Discovery and Synthesis Optimization of Isoreticular Al(III) Phosphonate-Based Metal-Organic Framework Compounds Using High-Throughput Methods
Summary
The targeted synthesis of new metal-organic frameworks (MOFs) is difficult, and their discovery depends on the knowledge and creativity of the chemist. High-throughput methods allow complex synthetic parameter fields to be explored quickly and efficiently, accelerating the process of finding crystalline compounds and identifying synthetic and structural trends.
Abstract
High-throughput (HT) methods are an important tool for the fast and efficient screening of synthesis parameters and the discovery of new materials. This manuscript describes the synthesis of metal-organic frameworks (MOFs) from solution using an HT reactor system, resulting in the discovery of various phosphonate-based MOFs of the composition [Al2H12-x(PMP)3]Clx∙6H2O (H4PMP = N,N '-piperazine bis(methylenephosphonic acid)) for x = 4, 6, denoted as Al-CAU-60-xHCl, containing trivalent aluminum ions. This was accomplished under solvothermal reaction conditions by systematically screening the impact of the molar ratio of the linker to the metal and the pH of the reaction mixture on the product formation. The protocol for the HT investigation includes six steps: a) synthesis planning (DOE = design of experiment) within the HT methodology, b) dosing and working with in-house developed HT reactors, c) solvothermal synthesis, d) synthesis workup using in-house developed filtration blocks, e) characterization by HT powder X-ray diffraction, and f) evaluation of the data. The HT methodology was first used to study the influence of acidity on the product formation, leading to the discovery of Al-CAU-60∙xHCl (x = 4 or 6).
Introduction
Metal-organic frameworks (MOFs) are porous, crystalline compounds whose structures consist of metal-containing nodes, like metal ions or metal-oxygen clusters, which are connected by organic molecules (linkers)1. By varying the metal-containing nodes as well as the linker, a variety of compounds can be obtained that exhibit a wide range of properties and therefore have potential applications in different fields1.
The stability of a material is important for its application1,2,3. Therefore, MOFs containing tri- or tetravalent metal ions, such as Al3+, Cr3+, Ti4+, or Zr4+, with carboxylate2 or phosphonate4 linker molecules have been the focus of many investigations5,6,7. In addition to the direct synthesis of stable MOFs, the enhancement of stability through post-synthetic modifications as well as the formation of composites is a field of interest2. Phosphonate-based MOFs have been less often reported compared to carboxylate-based MOFs8. One reason is the higher coordination flexibility of the CPO32- group compared to the -CO2– group, which often leads to the formation of dense structures and greater structural diversity8,9,10,11. In addition, phosphonic acids often must be synthesized, as they are rarely available on the market. While some metal phosphonates exhibit exceptional chemical stability10, systematic access to isoreticular metal phosphonate MOFs, which allows the tuning of properties, is still a topic of high relevance12,13. Different strategies for the synthesis of porous metal phosphonates have been investigated, such as incorporating defects into otherwise dense layers, for example, by partially replacing phosphonate with phosphate ligands4,14. However, as defective structures are poorly reproducible, and the pores are not uniform, other strategies have been developed. In recent years, the use of sterically demanding or orthogonalized phosphonic acids as linker molecules have emerged as a suitable strategy for the preparation of porous metal phosphonates4,8,10,11,13,15,16,17,18. However, a universal synthesis route for porous metal phosphonates has not yet been discovered. As a result, the synthesis of metal phosphonates is often a process of trial and error, requiring the investigation of many synthesis parameters.
The parameter space of a reaction system includes chemical and process parameters and can be vast19. It consists of parameters such as the type of starting material (metal salt), molar ratios of starting materials, additives for pH adjustment, modulators, type of solvent, solvent mixtures, volumes, reaction temperatures, times, etc.19,20. A moderate number of parameter variations can easily result in several hundred individual reactions, making a carefully considered synthesis plan and well-chosen parameter space necessary. For example, a simple study using six molar ratios of the linker to the metal (e.g., M:L = 1:1, 1:2, … to 1:6) and four different concentrations of an additive and keeping the other parameter constant, leads already to 6 x 4 = 24 experiments. Using four concentrations, five solvents, and three reaction temperatures would necessitate carrying out the 24 experiments 60 times, resulting in 1,440 individual reactions.
High-throughput (HT) methods are based on the concepts of miniaturization, parallelization, and automation, to varying degrees depending on the scientific question being addressed19,20. As such, they can be used to accelerate the investigation of multi-parameter systems and are an ideal tool for the discovery of new compounds, as well as synthesis optimization19,20. HT methods have been used successfully in different fields, ranging from drug discovery to materials science20. They have also been used for the investigation of porous materials such as zeolites and MOFs in solvothermal reactions, as recently summarized20. A typical HT workflow for solvothermal synthesis consists of six steps (Figure 1)19,20,21: a) selection of the parameter space of interest (i.e., the design of experiment [DOE]), which can be done manually or by using software; b) dosing of the reagents into the vessels; c) solvothermal synthesis; d) isolation and workup; e) characterization, which is typically done with powder X-ray diffraction (PXRD); and f) data evaluation, which is followed by step one again.
Parallelization and miniaturization are achieved in solvothermal reactions through the use of multiclaves, often based on the well-established 96-well plate format most commonly used in biochemistry and pharmacy19,20,22,23. Various reactor designs have been reported and several groups have constructed their own reactors19,20. Reactor choice depends on the chemical system of interest, especially the reaction temperature, (autogenous) pressure, and reactor stability19,20. For example, in a systematic study of zeolitic imidazolate frameworks (ZIFs), Banerjee et al.25 used the 96-well glass plate format to perform over 9600 reactions24. For reactions under solvothermal conditions, customized polytetrafluoroethylene (PTFE) blocks, or multiclaves with 24 or 48 individual PTFE inserts, have been described among others by the Stock group19,20. They are routinely employed, for example, in the synthesis of metal carboxylates and phosphonates. As such, Reinsch et al.25 reported the advantages of the methodology in the field of porous aluminum MOFs25. The in-house made HT reactor systems (Figure 2), which allow 24 or 48 reactions to be studied simultaneously, contain PTFE inserts with a total volume of 2.655 mL and 0.404 mL, respectively (Figure 2A,B). Usually, no more than 1 mL or 0.1 mL, respectively, is used. While these reactors are used in conventional ovens, microwave-assisted heating using SiC blocks and small glass vessels has also been reported26.
The automation of studies leads to time savings and improved reproducibility, as influence of the human factor is minimized20. The degree to which automation has been used varies strongly19,20. Fully automated commercial systems, including pipetting20 or weighting capabilities20, are known. A recent example is the use of a liquid-handling robot to study ZrMOFs, reported by the group of Rosseinsky27. Automated analysis can be performed by PXRD using a diffractometer equipped with an xy stage. In another example, a plate reader was used to screen solid-state catalysts, mainly MOFs, for HT screening of nerve agent degradation28. Samples can be characterized in a single run without the need for manual sample or position changes. Automation does not eliminate human error, but it reduces the possibility of its occurring19,20.
