A Comprehensive Approach to Analyze the Cell Components of Cerebral Blood Clots
Summary
This study describes a fast and effective method for the cell component analysis of cerebral blood clots through clot dissolving, cell staining, and routine blood examination.
Abstract
Cerebral thrombosis, a blood clot in a cerebral artery or vein, is the most common type of cerebral infarction. The study of the cell components of cerebral blood clots is important for diagnosis, treatment, and prognosis. However, the current approaches to studying the cell components of the clots are mainly based on in situ staining, which is unsuitable for the comprehensive study of the cell components because cells are tightly wrapped in the clots. Previous studies have successfully isolated a fibrinolytic enzyme (sFE) from Sipunculus nudus, which can degrade the cross-linked fibrin directly, releasing the cell components. This study established a comprehensive method based on the sFE to study the cell components of cerebral thrombus. This protocol includes clot dissolving, cell releasing, cell staining, and routine blood examination. According to this method, the cell components could be studied quantitatively and qualitatively. The representative results of experiments using this method are shown.
Introduction
Cerebrovascular disease is one of three major diseases that can threaten human health, among which ischemic cerebrovascular disease accounts for more than 80%. Cerebral thrombosis and cerebral vein thrombosis are the most concerned ischemic cerebrovascular diseases today, mainly caused by cerebral blood clots1,2. If the treatment is not done properly, it will have high disability and mortality rates and a high recurrence rate after discharge3.
Recently, a growing number of studies have shown that the cell components of cerebral blood clots are tightly correlated with the diagnosis, treatment, and prognosis of cerebral thrombosis4,5,6. Therefore, the availability of data on thrombus composition, especially the cell components, is important for clinical diagnosis and treatment. Unfortunately, the currently available methods cannot comprehensively analyze the blood clot component quantitatively and qualitatively. For example, Martius Scarlett Blue based in-situ staining can only study the red/white blood cells of certain slices of the clot7. Immunohistochemistry (IHC) based in-situ staining can only study limited blood components of certain slices of the clot using their antibodies8. The microscopic image-based methods are only concerned with the specific structure of the clot9. Moreover, all those methods are laborious and time-consuming10. To date, the procedures for quantitatively and qualitatively studying cerebral thrombi cell components have not been reported. It is widely acknowledged that the cross-linked fibrin tightly wraps the blood cells in the clots11. Consequently, the specific degradation of the cross-linked fibrin and release of the intact cells is critical for the accurate analysis of cell components.
Previous works isolated a fibrinolytic enzyme from Sipunculus nudus (sFE), which can degrade the fibrin specifically and quickly12. Herein, a method for analyzing the cell components of the cerebral thrombi based on the unique activity of sFE was proposed. This protocol utilized sFE to degrade the fibrin of clots first and then analyzed the cell components by Wright's Staining and routine blood examination13,14. According to this method, the cell components of cerebral thrombi can be quantitatively and qualitatively studied. This simple and effective protocol might be applied for the cell component analysis of other blood clots.
Protocol
The research was performed in compliance with the institutional guidelines of the Medical Ethics Committee of Huaqiao University. The cerebral blood clots were surgically removed and collected at Quanzhou First Hospital, affiliated to Fujian Medical University, with informed consent from the patients.
1. Blood clot pretreatment
- Place the clots on a clean dish, add 5 mL of physiological saline with a tweezer, shake the dish gently, and remove the saline with a pipette.
NOTE: Repeat the rinse three times. - Cut the clots into smaller pieces (smaller than 5 mm x 5 mm x 5 mm) with a scissor. Add 5 mL of physiological saline, shake the dish gently, and remove the saline with a pipette.
NOTE: Repeat the rinse three times. Ensure no clot pieces are aspirated during the rinse. - Transfer the clots to another clean U-plate.
NOTE: The protocol can be paused here (store the plate at 2-8 °C) and restarted later.
2. Thrombolysis
- Prepare the sFE working solution by adjusting the concentration of sFE to 2000 U/mL with physiological saline.
NOTE: The sFE was prepared according to the well-established protocols of Tang Lab15. - Add 300 µL of sFE working solution into the pretreated clots.
- Perform the first round of degradation.
- Incubate the mixture of sFE and clots at 37 °C for 0.5 h.
NOTE: The blot treated with physiological saline was set as negative control. Do not rotate the sample on the shaker. - Mix the sample gently. Transfer the liquid part to another clean tube with a clean pipette.
