Clean the ground glass joints of chambers and sensor plugs thoroughly by spraying 70% ethanol onto a laboratory wipe (not directly onto the joint) and wiping dust and old grease from the sensor plug (Figure 1A). Wipe off ethanol with a fresh laboratory wipe.
- Place 1 cm piece of cotton roll soaked in purified water into the bottom of the chamber to stabilize the humidity.
- Add enough water (~0.5 mL) to form a small pool at the bottom of the cotton roll.
- Wipe off any water that has spilled onto the joint of the chamber.
- Transfer the flies to labeled polypropylene tubes using a funnel.
- Plug the tube with a cotton roll.
NOTE: Tubes consist of a 5 mL polypropylene test tube, trimmed to 5.5 cm in length and perforated with a hot knife to allow the free exchange of air with the experimental chamber. CO2 anesthesia is known to cause metabolic abnormalities, so flies are transferred without anesthesia which requires more care to avoid losing the flies.
- Plug the tube with a cotton roll.
- Add one ventilated tube with flies into each respirometer chamber (on top of wet cotton).
- Fill soda lime cartridges (4-5 pellets per tube) and place them on the top of the tube containing flies inside the chamber.
NOTE: Soda lime cartridges consist of 800 µL centrifuge tubes perforated 4-5 times with a power drill. - Fill O2 generators with saturated copper sulfate (CuSO4) solution below level of vent holes
NOTE: O2 generators consist of screw-cap centrifuge tubes with 4 holes drilled below the threads. Platinum (Pt) and Copper (Cu) electrodes are soldered to two-pin connector, inserted into holes drilled in cap, and affixed with epoxy. Electrolysis of CuSO4 generates the O2 consumed by the experimental organism. CuSO4 is toxic to invertebrates, avoid spills or leakage and clean up immediately. - Connect the filled O2 generator to two-pin connector on the sensor plug.
NOTE: The copper cathode must connect with the negative output of the controller and the platinum anode to the positive wire. Reversed connections will cause the failure of the experiment. - Place two small dabs of clear silicone grease on opposite sides of the ground glass joint of the sensor plug.
- Insert the plug into the chamber and rotate the plug (or chamber) with moderate pressure to spread the grease in the joint.
- Wipe off excess grease with a laboratory wipe.
- Snap plastic Keck clamps onto joints to secure plugs in chambers. The assembled chamber should look like Figure 1C.
- Repeat the above steps for the number of chambers used for the day's experiment.
NOTE: The number of chambers that can be recorded is limited by the number of available chambers, controllers and USB inputs to the computer. For the present experiments, seven chambers were normally run in parallel. Experimental flies such as mutants should be matched with appropriate controls. A chamber set up identically but without flies should be included in each experiment as a control for environmental variation. Chambers containing different treatments (mutant, wildtype, no-fly) should be rotated between experiments. - Place assembled chambers into a rack in the water bath with stopcocks open (Figure 1E).
NOTE: To avoid circadian variation, chambers were placed into the bath between 9:30 and 9:50 am for all experiments described here. - Leave stopcocks open (Keep the handle parallel to the stopcock).
NOTE: Be careful not to allow water to enter the stopcocks. - Allow the chambers to equilibrate with stopcocks open for about 30 min.
NOTE: While the chamber is equilibrated, connect the electronics and set up data acquisition as described below.
3. Setting up controllers and computer
- Be sure that the switches supplying current to the O2 generators are in the OFF position (away from the connector; Figure 1D).
- Plug each controller box into an available Universal serial bus (USB) port.
NOTE: Construction and programming of controller units described elsewhere12. - Connect controllers to respirometer chambers using 6-conductor cables.
- Check that the organic light emitting diode (OLED) displays of the controllers (Figure 1D) are displaying environmental parameters.
- Briefly turn on O2 generators using the switch on the controller (Figure 1D).
- If the current value increases from zero to between 35 and 55 mA, the controller and chamber are ready for experiments.
- Determine which COM ports are being used by the controllers, as described below.,
- Click the Start Icon in Microsoft Windows.
- Click the Settings Icon.
- Click Bluetooth and Devices.
- Ensure that the controllers and their COM ports appear in the list of devices.
- Open PuTTY on the desktop and set up a log file for each channel of the respirometer as described below.
NOTE: PuTTY is a free secure shell and telnet client that is used to transfer data to the computer via COM ports.- Select COM port for a controller by typing the number of the port in the "Serial line" box (Figure 2A).
- Click on Logging.