Ideally, all steps in a HT workflow should be adapted in terms of parallelization, miniaturization, and automation to eliminate possible bottlenecks and maximize efficiency. However, if it is not possible to establish a HT workflow in its entirety, it may be helpful to adopt selected steps/tools for one's own research. The use of multiclaves for 24 reactions is particularly useful here. The technical drawings of the in-house made equipment used in this study (as well as others) are published for the first time and can be found in Supplementary File 1, Supplementary File 2, Supplementary File 3, and Supplementary File 4.
Protocol
In this protocol, the HT investigation of chemical systems to discover new crystalline materials, using Al-CAU-6029 as an example, is described.
1. Design of Experiment (DOE)
NOTE: The first step is to set up a synthesis plan, which requires knowledge of the reactor setup (Figure 2), reactants, and solvents used. This synthesis planning procedure is adapted to performing 24 or 48 reactions under a specific temperature-time program, for which in-house made steel multiclaves are used to perform 24 (Figure 2A) or 48 reactions (Figure 2B) at once. The reactors are in-house made PTFE inserts with a used reagent/solvent volume of 1 mL (PTFE reactor for carrying out 24 reactions in the steel multiclave) or 100 µL (PTFE reactor for carrying out 48 reactions in the steel multiclave). The technical drawings of the reactor setup can be found in Supplementary File 1 and Supplementary File 2 respectively.
- First, determine the parameter space to be investigated. Therefore, make decisions about an initial number of reactions, metal source, and linker molecule, as well as the use of additives and solvent.
- For the chosen example of Al-CAU-60, carry out 24 reactions using AlCl3∙6H2O as a metal source and N, N′-piperazine-bis(methylenephosphonic acid) (H4PMP) as a linker molecule. Furthermore, use aqueous solutions of NaOH and HCl as additives to study the influence of the pH of the reaction mixture on product formation.1
NOTE: The choice of parameters is usually based on published synthesis procedures or principles based on fundamental chemical knowledge. However, for the successful discovery of new materials, a broader variation of the reaction parameters should be applied (i.e., a certain degree of diversity of the reaction parameters should be considered). The number of parameters to be varied and the type of variations can be based on different principles. In the simplest form, only one parameter should be changed at a time. For example, a fixed metal salt concentration in combination with varying linker molecule concentrations can be used to investigate different linker-to-metal ratios. However, the investigation can also use different molar ratios of the linker to the metal and other solvents or additives. The accessible parameter space is limited by the solubility of the starting materials (amount and solvent type) in cases where only solutions are used21. The dosing of solids extends the accessible parameter space20.
- For the chosen example of Al-CAU-60, carry out 24 reactions using AlCl3∙6H2O as a metal source and N, N′-piperazine-bis(methylenephosphonic acid) (H4PMP) as a linker molecule. Furthermore, use aqueous solutions of NaOH and HCl as additives to study the influence of the pH of the reaction mixture on product formation.1
- Specify the parameter space. For this purpose, choose and calculate quantities of starting materials (molar ratios) and solvent volumes.
- For the chosen example of Al-CAU-60, vary the molar ratio of H4PMP to Al3+ between 4:1 and 0.3:1 in six steps: 4:1, 3:1, 2:1, 1:1, 0.5:1, 0.3:1. Carry out all six syntheses with different additive ratios; study one molar ratio of NaOH to Al3+ (1:1) and two molar ratios of HCl to Al3+ (20:1 and 40:1), as well as one without any additive. Use a spreadsheetto calculate the quantities of starting materials required for this, which can be found in the additional information.
2. Dosing and solvothermal synthesis
- Prepare the stock solutions in a fume hood by following the standard protocol for preparing stock solutions of the reagents.
CAUTION: H4PMP, AlCl3∙6 H2O, HCl, and NaOH are corrosive substances, which cause severe skin burns and eye damage on contact. Wear personal protective equipment when working with these substances.- For the chosen example of Al-CAU-60, prepare the following reagents according to the spreadsheet in the supporting information (Supplementary Table 1): hydrochloric acid solution with a concentration of 10 mol/L, sodium hydroxide solution with a concentration of 1 mol/L, and an AlCl3∙6H2O solution with a concentration of 1 mol/L.
NOTE: Product formation can also depend on the aggregation state of the added reagents. For solids, the particle size can have an effect due to the dissolution rate. A decision should be made at the beginning of the study whether to use solids or solutions to allow for systematic evaluation.
- For the chosen example of Al-CAU-60, prepare the following reagents according to the spreadsheet in the supporting information (Supplementary Table 1): hydrochloric acid solution with a concentration of 10 mol/L, sodium hydroxide solution with a concentration of 1 mol/L, and an AlCl3∙6H2O solution with a concentration of 1 mol/L.
- Insert the discs into the sample plate (Figure 3A).
- Transfer reagents, additives, and solvents into the PTFE inserts (Figure 3B).
- For the chosen example of Al-CAU-60, first add the linker H4PMP as a solid to the PTFE inserts, then add the aluminum chloride solution, the demineralized water, and the solution of additives (NaOH or HCl) with a pipette in accordance with the values calculated in the spreadsheet in the supporting information (Supplementary Table 1).
NOTE: The order in which the PTFE inserts are filled can also influence product formation; therefore, the order of starting materials should be chosen in advance and kept the same throughout the study to allow a systematic evaluation.
- For the chosen example of Al-CAU-60, first add the linker H4PMP as a solid to the PTFE inserts, then add the aluminum chloride solution, the demineralized water, and the solution of additives (NaOH or HCl) with a pipette in accordance with the values calculated in the spreadsheet in the supporting information (Supplementary Table 1).
- Insert the filled PTFE inserts into the sample plate.
- Mark the ground plate of the reactor in a way that allows the identification of the PTFE inserts later. Insert the sample plate with the filled PTFE inserts into the ground plate of the reactor (Figure 3C).
- Prepare two PTFE sheets (with a thickness of 0.1 mm) to cover the sample plates.
- Place the PTFE sheets on the sample plate (Figure 3D).
- Ensure that the PTFE sheet is correctly positioned and fits the head plate using the guide pins (Figure 3E), add the screws, and tighten them by hand.
- Seal the initially closed reactor with the help of, for example, a mechanical or hydraulic press (Figure 4A), far enough that the spring-loaded pressure pieces still have 2 mm of free space (Figure 4B). Then, tighten the screws by hand again (Figure 4C). Be aware that over-tightening can damage (bend) the multiclaves.