NOTE: The remaining clots were used for another round of degradation. - Centrifuge the liquid part at 200 × g for 5 min at 4 °C.
- Transfer the supernatant to another clean tube with a pipette to obtain the recovered sFE solution.
- Mix the cell precipitation with 50 µL of physiological saline gently.
NOTE: The cell mixture was stored 2-8 °C for later use.
- Incubate the mixture of sFE and clots at 37 °C for 0.5 h.
- Perform subsequent degradation.
- Degrade the remaining clots (step 2.3.2) with the recovered sFE solution (step 2.3.4) at 37 °C for 0.5 h.
NOTE: The rest steps were the same as the first degradation round. The degradation process was finished until the whole clots were degraded.
- Degrade the remaining clots (step 2.3.2) with the recovered sFE solution (step 2.3.4) at 37 °C for 0.5 h.
- Perform cell collection.
- Prepare the blood cell sample by collecting the cell mixture of each round of degradation.
3. Wright's staining
- Perform cell smear.
- Adjust cells to a density of 1 x 106 cells/mL.
- Add 5 µL of the cells to the poly-L lysine-coated glass slide, then smear them using the cover slip.
- Evaporate the liquid at room temperature.
- Perform the staining.
- Add 200 µL of Wright's dye (see Table of Materials) to the cell smear gently, and add 600 µL of Wright's dye B to mix evenly. Stain for 10 min at room temperature.
- Rinse the dye gently with clean water.
NOTE: The stained cell smear can be stored at room temperature for later use.
- Perform microscopic imaging.
- Examine the stained cells using a light microscope (see Table of Materials).
4. Routine blood examination
- Adjust cells to a density of 1 x 106 cells/mL.
- Analyze the cell components such as platelets, erythrocytes, white blood cells, lymphocytes, neutrophils, monocytes, eosinophils, basophils, and immature granulocytes using an autohematology analyzer according to the manufacturer's instructions (see Table of Materials).
Representative Results
In the initial stage of the degradation process, it was found that the blood clots had a red compact structure, and the working solution was colorless. After incubation for 30 min, the working solution turned light red, which indicated the crossed blood cells were released into the working solution. Most clots were dissolved when lengthening the incubation time to 5 h, and the working solution became light red. On the contrary, there was no significant change in the physiological saline group (NC) even after 10 h incubation (Figure 1). These results showed that the blood cells enclosed in the clots were released successfully by this protocol.
After Wright's stain, the cell components were examined clearly under a light microscope (Figure 2A). Among those released blood cells, a large number of mature red blood cells with slightly concave and disc-shaped surfaces could be observed. A great quantity of irregularly shaped small purple-red particles was observed on the glass slide, which were the platelets released from the blood clots. The white blood cells exhibited larger plasma and condensed nuclear. Several types of granulocytes, such as mature neutrophil granulocyte, eosinophil, and monocyte, were also observed.
With the aid of auto hematology analyzer, the cell components were analyzed quantitatively. Among the detected cells, platelets and erythrocytes accounted for 51.25% and 45.24%, respectively. These data are consistent with the results of Wright's stain. Other blood cells, such as white blood cells, lymphocytes, neutrophils, monocytes, eosinophils, basophils, and immature granulocytes, were also detected (Table 1). Thus, following this protocol, the cell components of a cerebral thrombus can be studied not only qualitatively but also quantitatively.
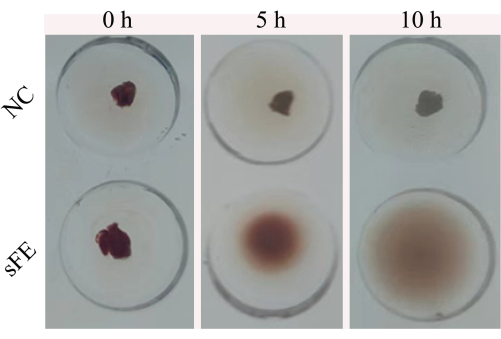
Figure 1: Dissolving process. The cerebral thrombus was dissolved in the saline (NC) and the sFE, respectively. Photography was taken at 0 h, 5 h, and 10 h. Please click here to view a larger version of this figure.