- Select Printable Output in "Session logging" (Figure 2B).
- Under Log File Name click Browse.
- In the folder of the choice, create a filename containing descriptive information (e.g., date, species, COM port number).
- Click Save.
- Click Open. A window will open showing comma-delimited data being logged (Figure 2C).
- Repeat for all other controllers in use for the experiment. Input to each COM port will appear as a separate window (Figure 2D).
4. Running experiments
- Once chambers have equilibrated for 30 min, seal them by closing stopcocks.
- Cover the bath and chambers with a polystyrene foam box to maintain a stable environment.
- Allow to equilibrate for another hour.
- Turn on the current to the O2 generator of each chamber using the switch on the controller box.
- Once the O2 generators are activated, ensure that the pressure increases to pre-set OFF pressure.
NOTE: 1017 hPa, which is slightly above atmospheric pressure, was used as the "OFF" pressure in this series of experiments. Return to the ambient pressure will indicate leakage of gas from the chambers. Further, it allows the same pressure to be used across experiments regardless of ambient barometric pressure. The "ON" pressure was 1016 hPa, meaning that pressure only needed to drop 1 hPa before the O2 generator was activated. This provided adequate sensitivity to measure O2 consumption in Drosophila. Once a chamber is pressurized to the "OFF" setting, current should drop to zero. - Let the experiment run for 3 or more h.
NOTE: Higher VO2 at elevated temperatures can allow for shorter experiment times. Monitor occasionally to ensure that equipment is functioning but avoid excessive activity near the chambers that may affect temperature stability.
5. Finishing experiment
- Turn off O2 generators on all controllers.
NOTE: Do first to avoid running the O2 generators while the chambers are open. - Open the stopcocks to unseal the chambers.
- Leave the PuTTY windows open for another 5-15 min to provide a final baseline.
- Close the PuTTY window for each controller, ending recordings.
NOTE: All experiments ended between 4:50 and 5:10 pm. - Disconnect sensors from cables.
- Move chambers to dry rack.
- Remove sensor plugs one at a time from the chambers.
- Disconnect the O2 generators and place them in the tube rack.
- Wipe grease off the sensor plug and keep it in the rack.
- Clean grease from chamber joints and remove tubes with flies and soda lime.
- Anesthetize flies in each tube with CO2, tap onto a weight boat and weigh on a microbalance.
- Log the weight and number of flies for each tube.
- Discard flies or set them aside for additional procedures.
- Dump soda lime from cartridges into the waste container.
- Open the O2 generator and discard the CuSO4 solution into the waste container.
- Rinse electrodes and tube with purified water.
- Place the tube racks for drying.
6. Analysis of charge transfer data
- Import Data as comma-delimited text into a spreadsheet, with each record comprising a separate worksheet.
- Record the current and time data for each pulse of the O2 generator. Starting with the first pulse after the chamber was pressurized, record the start time and end time (as row numbers) of each current pulse. That is the row number when the current goes above zero (usually to about 45-50 mA) to the last row that is above zero.
- Make a table on the worksheet to record the following data:
- The average current amplitude during the pulse: = AVERAGE([first row of pulse]:[last row of pulse]) for each pulse (from the current column).
- Pulse duration: ([Last row of pulse] – [first row of pulse[-one row]])/1000 for each pulse (from the time in milliseconds column).
- Total experiment time: [time at start of last pulse] – [time at end of first pulse after chamber pressurized] (from the time in minutes column).
- Then calculate charge transfer (Q) for each pulse (average current X duration)
- Sum the charge from all pulses to calculate Total Charge (Qtot).
7. Analysis of O2 consumption
- Set up a new spreadsheet for all data and enter or calculate the following for each chamber:
- Qtot (total charge)
- Moles (= Q ÷ 96485 × 4)
- mL O2 (= moles × 22413 mL/mol)
- Total time (from the data analysis above)
- mL min-1 (= ml O2 ÷ total time)
- Weight in grams (flies anesthetized and weighed measured after the experiment)
- mL min-1 g-1 (= mL min-1 ÷ weight in grams)
- mL/h/g (the above × 60)
- mg/fly (= weight of flies ÷ number of flies)
- μL fly-1 h-1 (= (mL min-1 × 3600) ÷ number of flies).
- Tabulate data for each treatment (genotype, e.g.)
- Compare treatments using ANOVA, t-test, or Mann-Whitney u-test 13.