- Place the multiclave in a programmable forced convection oven (Figure 4D), and then set and start the selected temperature-time program. It is advisable to use a convection oven to ensure uniform heating.
- For the discovery of Al-CAU-60, set the following temperature-time-program: Heat the oven to 160 °C in 12 h, maintain the target temperature for 36 h, and cool to room temperature (RT) in 12 h.
NOTE: The choice of the temperature-time program can influence product formation30. This includes the phases formed, but more often the crystal size and morphology30.
- For the discovery of Al-CAU-60, set the following temperature-time-program: Heat the oven to 160 °C in 12 h, maintain the target temperature for 36 h, and cool to room temperature (RT) in 12 h.
3. Isolation and workup
- Remove the multiclave from the oven when the temperature reaches room temperature.
- Place the multiclave, for example, in a mechanical or hydraulic press and gently compress it until the screws can be loosened by hand (Figure 5A).
- Place the multiclave in a fume hood and remove the head plate of the reactor, then remove the PTFE sheets and remove the sample plate with the PTFE inserts from the ground plate of the reactor (Figure 5B).
- Inspect the PTFE inserts and check for crystals (Figure 5C). If present, isolate some of them together with some mother liquor.
- Next, assemble the in-house high-throughput filtration block (Figure 6A): connect the filter block to a vacuum pump via two wash bottles, and place two filter papers between two silicone sealing mats with the corresponding recesses (Figure 6B-D) in the filter block. Place the PTFE filling block on top, making sure that the appropriate recesses match with the sealing mats and the filter block (Figure 6E). Tighten the layers using the clamping frame, which is held in place by four stud bolts. To properly seal the unit, use wing nuts on the stud bolts and tighten by hand (Figure 6F).
NOTE: The technical drawings of the filtration block are shown in the supporting information (Supplementary File 3). If a filter block is not available, the products can also be filtered individually. - Close the recesses of the filling block that are not to be filled with plugs (Figure 6F).
- Later in the process, seal the recesses that have already been drained. This allows the other wells to be drained as well.
- Turn on the membrane vacuum pump and set it to a mode in which it will pump down to the best possible vacuum (5-12 mbar).
- Using disposable pipettes, transfer the contents of the PTFE inserts into the designated wells of the filling block (Figure 7A).
NOTE: If harmful solvents (e.g., dimethylformamide ) are used, the products should be washed with ethanol or another less toxic and more volatile solvent to reduce contact with harmful substances during the following steps. - After all the inserts are empty, take a second look for crystals and isolate them if there are any (Figure 7B).NOTE: It is recommended to use an optical microscope with the possibility to use different magnifications in order to determine the size of the crystallites.
- Carefully disassemble the filtration block once all the wells are drained (Figure 7C).
- A so-called "product library" is now available on the filter paper (Figure 7D).
- Dry the product library by allowing it to air-dry in a fume hood; in the case of non-toxic and non-corrosive solvents, the PXRD measurements can be performed with wet products.
4. Characterization
NOTE: For the discovery of new crystalline compounds, the products obtained are characterized by HT-PXRD. New crystalline phases are identified and used for further characterization. Working with the powder X-ray diffractometer follows a standard procedure, which can be found in the operating manual. A standard powder X-ray diffractometer can also be used, which makes the characterization more tedious.
- Place the product library between two metal plates (base plate and cover plate; Figure 7E and Supplementary File 4) in a way that the recesses in the plates match the product locations to allow examination by PXRD. Carefully align the plates and secure them with two screws (Figure 7F).
- Insert the product library into the sample holder of the diffractometer (Figure 8A,B).
NOTE: Other sample holders may require different brackets. Refer to the user manual for further information. - Carefully place the loaded sample holder into the xy stage of the diffractometer and close the instrument (Figure 8C).
- The diffractometer is controlled via WinXPOW software31. In the Diffractometer Control window, set the measuring mode by clicking on the Ranges menu and choose Scan Mode. A new window opens; herein, choose Scan Mode: Transmission, PSD Mode: Moving, Scan Type: 2Theta, and Omega Mode: Fixed and confirm the dialog.
- To set the measuring parameters, click on the Ranges menu and choose Scan Range.
- A new window opens; herein, click on the Plus Icon and edit the appearing standard settings by double-clicking on it.
- To characterize the product library, perform a short 4 min measurement of each sample with the following settings: (a) 2Theta(Begin, End): 2, 47, (b) Step: 1.5, (c) Time/PSD Step [s]: 2, (d) Omega: 0. Confirm both dialogs.
- To choose the samples to be measured on the xy stage, click on the Ranges menu and choose Scan Usage.
- A new window opens; herein, set the Scan Usage to Multiple Samples and check the option Individual Ranges/Files.
- Next, click on the button Ranges/Files; a new window ("HT_Editor") with 48 selectable sample positions opens. Select all positions with samples on the sample plate by clicking on the position with the 'control' key pressed.
- To activate the positions, use the right-click on Measure Samples. Confirm both dialogs.
- Save the files by clicking on File in the menu and choose Save As. After choosing a directory and a filename, click on the Save button.
- Start the measurement by clicking on Measure in the menu and choose the first entry, Data Collection. A new window opens; click on the Ok button to start the measurement.
NOTE: The default settings and the procedure for calibrating the diffractometer should be taken from the user manual. The choice of measurement parameters (scanning angle, step size, time per scanning step) is also dependent on the density of the material, the weight of the diffracting atoms, etc., and may have to be adjusted. Absorption of the X-rays can be a problem if too much sample is formed and heavy elements are used.
5. Data evaluation
NOTE: An in-house procedure is used to evaluate the data; other procedures are conceivable. The PXRD data is obtained in ".raw" file format. To evaluate the diffractograms in other software, this file format must be converted, for example, to ".xyd" file format.
- Open the WinXPOW software31. To open the powder X-ray diffractograms, use the Raw Data menu and choose Raw Data Handling. A new window opens.
- Click on the icon for Batch Open and select all the files via Add Files. After selecting all the files, click on Open and confirm with Ok.
- Normalize the intensities to a maximum value of 10,000 by clicking on the Ranges and choosing Adapt Intensities; a new window opens. Choose the option Normalize Intensities to max. Int. and write 10000. Click Ok.
NOTE: WinXPOWsoftware31 overwrites the raw data when the data is changed; make sure to work on copies of the data. - Export the files via the Export icon in a file format suitable for evaluation programs. Choose an output directory and use the X/Y file format. Click Ok to finish the export.
- Display the PXRD data in a stacked or separated view in a suitable program. Identify the most crystalline products by examining the number of reflections, half-widths (full width at half maximum [FWHM]), and signal-to-noise ratio.
NOTE: For a first analysis, WinXPOWsoftware31 with the Graphics subroutine and the Search and Match function can also be used.