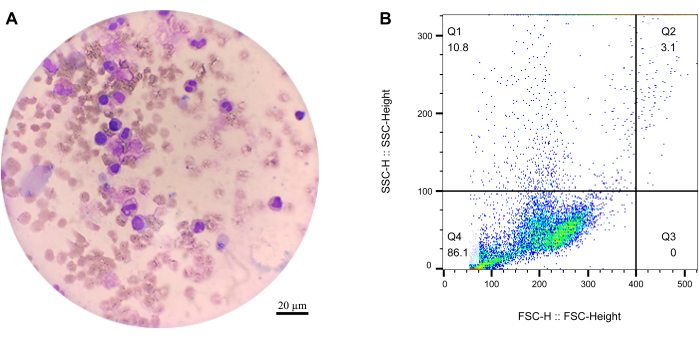
Figure 2: Microscopy and flow cytometry of the released cells. (A) The cells under the microscope after Wright's staining. Scale bar = 20 µm. (B) Flow cytometry results of the released cells. Please click here to view a larger version of this figure.
| Routine Blood Test | ||
| Parameters | Results | Unit |
| Immature granulocyte absolute number (IG#) | 1 | 109/L |
| Immature granulocyte percentage (IG%) | 3.7 | % |
| Erythrocyte Count (RBC) | 1.52 | 1012/L |
| Red blood cell specific volume(HCT) | 13.7 | % |
| Mean corpuscular volume (MCV) | 90.1 | f1 |
| White blood cell number (WBC) | 26.98 | 109/L |
| Lymphocytes number (LYM) | 1.41 | 109/L |
| Lymphocytes percentage (LYM%) | 5.2 | % |
| Neutrophils number (Neu#) | 24.08 | 109/L |
| Neutrophils percentage (Neu%) | 89.2 | % |
| Monocytes number (Mon#) | 1.02 | 109/L |
| Monocytes percentage (Mon%) | 3.8 | % |
| Eosinophils number (Eos#) | 0.34 | 109/L |
| Eosinophils percentage (Eos%) | 1.3 | % |
| Basophils number (Bas#) | 0.13 | 109/L |
| Basophils percentage (Bas%) | 0.5 | % |
| platelet count (PLT) | 1722 | 109/L |
| Mean platelet volume (MPV) | 11.6 | f1 |
Table 1: Routine blood examination.
Discussion
sFE is a fibrinolytic agent that can degrade the fibrin directly and effectively12,16. Here, sFE was employed to degrade the cross-linked fibrin of the cerebral blood clots, release the enclosed cells within the clots, and analyze the cell components of the clots qualitatively and quantitatively. The microscopy data and routine blood examination indicated that the enclosed cells were released from the blood clots. Furthermore, the cell types and structures of the released cells were not affected significantly after sFE treatment. Thus, a unique, simple, and effective method was created for the first time for analyzing the cell components of cerebral blood clots.
Because the released cells are not as stable as within the clots, the degradation conditions need to be optimized to decrease the broken ratio of released cells, such as temperature, the dose of sFE, speed of rotation, and degradation time. Wright’s staining results showed that the background of the 10 h degradation was more cloudy than that of 5 h. These differences are probably due to cell debris and other cell fragments. Moreover, the flow cytometry data indicated that the cell debris increased with the degradation time (Figure 2B). The cell debris rate of 10 h degradation (32.5%) was significantly higher than that of 5 h degradation (11.2%). Therefore, it was critical to separate the released cells from the working solution as soon as possible. So, in this study, the cells were collected every 0.5 h. Another key point was the rotation, which could accelerate degradation but is bad for keeping the intact cell structure of the released cells.
Although the degradation conditions have been optimized, some cells may be lysed in this process. Cell debris may be adverse to cell microscopy and routine blood examination17. Therefore, separating those cell debris from the intact cells is recommended. Another limitation of this technique is the possibility of the microbe contamination. The underlying reason for this phenomenon is probably the long degradation time in couple with the nonsterile operation. So, some suitable antibiotics should be added in future studies18. It is well known that different blood clots share similar clots structures. Thus, the protocol reported here will be suitable for cell component analysis of other blood clots. Moreover, with the aid of this technique, not only the cell components but also the plasma with the clots could be analyzed qualitatively and quantitatively.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was funded by the Science and Technology Bureau of Xiamen City (3502Z20227197), and the Science and Technology Bureau of Fujian Province (No. 2019J01070, No.2021Y0027).