Representative Results
The pressure and current outputs of the respirometer controller are shown for one chamber in one experiment in Figure 3A. The first, long current pulse pressurized the chamber from ambient pressure (approximately 992 hPa) to the pre-set OFF threshold of 1017 hPa. As the flies consumed O2 and CO2 was absorbed, pressure decreased slowly until it reached the ON threshold of 1016 hPa, which activated current through the O2 generator. In the example shown, the average amplitude of each pulse is 50.1 mA, the duration is 16.1 s, yielding a charge transfer of about 0.81 coulombs (C) per pulse. The total charge transfer for this chamber was 3.28 C over a total time of 240.0 min. Using the calculations described in Procedures with the mass and number of flies (23 flies weighing 14.9 mg total), O2 consumption for the group in this chamber was 3.19 mL h-1 g-1 or 2.07 µL h-1 fly-1.
The equipment can be set up easily, with a minimum of training, and performs reliably for many cycles of assembly and shutdown. Nonetheless, equipment must be maintained and inspected regularly, and experimental conditions must be controlled carefully. For example, the loss of a gastight seal, due to failure of a joint or stopcock, can lead to rapid pressurization cycles and spuriously high VO2 (Figure 3B). Additionally, temperature and humidity must remain stable inside the chamber. If temperature or humidity decreases, the resulting pressure drop will be interpreted erroneously as a O2 being consumed. Conversely, upward drift in temperature or humidity will counteract the pressure decrease caused by O2 consumption, and artificially reduce or eliminate the VO2 signal (Figure 3C).
The method was used to test VO2 of CASKΔ18 mutants, which were generated by imprecise excision of a transposable element from the CASK locus14, and in which locomotion is drastically reduced14,16. In wild-type w(ex33) controls, generated by precise excision of the transposable element, average mass-specific O2 consumption was 3.65 ± 0.24 mL·g-1·h-1 (n = 16 chambers; Figure 4A).
Despite their visibly reduced locomotion, CASKΔ18 mutants' VO2 was slightly but not significantly lower than that of controls (mean ± s.e.m.= 3.23 ± 0.13 mL·g-1·h-1; n = 11 groups; P = 0.08 Mann-Whitney u-test).