Representative Results
The PXRD data is shown in Figure 9. For the first evaluation, the results obtained are linked to the synthesis parameters of the investigated parameter space. The investigation was carried out using six different molar ratios of linker to metal and four different molar ratios of NaOH/HCl to Al3+. By linking this information with the obtained PXRD data (Figure 9), it can be seen that products of low crystallinity were obtained from syntheses at a molar ratio of NaOH:Al3+ of 1:1 (series A1 to A6) and in the absence of NaOH or HCl (series C1 to C6). This is reflected by the small number of reflections, the high signal-to-noise ratio, and the large half-width (FWHM) of the reflections. The number and position of the reflections vary in the individual powder patterns, which indicates the formation of different products or phase mixtures. Within these series, the syntheses at medium or low molar ratios of linker to metal (2:1, 1:1, 0.5:1) in particular show products of higher crystallinity.
In the reactions carried out at the two highest molar ratios of HCl:Al3+ of 20:1 and 40:1, very similar reaction products are formed. Looking at the data series E1 to E6 (HCl:Al3+ = 20:1), lower signal-to-noise ratios in the PXRD data of the products prepared with a high molar ratio of linker to metal is observed. Furthermore, the diffraction patterns of the products obtained with a lower molar ratio of linker to metal (E5 and E6) show additional reflections, indicating the presence of a different phase or a phase mixture. Analyzing the G1 to G6 series (HCl:Al3+ = 40:1), the same crystalline phase is obtained in all reactions. Again, the signal-to-noise ratio increases with decreasing molar ratios of linker to metal.
In the next step, the PXRD patterns with the highest signal-to-noise ratio and the smallest half-widths (here, sample G1 from the series E1 to E6 and G1 to G6) are compared with calculated powder patterns. To do so, crystallographic databases can be searched for compounds with the same linker molecule. For example, the MOF subset of the CSD database of the CCDC can be used32. The CSD database can be searched using the ConQuest33 program or directly from the CCDC website32. The use of ConQuest33allows, among other features, to restrict the search to MOF subsets and further to crystal structures, which, for example, contain or explicitly do not contain certain elements or functional groups. In this case, compounds containing a trivalent metal ion and the linker molecule are of interest, and Al-MIL-9134 is one compound of interest. The matching entry is downloaded and a PXRD pattern is calculated in the WinXPOWsoftware31. In Figure 10, the calculated PXRD pattern of Al-MIL-91 is compared with the measured PXRD patterns. By comparing the reflection positions, some powder patterns can be identified, in which reflections that may be assigned to Al-MIL-91 appear (Figure 10; e.g.; A4), but not as a pure phase in any synthesis. The PXRD patterns of products obtained using HCl as an additive are completely different from those of MIL-91 and other compounds containing the linker molecule. This information is summarized in Figure 11, which can be called a discovery library. Table 1 lists the molar ratios of the reagents, the concentrations of the solutions, the used volumes, and the amount of linker. A simplified form of the experimental table and the color scheme showing only the molar ratios is represented in Table 2 and Supplementary Figure 1, respectively.
PXRD patterns of samples obtained with NaOH as the additive (A1 to A6) or the absence of an additive (C1 to C6) are not suitable for identifying clear reaction trends. Nevertheless, with experience, one can extract some information. For example: a) the same PXRD patterns are observed for the product in the A and C series (e.g., A2 and C2), and b) phase mixtures are found (A3 could be a mixture of A2 and A4 and C3 could be a mixture of C2 and C4). Therefore, in the next step of the investigation, the parameter space should be modified in such a way that smaller steps in the variation of the molar ratios of ligand to metal to additive are used.
In summary, clear trends can be seen in the series of syntheses carried out with HCl as an additive. An excess of the linker (molar ratio of the linker to metal of 4:1) and a high molar ratio of HCl to metal (40:1) lead to a new, highly crystalline compound. Further investigations allowed us to obtain single crystals suitable for single-crystal X-ray diffraction, which led to the structural elucidation of the new compound.
From the results presented here, a key factor for the successful use of HT methods is the sensible selection of the parameter space to be investigated and the linking of the design of the experiment (parameter space) to the characterization data.
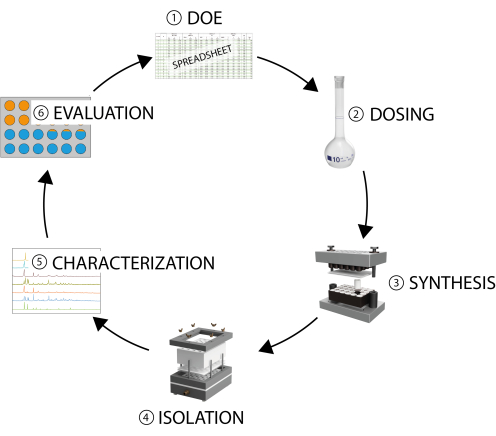
Figure 1: The steps in an HT workflow. 1) DOE, selecting the parameter space of interest; 2) dosing of the reagents; 3) solvothermal synthesis; 4) isolation and workup; 5) characterization, which is typically done with PXRD; 6) data evaluation, which is followed by step 1 again. Please click here to view a larger version of this figure.
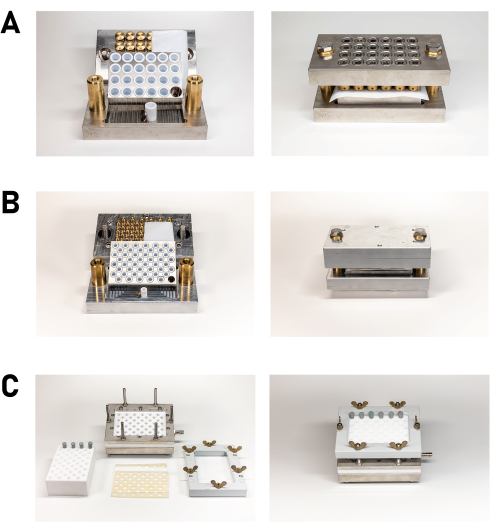
Figure 2: The individual parts of the equipment on the left and the assembled apparatus on the right. (A) The in-house built HT reactor for 24 reactions consists of a base plate flanked by two guide pins on the short sides, and has a recess into which the sample plate, recessed to hold the 24 PTFE inserts, can be inserted. The supporting information contains the technical drawings (Supplementary File 1). (B) The in-house made steel multiclaves for 48 syntheses. The design is basically identical to that of the 24 reaction reactor. The supporting information contains technical drawings (Supplementary File 2). (C) The in-house made filtration block for the filtration of 48 reaction mixtures; the individual parts are shown on the left, and the assembled filter block is on the right. The supporting information contains the technical drawing (Supplementary File 3). Please click here to view a larger version of this figure.
