Ex Vivo Culture of Circulating Tumor Cells in the Cerebral Spinal Fluid from Melanoma Patients to Study Melanoma-Associated Leptomeningeal Disease
Summary
This article describes a protocol for the propagation of cerebral spinal fluid-circulating tumor cells (CSF-CTCs) collected from patients with melanoma-associated leptomeningeal disease (M-LMD) to develop preclinical models to study M-LMD.
Abstract
Melanoma-associated leptomeningeal disease (M-LMD) occurs when circulating tumor cells (CTCs) enter into the cerebral spinal fluid (CSF) and colonize the meninges, the membrane layers that protect the brain and the spinal cord. Once established, the prognosis for M-LMD patients is dismal, with overall survival ranging from weeks to months. This is primarily due to a paucity in our understanding of the disease and, as a consequence, the availability of effective treatment options. Defining the underlying biology of M-LMD will significantly improve the ability to adapt available therapies for M-LMD treatment or design novel inhibitors for this universally fatal disease. A major barrier, however, lies in obtaining sufficient quantities of CTCs from the patient-derived CSF (CSF-CTCs) to conduct preclinical experiments, such as molecular characterization, functional analysis, and in vivo efficacy studies. Culturing CSF-CTCs ex vivo has also proven to be challenging. To address this, a novel protocol for the culture of patient-derived M-LMD CSF-CTCs ex vivo and in vivo is developed. The incorporation of conditioned media produced by human meningeal cells (HMCs) is found to be critical to the procedure. Cytokine array analysis reveals that factors produced by HMCs, such as insulin-like growth factor-binding proteins (IGFBPs) and vascular endothelial growth factor-A (VEGF-A), are important in supporting CSF-CTC survival ex vivo. Here, the usefulness of the isolated patient-derived CSF-CTC lines is demonstrated in determining the efficacy of inhibitors that target the insulin-like growth factor (IGF) and mitogen-activated protein kinase (MAPK) signaling pathways. In addition, the ability to intrathecally inoculate these cells in vivo to establish murine models of M-LMD that can be employed for preclinical testing of approved or novel therapies is shown. These tools can help unravel the underlying biology driving CSF-CTC establishment in the meninges and identify novel therapies to reduce the morbidity and mortality associated with M-LMD.
Introduction
Leptomeningeal disease (LMD) occurs when circulating tumor cells (CTCs) disseminate into the cerebral spinal fluid (CSF) and establish in the meninges, the membrane surrounding the brain and spinal cord1,2. LMD can occur in several cancers but is particularly prevalent in melanoma. In advanced stages of melanoma, approximately 5% of patients will develop melanoma-associated M-LMD2,3. While relatively low in regard to other sites of metastasis, the consequences of M-LMD are devastating, with overall survival ranging from weeks to months, and is a significant contributor to patient morbidity1,3,4. This is primarily due to a paucity of effective treatment options combined with major gaps in our knowledge regarding how the leptomeninges are colonized by melanoma cells2. Therefore, understanding the biology of M-LMD will facilitate in designing novel therapies to improve clinical outcomes.
Recent reports have shown how CTCs colonize the unique CSF microenvironment. For example, Complement C3 promotes the invasion of tumor cells into the CSF via the choroid plexus, an intricate network of blood vessels in each ventricle of the brain5. Further, in response to the scarce micronutrients in the CSF, CTCs can upregulate lipocalin-2, an iron-scavenging protein, and its receptor SLC22A17 to enhance survival6. Using omic-based analyses of CSF, our group also found that the CSF is enriched with proteins that regulate insulin-like growth factor (IGF) signaling, as well as innate immunity3. Together, these data emphasize the value of CSF-CTCs from liquid biopsies to study M-LMD.
While CSF-CTCs can sometimes be identified by sampling patient CSF via lumbar puncture, Ommaya reservoir, or rapid autopsies, a major limitation is obtaining sufficient numbers of these rare and fragile cells1,7. For example, using the CTC enumeration technique, only several hundred to several thousand tumor cells are identifiable per patient CSF sample7, which makes it difficult to perform molecular and functional analyses in vitro or in vivo. Though there have been reports of success in briefly growing CTCs ex vivo from peripheral blood (i.e., breast cancer CTCs)8,9,10, these cells usually only grow for the short term, and there have been no reported cases of being able to grow melanoma CTCs in the CSF. Hence, finding ways to propagate melanoma CSF-CTCs, or CTCs in general, will be highly beneficial to study the biology of M-LMD7,11.