Because the validity of expressing metabolic rate in terms of body mass has been questioned18, O2 consumption was also analyzed on a per-fly basis (Figure 4B). Using this analysis, VO2 was significantly reduced in CASKΔ18 compared to wild-type controls (ex33: 2.22 ± 0.13 µL·fly-1·h-1; CASKΔ18: 1.58 ± 0.10 µL·fly-1·h-1; P = 0.0003, Mann-Whitney u-test). However, the mean mass of CASKΔ18 flies was >20% lower than that of ex33 controls (Figure 4C; ex33 0.61 ± 0.01 mg; CASKΔ18 0.51± 0.02 mg; P = 0.0005, Mann-Whitney u-test), so the difference in metabolic rate between genotypes is probably due to the difference in their sizes.
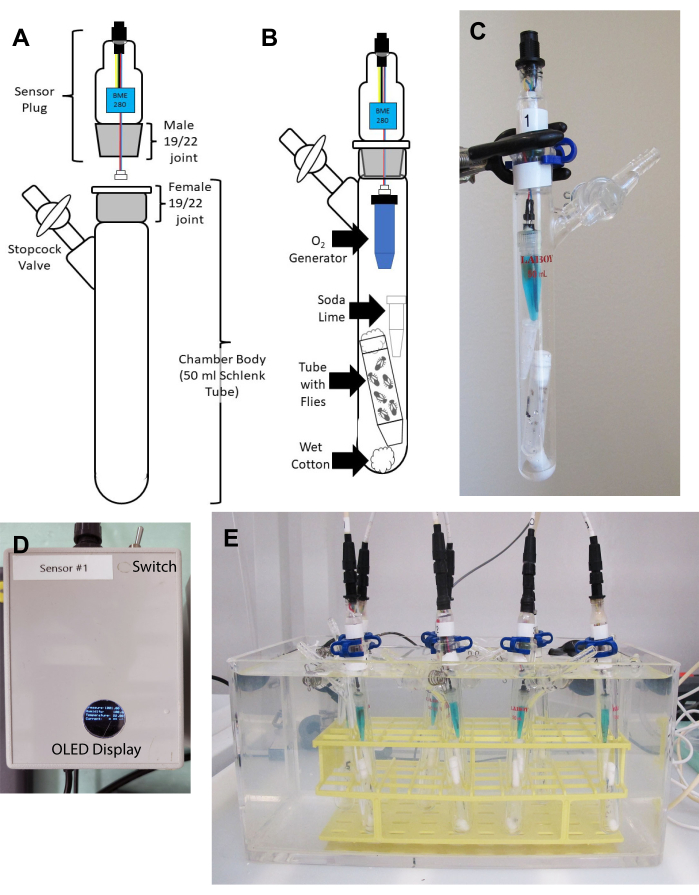
Figure 1: Respirometer setup. (A) Diagram of sensor plug (above) and 50 mL chamber (consisting of a 50 mL Schlenk tube, below) before assembly. Note the locations of the 19/22 ground glass joints that will connect the chamber and sensor plug, and that must be cleaned before each experiment. The stopcock, which is necessary for opening or sealing the chamber, is also indicated. (B) Diagram of chamber and components, assembled and ready for the experiment, showing: wet cotton roll, polypropylene tube containing flies, plugged with a cotton stopper, soda-lime cartridge, and O2 generator filled with CuSO4. (C) Photograph of the assembled chamber. The Keck clamp securing the plug to the chamber is partially obscured by the ring stand clamp holding the chamber. (D) Photograph of controller showing switch controlling current through O2 generator and window for viewing OLED display. (E) Assembled chambers in a water bath. Seven chambers are shown, with three containing mutants, three with wildtype controls, and one chamber containing all components except flies. Please click here to view a larger version of this figure.
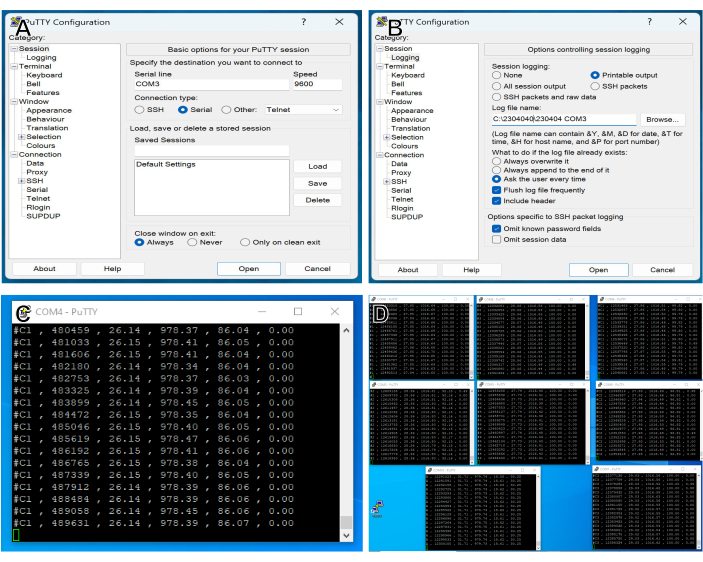
Figure 2: Data acquisition setup. (A) PuTTY interface for selecting the serial port for data acquisition. COM3 has been selected, with a BAUD rate of 9600 to match the output of the controller. (B) PuTTY interface for setting up log file. "Printable output" is selected to enable logging of data to a text file, data folder is selected using the "Browse" button, and a filename is created. (C) PuTTY log file during an experiment. Data are acquired approximately twice per second, and each line contains the following comma-delimited information: Sensor Number, time (ms) since the beginning of acquisition, chamber temperature (°C), chamber pressure (hPa), Humidity (percent relative), and current (mA). (D) Data logging during a typical experiment, with seven windows for experimental chambers, plus one channel recording bath temperature, ambient air temperature, pressure, and humidity. Please click here to view a larger version of this figure.