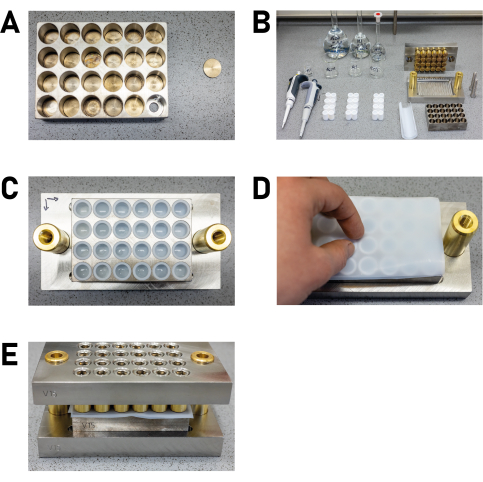
Figure 3: Assembling the HT reactor. (A) A total of 24 discs with a height of 2 mm are inserted into the sample plate to cover the holes. This allows the PTFE inserts to fit correctly and are easily removable. (B) After filling the PTFE insert with the solid, the solutions are added using pipettes. The reactor is then assembled. (C) The sample plate with the PTFE inserts is inserted into the base plate. The base plate is marked to identify the PTFE inserts (top left). (D) The PTFE sheets are placed on the sample plate. (E) The head plate is placed on top of the base plate that contains the sample plate and two PTFE sheets. Please click here to view a larger version of this figure.
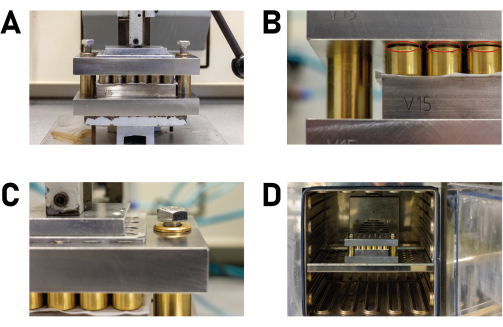
Figure 4: Sealing and placing the reactor in the forced convection oven. (A) The multiclave is placed in a press that applies just enough pressure to the reactor to leave 2 mm of free space (marked with red cycles) in the spring-loaded pressure pieces. (B) In the press, pressure is applied to the reactor so that the spring-loaded pressure pieces have 2 mm of free space. (C) After pressure has been applied, the screws are tightened by hand. (D) The reactor is placed in the forced convection oven. This ensures uniform and continuous heating. Please click here to view a larger version of this figure.
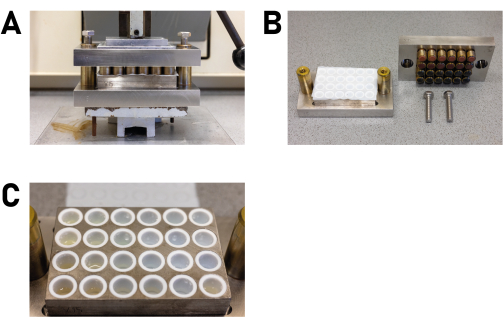
Figure 5: Removing the multiclave and checking for crystals. (A) In the press, the reactor is pressurized to the point where the screws can be loosened by hand. (B) The reactor is carefully disassembled in the fume hood. (C) The PTFE inserts are now examined for the presence of crystallites; if present, these should be isolated with some mother liquor. Please click here to view a larger version of this figure.
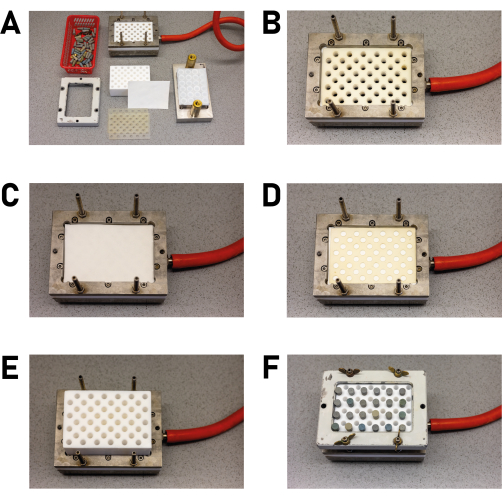
Figure 6: Assembling the HT filtration block. (A) Parts of the filtration block and the reactor. Two filter papers are cut to fit into the filter block. The filtration block is also connected to the vacuum pump. (B) A sealing mat (made of silicone) is inserted into the filter block. (C) The two filter papers are placed on top of the sealing mat. (D) The second sealing mat (made of silicone) is inserted into the filter block. (E) The filling block (made of PTFE) is placed on top of the sealing mats into the filter block. (F) A steel frame is placed on top and fixed with wing screws. Unused filter openings are closed with rubber plugs. Please click here to view a larger version of this figure.
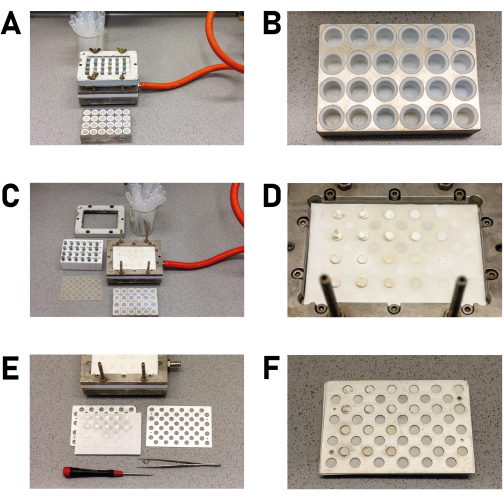
Figure 7: Transferring the contents of the PTFE inserts, disassembling the filtration block, and preparing the product library for characterization using PXRD. (A) The sample holder with the PTFE inserts is now placed in front of the filter block. With the help of disposable pipettes, the contents of the PTFE inserts are transferred to the corresponding holes of the filtration block. (B) The PTFE inserts are examined a second time for non-transferred crystallites. (C) The filtration block is now carefully disassembled. Special care must be taken not to contaminate the neighboring samples. This can happen if the filtration block is not lifted off vertically or if half of the filter paper remains attached to the filtration block. (D) The product library on filtration paper. (E) The product library is carefully removed from the filtration block and placed on a metal plate (the supporting information contains technical drawings; Supplementary File 4) in such a way that the holes are aligned with the positions of the samples. Two screws are used to secure the top section to the bottom plate. (F) The product library is now fixed between two metal plates. The individual reaction products could now be examined a third time under a light microscope for the presence of crystallites. Please click here to view a larger version of this figure.