For the first time, a novel technique to propagate CSF-CTCs from M-LMD patients ex vivo is described. Here in this report, a detailed protocol was developed that allows for the culture and expansion of CSF-CTCs from M-LMD patients. Since the meninges secrete a variety of growth factors such as FGF, IGF, VEGF-A, and IGFBPs that support the growth surrounding its environment12,13,14,15,16, it was rationalized that CSF-CTCs may require these components to grow in ex vivo conditions. Therefore, this protocol uses conditioned media generated by culturing human meningeal cells- (HMCs-) in vitro. For in vivo inoculation, patient-derived cells are inoculated into immunodeficient mice to generate patient-derived CSF-CTCs (PD-CSF-CTCs) lines. The availability of patient-derived M-LMD cells will support cellular, molecular, and functional assays to study M-LMD and propose novel treatment strategies for this deadly disease.
Protocol
The collection of deidentified patient CSF specimens was approved by the University of South Florida's Institutional Review Board (IRB) (MCC 50103, 50172, and 19332). Patients with M-LMD may be diagnosed in several ways, including positive CSF cytology, a characteristic magnetic resonance imaging (MRI) of the brain and/or spine, or a combination of clinical findings with suggestive MRI findings. CSF from these M-LMD patients were collected routinely as a part of their standard clinical care. No procedure is performed unless there is a clinical indication. Informed consent was obtained from patients for sample collection and using them for research and publication. The generation of in vivo murine-LMD models was approved by the University of South Florida Institutional Animal Care and Use Committee (IACUC# IS00010398). The overall scheme of this protocol is summarized in Figure 1. The details of the reagents and equipment used in the study are listed in the Table of Materials.
1. Preparation of HMC-conditioned media
- Precoat a T175 flask with poly-l-lysine at 2 µg/cm2.
- Place the flask in a 37 °C incubator for 1 h.
- Aspirate the poly-l-lysine solution using a sterile serological pipette. It is not required to rinse the flask, and is ready for the HMC culture.
- Culture approximately 1.0 x 106 HMCs in 30 mL of complete Meningeal Culture Medium (MenCM), which contains 5% fetal bovine serum, 1% meningeal cell growth supplement, and 100 I.U./mL penicillin-streptomycin antibiotic solution per flask. Culture the cells in the cell culture incubator at standard tissue culture conditions at 37 °C and 5% CO2.
- Change media every 3 days.
- When cells reach approximately 75%-80% confluency, collect and save the HMC cultured media in a 50 mL conical tube.
- Split HMCs into new T175 flasks and fresh complete MenCM if more HMC cultured media is needed.
- To the HMC cultured media, add a 1:1 ratio of complete MenCM.
- Add 20 ng/mL fibroblast growth factor (FGF), and 20 ng/mL epidermal growth factor (EGF), which will become the HMC-conditioned media for CSF-CTCs.
NOTE: It is recommended to add fresh FGF and EGF when CTCs are ready for culturing. - Store HMC-conditioned media in 50 mL aliquots at 4 °C.
NOTE: It is recommended that HMC-conditioned media in aliquots be stored at 4 °C but not more than 4 weeks.
2. Collection of CSF and sample processing
- Prechill centrifuge by setting it to 4 °C.
- Once drawn from the patient, place the CSF sample in a 15 mL conical tube on ice immediately to keep it cool.
NOTE: Our IRB approved protocol allows drawing 7.5 mL CSF from the consented patient. - Centrifuge the CSF at 257 x g for 5 min at 4 °C.
- Remove, save, and make aliquots of the CSF supernatant without disturbing the cell pellet at the bottom. CSF supernatant aliquots can be stored frozen at -80 °C if needed for further analysis.
NOTE: The pellet may not be visible to the naked eye; hence, it is suggested to leave ~40-50 µL at the bottom of the tube. - In the same tube, add 1 mL of sterile phosphate-buffered saline (PBS) to resuspend and rinse cells, and repeat spin at 257 x g for 5 min at 4 °C.
NOTE: (Optional) Perform red blood cell (RBC) lysis if the sample contains blood contamination. However, keep in mind that some cells could be lost during the process, including CTCs. CTCs can be propagated without the RBC lysis procedure. - Remove and discard PBS without disturbing the cell pellet, and leave ~50 µL at the bottom.
- Perform cell count to determine cell viability. From here, there are two options to proceed with growing CSF-CTCs: in vitro culture (step 3) or attempt in vivo patient-derived xenograft expansion (step 4).
NOTE: If CSF-CTCs are to be cultured at a later time, cryopreserve the cells in cell culture freezing media until it is ready for propagation. Cell culture freezing media can be made using 90% FBS + 10% DMSO. If excess CSF is available (i.e., more than one CSF collection from patients or CSF is collected at autopsy), CTCs can be evaluated by submitting the sample for CTC enumeration assay17, or immunofluorescence (IF) staining for melanoma marker (i.e., anti-MLANA) which may provide insights on the quantity and viability of CTCs. Cells cannot be recovered after performing these experiments. Therefore, it is not recommended if there are no backup CSF samples from the patient.
3. In vitro culture and expansion of CSF-CTCs
- Resuspend CSF-CTCs in HMC-conditioned media. If CSF-CTCs are cryopreserved, thaw the cells, spin them, and gently wash them with PBS.