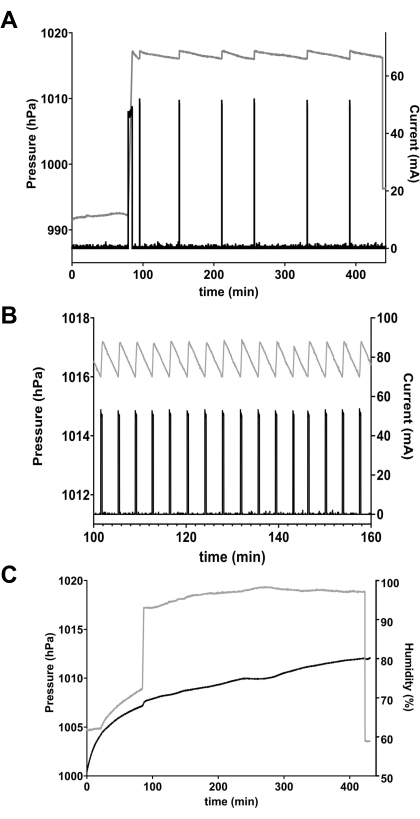
Figure 3: Data from microrespirometer. (A) Pressure (grey line, left axis) and current across the O2 generator (black line, right axis) in a single respirometer chamber containing 23 w(ex33) flies. At the beginning, a long current pulse is required to pressurize the chamber from ~992 hPa to the OFF threshold of 1017 hPa. As the flies consumed O2, pressure dropped until it reached the ON threshold of 1016 hPa, which activated current through the O2 generator, which re-pressurized the chamber to 1017 hPa. The process was repeated six times in this experiment. (B) An example of a leaky chamber caused by a damaged stopcock, taken from a different series of experiments. The chamber failed to maintain pressure (grey line), resulting in constant cycling of electrolytic current (black line). Note different timescale from panel A. (C) Effect of drift in humidity. O2 consumption by the 20 mg lady beetle (Hippodamia convergens) in the chamber should have produced a cycling pattern of pressure similar to Figure 3A, but the steady increase in humidity (black line) caused an artifactual increase in chamber pressure (grey line) that masked VO2. Please click here to view a larger version of this figure.
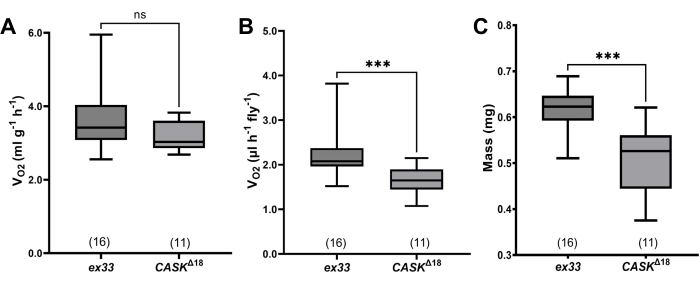
Figure 4: Quantitative data from wild-type and CASK mutant D. melanogaster. (A) Mass-specific VO2 for wild-type (w(ex33)) and mutant (CASKΔ18) flies. In all plots, bottoms and tops of boxes indicate first and third quartiles, respectively, whiskers indicate extreme values, and the sample sizes (numbers of chambers, each containing 17-24 flies) are given in parentheses above the genotypes. CASKΔ18 flies are not statistically significantly different from ex33 (median: ex33: 3.420 mL·g-1·h-1, CASKΔ18: 3.029 mL·g-1·h-1; p = 0.08 Mann-Whitney u-test). (B) Fly-specific VO2. CASKΔ18 flies consumed significantly less O2 (median 1.650 mL·fly-1·h-1) than w(ex33) (2.078 mL·fly-1·h-1; p = 0.0003, Mann-Whitney u-test; significance indicated by asterisks). (C) Mass differed between CASKΔ18 and the wildtype (median: 0.526 mg, ex33: 0.623 mg; p = 0.0005, Mann-Whitney u-test; significance indicated by asterisks). Please click here to view a larger version of this figure.
Table 1. Survey of Drosophila respirometry data from wildtype flies at 25 °C. With one exception18, studies are limited to those measuring O2 consumption. In most cases, it was necessary to estimate VO2 from graphs, and figure numbers from the original papers are provided. Although all genotypes were considered to be "wildtype" the sources and propagation methods varied. Please click here to download this Table.
Discussion
The above procedure demonstrates measurement of O2 consumption in D. Melanogaster using an electronic coulometric microrespirometer. The resulting data for O2 consumption in wild-type D. melanogaster were within the ranges described in most previous publications using diverse methods (Table 1) although somewhat lower than that reported by others3,6.
Critical steps addressed the two absolute requirements of the method: gastight seal and environmental stability. Maintaining a gastight environment is straightforward but requires care. Schlenk flasks and tubes are ideally suited as respirometry chambers. Glass construction avoids gas permeability present in many types of plastic19, which is especially critical in prolonged experiments. The standard joints allow gastight connections with the plugs containing the sensors and electronic connections. Stopcocks provide reliable seals and can be opened or closed as needed during experiments. To ensure gastight seals for each experiment, stopcocks were inspected, joints were cleaned thoroughly, judicious amounts of clean silicone grease were applied, and joints were secured with Keck clamps.