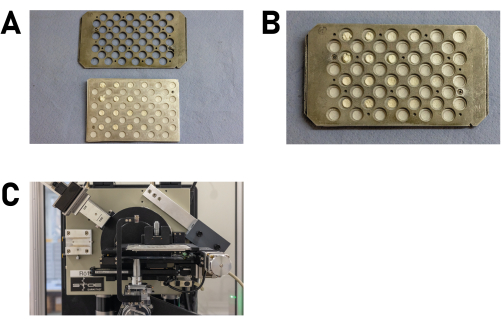
Figure 8: HT-PXRD measurement. (A) For the HT-PXRD measurement, the product library between the metal plates (bottom) is attached to the sample holder (top) with two screws. (B) Product library in the HT-PXRD sample holder. (C) Powder X-ray diffractometer with an xy stage. The Xray tube is on the bottom, and the detector is on the top left. Please click here to view a larger version of this figure.
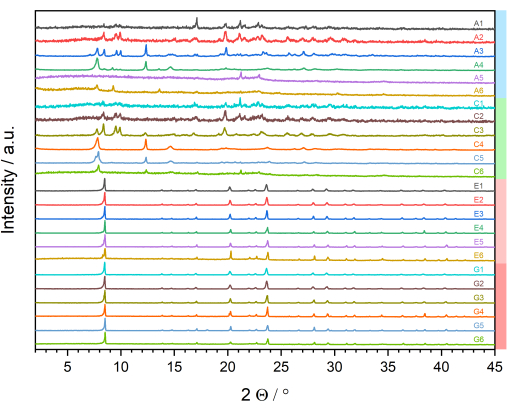
Figure 9: Stacked plot of all measured PXRD patterns. The diffractograms are labeled according to Table 1, which contains the molar ratios of the reagents. Information from Table 1 is added as bars on the right side of the plot, highlighting the additives-blue: NaOH; green: no additive; red: HCl-in accordance with the color scheme used in Table 2. Please click here to view a larger version of this figure.
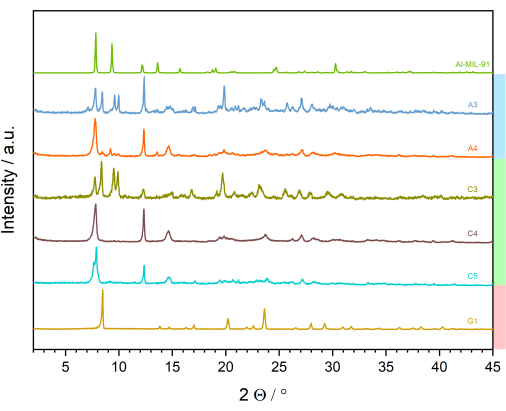
Figure 10: Comparison of the PXRD patterns of the most crystalline phases with the calculated PXRD pattern of Al-MIL-9134. Bars on the right side of the plot highlight the additives that were used-blue: NaOH; green: no additive; red: HCl (in accordance with the color scheme used in Table 2). Please click here to view a larger version of this figure.
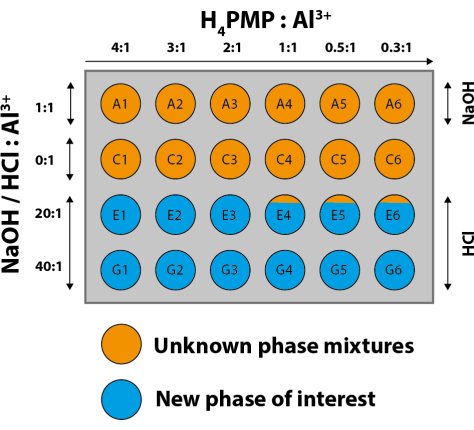
Figure 11: Discovery library and the results of the first HT study in the chemical system AlCl3∙6H2O/H4PMP/NaOH/HCl/H2O. The numbering follows the numbering of the diffractometer software and corresponds to Table 1, Figure 9, and Figure 10. Please click here to view a larger version of this figure.
Table 1: The molar ratios of the reagents, concentrations of the solutions, used volumes, and amount of linker. The full table can be found in the supporting information (Supplementary Table 1). Please click here to download this Table.
Table 2: A simplified form of the experimental table, showing only the molar ratios. Syntheses 1-6 are performed with NaOH as an additive (shown as bluish and white stripes). Syntheses 7-12 take place without any additive (green and white stripes) and syntheses 13-24 are carried out with HCl as an additive in two different molar ratios to the metal (both with reddish and white stripes). Please click here to download this Table.
Supplementary Figure 1: Color scheme representing the molar ratios. Please click here to download this File.
Supplementary Table 1: The full table with the molar ratios, concentrations, used volumes, and amount of linker used for preparing the reagents. Please click here to download this File.
Supplementary File 1: The technical drawings of the 24-reaction reactor. Please click here to download this File.
Supplementary File 2: The technical drawings of the 48-reaction reactor. Please click here to download this File.
Supplementary File 3: The technical drawings of the filtration block. Please click here to download this File.
Supplementary File 4: The technical drawings of the sample holder for HT-PXRD. Please click here to download this File.
Discussion
Due to the complexity of the HT method, the individual steps and the method itself are discussed in the following sections. The first part covers the critical steps for each working step of the HT workflow (Figure 1), possible modifications, and limitations of the technique, where applicable. At the end, a general discussion also including the significance of the HT method with respect to existing methods and future applications is presented.
In the first step of the HT workflow, the DOE, one has to choose the relevant parameters for the study well to gain maximum information about a certain experiment since “a poorly designed experiment will give bad information with unprecedented speed and in outstanding quantity”35. Once the parameters are fixed, spreadsheet software is often used to calculate the amounts of starting materials and solvent masses and volumes. Small mistakes, for example, in molar masses, formulae, etc., result in a set of unintended reaction parameters: “if anything, planning must be done even more carefully since we now have the possibility of going in the wrong direction faster than ever”35. The adaptation of the HT methodology to solvothermal syntheses using in-house made multiclaves has some general limitations on the types of experiments that can be performed. The working temperature of the PTFE insert is limited not only because PTFE exhibits high creep, but also due to degradation processes36. For information about the maximum working temperature, the PTFE manufacturers’ technical information must be addressed. In addition, the autogenous pressure at the reaction temperature or the formation of volatile reaction products must be taken into account. The pressure may become too high for the seals and leakage may occur. For general limitations of the multiclaves, the manufacturing shop that made the reactors should be questioned. Future developments in materials science could expand the accessible temperature and pressure ranges of PTFE vessels and multiclaves.