Materials
| Agglutination Reaction Plate | ROTEST | RTB-4003 | |
| Auto Hematology Analyzer | SYSMEX | XNB2 | |
| Automatic Vertical Pressure Steam Sterilizer | SANYO | MLS-3750 | |
| Centrifuge Tube (1.5 mL) | Biosharp | BS-15-M | |
| Clean bench | AIRTECH | BLB-1600 | |
| Constant Temperature Incubator | JINGHONG | JHS-400 | |
| Culture Dish (100 mm) | NEST | 704001 | |
| DHG Series Heating and Drying Oven | SENXIN | DGG-9140AD | |
| Electronic Analytical Balance | DENVER | TP-213 | |
| Filter Membrane (0.22 µm) | Millex GP | SLGP033NK | |
| Micro Refrigerated Centrifuge | Cence | H1650-W | |
| Microscope Slides | CITOGLAS | 01-30253-50 | |
| Milli-Q Reference | Millipore | Z00QSV0CN | |
| Normal Saline | CISEN | H37022337 | |
| Optical Microscope | Nikon | ECLIPSE E100 | |
| Parafilm | Bemis | PM-996 | |
| Phosphate-Buffered Saline | Beyotime | C0221A | |
| Pipette Tip (1 mL ) | Axygene | T-1000XT-C | |
| Pipette Tip (200 µL) | Axygene | T-200XT-C | |
| Pipettor (1 mL) | Thermo Fisher Scientific | ZY18723 | |
| Pipettor (200 µL) | Thermo Fisher Scientific | ZY20280 | |
| Scalpel | MARTOR | 23111 | |
| Small-sized Vortex Oscillator | Kylin-Bell | VORTEX KB3 | |
| Tweezer | Hystic | HKQS-180 | |
| Wright Staining Solution | Beyotime | C0135-500ml |
References
- Park, D. W., et al. Edoxaban versus dual antiplatelet therapy for leaflet thrombosis and cerebral thromboembolism after TAVR: The ADAPT-TAVR Randomized clinical trial. Circulation. 146 (6), 466-479 (2022).
- Devasagayam, S., Wyatt, B., Leyden, J., Kleinig, T. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke. 47 (9), 2180-2182 (2016).
- Sacco, R. L., et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 44 (7), 2064-2089 (2013).
- Thalin, C., Hisada, Y., Lundstrom, S., Mackman, N., Wallen, H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arteriosclerosis Thrombosis and Vascular Biology. 39 (9), 1724-1738 (2019).
- Mocsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. Journal of Experimental Medicine. 210 (7), 1283-1299 (2013).
- Dhanesha, N., et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. 139 (8), 1234-1245 (2022).
- Ducroux, C., et al. Thrombus neutrophil extracellular traps content impair tpa-induced thrombolysis in acute ischemic stroke. Stroke. 49 (3), 754-757 (2018).
- Solomon, C., Ranucci, M., Hochleitner, G., Schochl, H., Schlimp, C. J. Assessing the methodology for calculating platelet contribution to clot strength (platelet component) in thromboelastometry and thrombelastography. Anesthesia and Analgesia. 121 (4), 868-878 (2015).
- Daraei, A., et al. Automated fiber diameter and porosity measurements of plasma clots in scanning electron microscopy images. Biomolecules. 11 (10), 1536 (2021).
- Abbasi, M., et al. Diverse thrombus composition in thrombectomy stroke patients with longer time to recanalization. Thrombosis Research. 209, 99-104 (2022).
- C W Francis, a., Marder, V. J. Concepts of clot lysis. Annual Review of Medicine. 37 (1), 187-204 (1986).
- Xu, R., Ma, G., Chen, L., Cui, X. . Preparation and application of natural fibrinolytic enzyme from peanut worm. , (2019).
- Fotso Fotso, A., Drancourt, M. Laboratory Diagnosis of tick-borne african relapsing fevers: latest developments. Front Public Health. 3, 254 (2015).
- Liou, G. Y., Byrd, C. J. Diagnostic bioliquid markers for pancreatic cancer: What we have vs. what we need. Cancers (Basel). 15 (9), 2446 (2023).
- Tang, M., Lin, H., Hu, C., Yan, H. Affinity purification of a fibrinolytic enzyme from Sipunculus nudus. Journal of Visualized Experiments. 196, e65631 (2023).
- Ge, Y. H., et al. A Novel antithrombotic protease from marine worm Sipunculus Nudus. International Journal Of Molecular Sciences. 19 (10), 3023 (2018).
- Talukder, M. A., Menyuk, C. R., Kostov, Y. Distinguishing between whole cells and cell debris using surface plasmon coupled emission. Biomedical Optics Express. 9 (4), 1977-1991 (2018).
- Shapiro, D. J., Hicks, L. A., Pavia, A. T., Hersh, A. L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. Journal of Antimicrobial Chemotherapy. 69 (1), 234-240 (2014).