The sensitivity of the method is limited by the stability of temperature and humidity within the chamber. Changes in either parameter will result in pressure fluctuations that interfere with the VO2 signal. Use of an environmental chamber12 or circulating water bath provides more stable temperature control. Previous work by others has demonstrated that the method can measure O2 consumption at the nmol·h-1 level when temperature was maintained within ±0.01 °C20. To stabilize humidity, pieces of cotton roll immersed in water maintained humidity at nearly 100%. Temperature and humidity were allowed to stabilize for at least 90 min before chambers were pressurized with electrolytically generated O2 and recordings begun. All experiments included one chamber that was assembled with all components but did not contain flies, in order to monitor environmental conditions.
Constantly monitoring the temperature, pressure and humidity regularly greatly simplifies troubleshooting. For example, the artificially high apparent VO2 caused by a damaged stopcock (Figure 3B) or the apparent loss of VO2 due to unstable chamber humidity (Figure 3C) were able to be detected and corrected.
The coulometric microrespirometer described here has several advantages. First, it is relatively inexpensive, with an approximate total cost of $100 per channel (comprising chamber, sensor and controller). Second, because each channel acquires and transmits data independently, the system is scalable, with the number of chambers only limited by the size of the environmental controller (water bath or environmental chamber) and the number of available USB ports in the computer (generally expandable to >100). Also, because the chambers and controllers are independent, failure of any of them will not affect the others. Third, controllers can be easily reprogrammed to adapt to ambient pressure or to adjust sensitivity as needed. Related to this, the pressure inside the chambers is set to a fixed value, so experimental pressure will be constant across experiments regardless of ambient barometric pressure. Fourth, the steady stream of data regarding time, temperature, pressure, humidity, and current allows detailed analysis of these parameters for each chamber, facilitating troubleshooting or analysis of temporal changes in VO2 in longer experiments. Finally, the environmental conditions inside the chamber can be maintained at a constant level for many hours or days, because consumed O2 is continually replaced. In the present study, chamber pressure fluctuated from 1016 to 1017 hPa, which is less than 0.1%, with the resulting variation in PO2 being less than 0.5%. Based on the amount of CuSO4 in each O2 generator, which can generate 28 mL of O2, and the average VO2 of groups of flies in this study, 1.15 mL/day (mean = 21.8 flies per chamber), metabolic rate can be studied for up to 24 days at a time. Assuming the flies are provided with adequate nutrition, this means that metabolism can be studied continuously for most of a fly's lifespan.
At present, most studies of metabolism in D. melanogaster are based on gas analysis or manometry. Methods that rely on gas analysis, such as stop-flow and continuous flow respirometers2,3,6 have the advantage that the method is well-established and equipment is available commercially. However, they require expensive and complex equipment, including manifolds and molecular flow sensors to regulate flow, and gas analyzers to measure gas concentrations. Alternatively simple manometers9 are far less expensive. In the simplest form, commonly in use for D. melanogaster, flies are anesthetized and placed in a small chamber which contains CO2 absorbent material and which is connected to a fluid reservoir by a capillary tube. As O2 is consumed, and the resulting CO2 absorbed, pressure drops in the chamber, which draws fluid into the capillary tube. The height of the fluid is then proportional to the amount of O2 consumed. Because consumed O2 is not replaced during the experiment, PO2 decreases continuously over the course of the experiment. Although routine experiments may not reduce PO2 sufficiently to negatively affect VO2, prolonged experiments, or experiments at elevated temperatures could deplete O2 and reach the critical PO2 at which VO2 declines sharply3. Another issue is that the weight of the fluid in the capillary exerts a downward force, reducing the height of the fluid in proportion to the downward force exerted, resulting in potential errors. Methods to compensate for this effect have been described4,21, but are cumbersome and are not in widespread use. Direct comparison of gas analysis and manometry indicated that the two methods produced significantly different results, but the authors were unable to produce a satisfactory explanation for the dicrepancy22.
Despite its advantages, the coulometric microrespirometer also has limitations. First, unlike some stop-flow gas analysis systems, it is not commercially available. The components are easy to acquire and neither the design nor the programming are complicated, but it is potentially more complex to construct than the simple manometric systems9. Second, acquisition of accurate data must include multiple cycles of O2 generation, so each experiment must last for several hours. This is also true of experiments using manometry. Finally, again like manometry, temperature and humidity inside the chambers must be stable. Nonetheless, the coulometric microrespirometer offers a middle ground with regard to cost and complexity, is robust and reliable once assembled, and measures O2 consumption precisely.