The second step in the HT workflow is the dosing of small amounts of reagents. Stock solutions of defined concentrations must be accurately prepared before adding the starting materials to the reaction vessels, as small dosing errors combined with errors in the dosing of small amounts can result in large deviations from the specified molar ratios of the starting materials19,20. The dosing of solids at the milligram scale is very challenging, and so highly precise scales must be used. In addition, deviations from the specified amounts (defined in section 1) must be documented. The order of dosing the reagents and solvents should always remain the same. Only starting materials of high purity should be used and reagent solutions should be freshly prepared (or at least the day of preparation should be documented), since aging can, for example, result in polycondensation reactions or the precipitation of metal species. In some cases, homogenization of the reaction mixtures is mandatory, although usually this is not done. The easiest mistake to make is to be careless when filling the autoclaves; as it can be very monotonous to add two or more reactants in different amounts to the 24 or 48 reaction vessels, one must be very accurate and careful. Automation using dosing robots would eliminate this source of error, but the suitable equipment is complex and therefore expensive, and requires extensive maintenance.
The third step, the solvothermal synthesis, leads to formation of the products under a certain temperature-time program. The individual PTFE inserts, as well as the whole multiclaves within a systematic study, should be treated in the same way. Different aging times (e.g., through waiting times between assembling the autoclaves and placing them into the oven) can have an impact on product formation. In addition, the position of the multiclave in the oven can play a role due to temperature gradients inside the oven. This is less important in cases where forced convection ovens are used. Regular calibration of the oven is also advised. Regarding the temperature-time program, one must keep in mind that it takes hours to heat the steel multiclaves to the required reaction temperature, and thus short reaction times of only a few hours are not advised.
The fourth step, product isolation and workup, is carried out manually. Cross-contamination during the filtration step can lead to outliers in the trends that cannot be explained. Especially in synthesizing porous materials, the workup procedures including the type of solvents for washing or different drying methods should be kept the same throughout the study. One should also visually inspect the reaction vessels for crystallites at the bottom of the walls, since they are sometimes not transferred in the filtration step.
The fifth step of the HT workflow, product characterization, is performed on the reaction product from section 4. The identification of crystalline phases by PXRD and data quality can be hampered by the amount, morphology, and crystallinity of the product19,20. Small amounts result in data with a large signal-to-noise ratio, while large amounts can lead to X-ray absorption, especially when the reaction product contains heavy elements. The preferred orientation can be a problem when highly anisotropic crystal shapes occur, since this leads to significant changes in relative intensities in the PXRD pattern. The same applies to large crystals, but sharp reflections of high intensity will usually be observed. It is therefore advisable to inspect the samples under an optical microscope before data collection and grind the sample if larger crystals are present. Another aspect to consider when comparing the measured PXRD pattern with those calculated from known crystal structures from a structural database is the fact that some structures may not have been published. Sometimes, structures have been published with different cell parameters or for compounds containing different solvents or counter ions. A rare case specific to MOFs is their possible structural flexibility (i.e., the amount and type of guest molecule lead to strong changes of the framework), which is reflected in large changes in the positions and relative intensities in the PXRD patterns. In these cases, the samples must be treated the same way. In addition, other HT characterization methods (catalytic reactions, gas sorption measurements) have also been reported, but PXRD is mandatory for the discovery of new crystalline compounds.
The last step of the HT workflow is the data evaluation. Due to a large amount of data, in this case the number of PXRD patterns, careful evaluation is mandatory, especially when phase mixtures are present. This becomes even more difficult as new compounds are formed, but with some practice it is possible to identify phase mixtures. This requires correlating the chemical parameters from section one with the resulting reaction products (PXRD patterns); usually, trends can be identified between them. While data evaluation can be carried out by visual inspection of the PXRD patterns, software for qualitative phase analysis can be used as well.
Finally, there are some general remarks on the use of HT methods. They allow the systematic investigation of complex parameter fields and the extraction of information on fields of formation and synthesis trends. Depending on the available HT setup, this can be done with various degrees of parallelization, miniaturization, and automation19,20. In all cases, investigations are sped up, the consumption of starting materials is reduced, and reproducibility is improved by reducing human error19,20. An important advantage of many data points is that outliers (i.e., results that do not fit the trends) indicate that there could have been some error in the dosing of starting materials (e.g., wrong amounts) or unwanted impurities in the reactors. The latter can easily happen when reusing PTFE reactors. Nevertheless, several pitfalls can occur, which are related to the six steps of the HT workflow, as mentioned above. In general, caution is advised as errors propagate, making reproducibility challenging. Other general aspects to consider are the scale-up of reactions and the use of other reactor systems, which must be also considered as additional reaction parameters. These can change the kinetics of the reaction, but in other cases, for example, for CAU-1037, scale-up and the use of other reactors have been accomplished from the milliliter to the liter range using PTFE or glass reactors37. The study presented here is only one example. The methodology can be applied to any reaction in solution as long as the limiting parameters are taken care of.
While various reactor designs have been reported19,20, the adaptation of HT methods in general is the only way to manage vast experimental parameter spaces. Future development of the reaction vessels and the multiclaves will expand the accessible parameter space by means of accessible temperature and pressure ranges. Also, as other HT systems such as dosing robots or HT characterization systems and new software tools become more affordable and easier to use, more and more steps of the HT workflow will be optimized, thus accelerating the discovery of new compounds or unknown properties of known compounds.
With this contribution, we want to share our methodology in detail with the scientific community.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The work was supported by the Christian-Albrechts-University, the State of Schleswig-Holstein, and the Deutsche Forschungsgemeinschaft (especially STO-643/2, STO-643/5 and STO-643/10).
Norbert Stock would like to thank the B.Sc., M.Sc., and doctoral students, as well as the cooperation partners who have carried out many interesting projects using the high-throughput methodology, in particular Prof. Bein from the Ludwig-Maximilians-Universität in Munich, who played a major role in the development of the reactors.
Materials
| AlCl3·6H2O | Grüssing | N/A | 99% |
| Filter block for filtration of max. 48 reaction mixtures | In-house made | N/A | Technical drawings in the supplementary files |
| Hydrochloric acid | Honeywell | 258148 | Conc. 37 %, p.a. |
| Multiclaves with 24 individual Teflon inserts | In-house made | N/A | Technical drawings in the supplementary files |
| N,N ‘-piperazine bis(methylenephosphonic acid | Prepared by coworkers | N/A | H4PMP, Prepared by coworkers with the method reported by Villemin et al.: D. Villemin, B. Moreau, A. Elbilali, M.-A. Didi, M.’h. Kaid, P.-A. Jaffrès, Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 2511. |
| Sample Plate for PXRD | In-house made | N/A | Technical drawings in the supplementary files |
| Sodium hydroxide | Grüssing | N/A | 99% |
| Stoe Stadi P Combi | STOE | Stadi P Combi | Cu-Kα1 radiation (λ = 1.5406 Å); transmission geometry; MYTHEN2 1K detector; opening angle 18°; curved monochromator; xy-table |
| Forced convection oven | Memmert | UFP400 |
References
- Kaskel, S. . The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications. , (2016).