The experiments with CASKΔ18 mutants demonstrate both negative (mass-specific VO2) and positive results (fly-specific VO2). If the >50% reduction in walking of CASKΔ18 mutants resulted from compromised metabolism, it might be predicted that VO2 would be reduced by a similar amount. Yet the change in mass-specific O2 consumption was modest (9.6 % reduction in median VO2), and not statistically significant. This negative result is consistent with CASKΔ18 mutants having relatively normal metabolism, with the behavioral phenotype resulting from decreased locomotory drive. Although it is possible that the method lacks sufficient sensitivity, the reduction of VO2 caused by a relatively small difference in size (15.5% reduction in median mass) was highly significant.
Walking is associated with significantly increased energy expenditure5,23, so why is VO2 relatively normal in CASK mutants? It is possible that that excessive grooming in CASK mutants16 could counterbalance the reduction in walking, or the walking phenotype is less apparent when flies are tested in groups in small tubes, but these hypotheses await experimental verification. Nonetheless, we conclude that mutations in the CASK locus do not have strong, direct effects on metabolism, and that the coulometric microrespirometer is an effective tool for studying metabolism in D. melanogaster.
Because this apparatus is easily constructed from common components, measures O2 consumption precisely, is highly portable, can be used in any environment with stable temperature, and has been used with organisms as small as 0.5 mg flies (the present study) and as large as 500 mg scorpions (DJS unpublished), it adds a versatile tool for studying the metabolism of diverse organisms.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Linda Restifo at the University of Arizona for suggesting testing the O2 consumption of CASK mutants and for sending CASK mutants and their congenic controls. Publication fees were provided by the Departmental Reinvestment Fund from the Biology Department at the University of College Park. Space and some equipment were provided by the Universities at Shady Grove.
Materials
| 19/22 Thermometer Adapter | Wilmad-Labglass | ML-280-702 | Sensor Plug |
| 2 ml Screwcap Tubes | Fisher | 3464 | O2 generator |
| 2-Pin Connector | Zyamy | 40PIN-RFB10 | O2 generator: cut to 2-pin |
| 4-Pin Female Connector | TE Connectivity | 215299-4 | Sensor Plug |
| 5 ml Polypropylene Tube | Falcon | 352063 | Cut to 5.5 cm and perforated |
| 50 ml Schlenk Tube 19/22 Joint | Laboy | HMF050804 | Chamber |
| 6-Conductor Cable | Zenith | 6-Conductor 26 ga | Cable |
| 6-Pin Female Bulkhead Connector | Switchcraft | 17982-6SG-300 | Controller |
| 6-Pin Female Connector | Switchcraft | 18982-6SG-522 | Sensor plug |
| 6-Pin Male Connector | Switchcraft | 16982-6PG-522 | Cable |
| 800 ul centrifuge tube | Fisher | 05-408-120 | Soda Lime Cartridge |
| ABS Plastic Enclosure | Bud Industries | PS-11533-G | Controller |
| Arduino Nano Every | Arduino LLC | ABX00028 | Controller |
| BME 280 Sensor | DIYMall | FZ1639-BME280 | Sensor Plug |
| Circuit Board | Lheng | 5 X 7 cm | Controller |
| Copper Sulfate | BioPharm | BC2045 | O2 Generator |
| Computer | Azulle | Byte4 | Data Acquisition |
| Cotton Rolls | Kajukajudo | #2 | Cut in half to plug fly tubes Cut in quarters for humidity |
| Environmental Chamber | Percival | I30 VLC8 | Fly Care |
| Epoxy | JB Weld | Plastic Bonder | Secure Electrodes in O2 Generator |
| Fly Food | Lab Express | Type R | Fly Care |
| Keck Clamps | uxcell | a20092300ux0418 | Secures glass joint of chamber to plug |
| Low-Viscosity Epoxy | Loctite | E-30CL | Sensor Plug |
| OLED Display | IZOKEE | IZKE31-IIC-WH-3 | Controller |
| Platinum Wire 24 ga | uGems | 14349 | O2 generator |
| Silicone grease | Dow-Corning | High Vacuum Grease | Seals chamber-plug connection |
| Soda Lime | Jorvet | JO553 | CO2 absorption |
| Toggle Switch | E-Switch | 100SP1T1B1M1QEH | Controller |
| USB Cable | Sabrent | CB-UM63 | Controller |
| USB Hub | Atolla | Hub 3.0 | Connect controllers to computer |
| Water bath | Amersham | 56-1165-33 | Temperature Control |
| Water Bath Tank | Glass Cages | 15-liter rimless acrylic | Bath for Respirometers |
References
- Arking, R., Buck, S., Wells, R. A., Pretzlaff, R. Metabolic rates in genetically based long lived strains of Drosophila. Experimental Gerontology. 23 (1), 59-76 (1988).