- Ding, M., Cai, X., Jiang, H. -. L. Improving MOF stability: approaches and applications. Chemical Science. 10 (44), 10209-10230 (2019).
- Stock, N., Biswas, S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chemical Reviews. 112 (2), 933-969 (2012).
- Shimizu, G. K. H., Vaidhyanathan, R., Taylor, J. M. Phosphonate and sulfonate metal organic frameworks. Chemical Society Reviews. 38 (5), 1430-1449 (2009).
- Yuan, S., Qin, J. -. S., Lollar, C. T., Zhou, H. -. C. Stable metal-organic frameworks with group 4 metals: current status and trends. ACS Central Science. 4 (4), 440-450 (2018).
- Devic, T., Serre, C. High valence 3p and transition metal based MOFs. Chemical Society Reviews. 43 (16), 6097-6115 (2014).
- Rhauderwiek, T., et al. Highly stable and porous porphyrin-based zirconium and hafnium phosphonates-electron crystallography as an important tool for structure elucidation. Chemical Science. 9 (24), 5467-5478 (2018).
- Steinke, F., Otto, T., Ito, S., Wöhlbrandt, S., Stock, N. Isostructural family of rare-earth MOFs synthesized from 1,1,2,2-Tetrakis(4-phosphonophenyl)ethylene. European Journal of Inorganic Chemistry. 2022 (34), 2022005562 (2022).
- Zhu, Y. -. P., Ma, T. -. Y., Liu, Y. -. L., Ren, T. -. Z., Yuan, Z. -. Y. Metal phosphonate hybrid materials: from densely layered to hierarchically nanoporous structures. Inorganic Chemistry Frontiers. 1 (5), 360-383 (2014).
- Glavinović, M., Perras, J. H., Gelfand, B. S., Lin, J. -. B., Shimizu, G. K. H. Orthogonalization of polyaryl linkers as a route to more porous phosphonate metal-organic frameworks. Chemistry. 28 (31), 202200874 (2022).
- Yücesan, G., Zorlu, Y., Stricker, M., Beckmann, J. Metal-organic solids derived from arylphosphonic acids. Coordination Chemistry Reviews. 369, 105-122 (2018).
- Wharmby, M. T., Mowat, J. P. S., Thompson, S. P., Wright, P. A. Extending the pore size of crystalline metal phosphonates toward the mesoporous regime by isoreticular synthesis. Journal of the American Chemical Society. 133 (5), 1266-1269 (2011).
- Zheng, T., et al. Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system. Nature Communications. 8, 15369 (2017).
- Dines, M. B., Cooksey, R. E., Griffith, P. C., Lane, R. H. Mixed-component layered tetravalent metal phosphonates/phosphates as precursors for microporous materials. Inorganic Chemistry. 22 (6), 1003-1004 (1983).
- Hermer, N., Reinsch, H., Mayer, P., Stock, N. Synthesis and characterisation of the porous zinc phosphonate [Zn2(H2PPB)(H2O)2]·xH2O. CrystEngComm. 18 (42), 8147-8150 (2016).
- Rhauderwiek, T., et al. Crystalline and permanently porous porphyrin-based metal tetraphosphonates. Chemical Communications. 54 (4), 389-392 (2018).
- Steinke, F., et al. Synthesis and structure evolution in metal carbazole diphosphonates followed by electron diffraction. Inorganic Chemistry. 62 (1), 35-42 (2023).
- Taddei, M., et al. The first route to highly stable crystalline microporous zirconium phosphonate metal-organic frameworks. Chemical Communications. 50 (94), 14831-14834 (2014).
- Stock, N. High-throughput investigations employing solvothermal syntheses. Microporous and Mesoporous Materials. 129 (3), 287-295 (2010).
- Clayson, I. G., Hewitt, D., Hutereau, M., Pope, T., Slater, B. High throughput methods in the synthesis, characterization, and optimization of porous materials. Advanced Materials. 32 (44), 2002780 (2020).
- Clearfield, A., Demadis, K. . Metal Phosphonate Chemistry: From Synthesis to Applications. , (2011).
- Mennen, S. M., et al. The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Organic Process Research & Development. 23 (6), 1213-1242 (2019).
- Yang, L., et al. High-throughput methods in the discovery and study of biomaterials and materiobiology. Chemical Reviews. 121 (8), 4561-4677 (2021).
- Banerjee, R., et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science. 319 (5865), 939-943 (2008).
- Reinsch, H., Stock, N. High-throughput studies of highly porous Al-based MOFs. Microporous and Mesoporous Materials. 171, 156-165 (2013).
- Reimer, N., Reinsch, H., Inge, A. K., Stock, N. New Al-MOFs based on sulfonyldibenzoate ions: a rare example of intralayer porosity. Inorganic Chemistry. 54 (2), 492-501 (2015).
- Tollitt, A. M., et al. High-throughput discovery of a rhombohedral twelve-connected zirconium-based metal-organic framework with ordered terephthalate and fumarate linkers. Angewandte Chemie. 60 (52), 26939-26946 (2021).
- Palomba, J. M., et al. High-throughput screening of solid-state catalysts for nerve agent degradation. Chemical Communications. 54 (45), 5768-5771 (2018).
- Reichenau, T. M., et al. Targeted synthesis of an highly stable aluminium phosphonate metal-organic framework showing reversible HCl adsorption. Angewandte Chemie. , (2023).
- Biemmi, E., Christian, S., Stock, N., Bein, T. High-throughput screening of synthesis parameters in the formation of the metal-organic frameworks MOF-5 and HKUST-1. Microporous and Mesoporous Materials. 117 (1), 111-117 (2009).
- STOE & Cie GmbH. WinXPOW v.3.1. STOE & Cie GmbH. , (2016).
- Groom, C. R., Bruno, I. J., Lightfoot, M. P., Ward, S. C. The Cambridge structural database. Acta Crystallographica Section B, Structural Science. Crystal Engineering and Materials. 72, 171-179 (2016).
- Bruno, I. J., et al. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallographica. Section B, Structural Science. 58, 389-397 (2002).
- Hermer, N., Wharmby, M. T., Stock, N. . CCDC 1499757: Experimental Crystal Structure Determination. , (2017).
- Cawse, J. N. . Experimental Design for Combinatorial and High Throughput Materials Development. , (2003).
- Dhanumalayan, E., Joshi, G. M. Performance properties and applications of polytetrafluoroethylene (PTFE)-a review. Advanced Composites and Hybrid Materials. 1, 247-268 (2018).
- Lenzen, D., et al. Scalable green synthesis and full-scale test of the metal-organic framework CAU-10-H for use in adsorption-driven chillers. Advanced Materials. 30 (6), 1705869 (2018).