- Henry, Y., Overgaard, J., Colinet, H. Dietary nutrient balance shapes phenotypic traits of Drosophila melanogaster in interaction with gut microbiota. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 241, 110626 (2020).
- Burggren, W., Souder, B. M., Ho, D. H. Metabolic rate and hypoxia tolerance are affected by group interactions and sex in the fruit fly (Drosophila melanogaster): new data and a literature survey. Biology Open. 6, 471-480 (2017).
- Lighton, J. R. B. . Measuring Metabolic Rates. , (2019).
- Fiorino, A., et al. Parallelized, real-time, metabolic-rate measurements from individual Drosophila. Scientific Reports. 8 (1), 14452 (2018).
- Berrigan, D., Partridge, L. Influence of temperature and activity on the rate of adult Drosophila melanogaster. Comparative Biochemistry and Physiology. 118 (4), 1301-1307 (1997).
- Hulbert, A. J., et al. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Experimental Gerontology. 39 (8), 1137-1143 (2004).
- Botero, V., et al. Neurofibromin regulates metabolic rate via neuronal mechanisms in Drosophila. Nature Communications. 12 (1), 4285 (2021).
- Yatsenko, A. S., Marrone, A. K., Kucherenko, M. M., Shcherbata, H. R. Measurement of Metabolic Rate in Drosophila using Respirometry. Journal of Visualized Experiments. (88), 51681 (2014).
- Ross, R. E. Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. Journal of Insect Physiology. 46 (11), 1477-1480 (2000).
- Brown, E. B., Klok, J., Keene, A. C. Measuring metabolic rate in single flies during sleep and waking states via indirect calorimetry. Journal of Neuroscience Methods. 376, 109606 (2022).
- Sandstrom, D. J., Offord, B. W. Measurement of oxygen consumption in Tenebrio molitor using a sensitive, inexpensive, sensor-based coulometric microrespirometer. Journal of Experimental Biology. 225 (9), jeb243966 (2022).
- Becker, M., et al. Presynaptic dysfunction in CASK-related neurodevelopmental disorders. Translational Psychiatry. 10 (1), 312 (2020).
- Slawson, J. B., et al. Central Regulation of Locomotor Behavior of Drosophila melanogaster Depends on a CASK Isoform Containing CaMK-Like and L27 Domains. Genetics. 187 (1), 171-184 (2011).
- Slawson, J. B., et al. Regulation of dopamine release by CASK-Î2 modulates locomotor initiation in Drosophila melanogaster. Frontiers in Behavioral Neuroscience. 8, (2014).
- Andrew, D. R., et al. Spontaneous motor-behavior abnormalities in two Drosophila models of neurodevelopmental disorders. Journal of Neurogenetics. 35 (1), 1-22 (2021).
- Ueno, T., Tomita, J., Kume, S., Kume, K. Dopamine Modulates Metabolic Rate and Temperature Sensitivity in Drosophila melanogaster. PLoS ONE. 7 (2), e31513 (2012).
- Van Voorhies, W. A., Khazaeli, A. A., Curtsinger, J. W. Lack of correlation between body mass and metabolic rate in Drosophila melanogaster. Journal of Insect Physiology. 50 (5), 445-453 (2004).
- Norton, F. J. Permeation of gases through solids. Journal of Applied Physics. 28 (1), 34-39 (1957).
- Hoegh-Guldberg, O., Manahan, D. T. Coulometric measurement of oxygen-consumption during development of marine invertebrate embryos and larvae. Journal of Experimental Biology. 198 (1), 19-30 (1995).
- Sohal, R. S., Agarwal, A., Agarwal, S., Orr, W. C. Simultaneous overexpression of copper- and zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. Journal of Biological Chemistry. 270 (26), 15671-15674 (1995).
- Van Voorhies, W. A., Melvin, R. G., Ballard, J. W. O., Williams, J. B. Validation of manometric microrespirometers for measuring oxygen consumption in small arthropods. Journal of Insect Physiology. 54 (7), 1132-1137 (2008).
- Herreid, C. F., Full, R. J. Cockroaches on a treadmill: Aerobic running. Journal of Insect Physiology. 30 (5), 395-403 (1984).