- Split all the cells in triplicate wells in a 96-well plate with 150 µL volume per well. Only viable cells will lightly adhere to the surface overnight.
NOTE: The numbers of CSF-CTCs can vary greatly per single patient sample (Table 1). For patients with low CTC counts, a 96-well plate is used as a starting point for culturing, and the entire pellet is plated without counting for fear of losing CTCs. However, if there is a larger quantity of CSF (i.e., obtained from an autopsy), it is possible to count the cells. Not all tumor cells can grow ex vivo; some will expand slowly for several passages before they become static. Currently, the chance of success of growing melanoma CSF-CTCs ex vivo is approximately 60%7. - For every 3 days, top up by adding fresh HMC-conditioned media or gently remove the media by placing the pipette tip on the side of the well, leaving some liquid behind without disturbing the bottom of the well, and then replace it with fresh HMC-conditioned media.
- When ex vivo CSF-CTCs expand and become 90% confluent, trypsinize and transfer the whole well to a new well in a 24-well plate. When the well in a 24-well is confluent, transfer it to a 12-well plate, then a 6-well plate and so forth.
NOTE: After trypsinization, consider cryopreserving a small subset of CTCs in cell culture freezing media (10% DMSO + 90% FBS) before plating as backup. - Continue culturing CTCs. Some cells may propagate for the short term and eventually become static. However, one or more clones may transform and expand exponentially (Figure 2A). Select these clones, which will become the in vitro patient-derived CSF-CTC (PD-CSF-CTCs) cultures.
NOTE: If these clones become overcrowded or cluster, trypsinize and replate the cells in a fresh tissue culture plate/flask.
4. In vivo inoculation of CSF-CTCs to generate cell line-derived xenograft (CDX) or patient-derived xenograft (PDX) model
NOTE: A PDX model involves the engraftment of cancer cells directly from a cancer patient (without ex vivo culture), whereas the CDX model uses cancer cell lines or, in this case, CTCs that have been propagated and immortalized18.
- Use 6-8 weeks female immunodeficient NOD SCID gamma (NSG) mice for CSF-CTCs inoculation. NSGs are used because they are severely immunodeficient and are very receptive to human tumor cell engraftment19. Due to their immune deficiencies, these mice should be kept in a strictly controlled hygienic environment and should be housed in isolation from other mouse strains. The method to render murine-LMD has been described in detail elsewhere20.
NOTE: ex vivo cells (patients' CSF-CTCs that have only been processed in step 2 without culturing) are used to generate the PDX model; physical observation of the animal and MRI of the brain are needed to determine LMD progression. On the other hand, with the CDX model using in vitro, PD-CSF-CTCs can be labeled with a luciferase reporter, and the status of LMD can be assessed by bioluminescent imaging (BLI). The cell labeling system used in this report is a NanoLuc (NL) reporter that utilizes furimazine as a substrate, which has been shown to increase sensitivity in proportion to tumor growth21. An interference of CTC cell growth (in vitro or in vivo) by NL expression was not observed. - Check for signs of LMD progression using these methods: physical observation: weight loss, head tilt, and hunched back. MRI: enlarged ventricles and signs of hydrocephaly (Figure 2B). BLI: positive bioluminescent signals in the CNS region (Figure 2C).
5. CSF collection from mice with LMD for subsequent clone expansion
- Anesthetize the NSG mouse with LMD with 4% isoflurane (following institutionally approved protocols) until it shows no signs of the righting reflex.
- Prepare the mouse by shaving the fur of the entire ventral surface of the head and prepare the skin using sterile technique.
- Position the nose using a modified L-shaped nose cone of the stereotactic apparatus, ensuring the nostrils stay unobstructed. Secure the skin by gently pulling it forward across the ventral surfaces of both pinnae with tape, affixing it to the nose cone, and then bending the neck at roughly a 90° angle after securing it. Administer 1.5%-3% isoflurane to maintain anesthesia.
- Fully extending the neck and beginning just between the pinnae, guide the surgical scissor tips downward across the occipital bone with slight pressure.
NOTE: In this midline position, a subtle depression is discernible as the scissor tips enter the concave area over the cisterna magna. - Create a small midline incision measuring 5-7 mm just above the palpated concavity.
- Use blunt-tipped forceps with 1-2 mm tips to gently apply pressure on the cisterna magna. Introduce tips in a closed position and open them while exerting downward pressure on the dura.
- Repeat the blunt dissection process as outlined in step 6 until the dural membrane is clearly discernible, and the associated blood vessels are visible within the exposed area.
- While keeping the forceps open to retract the surrounding musculature, insert a 27-29 G needle attached to a 1 mL syringe beneath the dura to visualize the bevel. Ensure the needle penetrates just beyond the bevel. Gradually retract the syringe plunger.
- Collect as much CSF as possible (usually between 15-30 µL) prior to mouse euthanasia.
NOTE: Euthanasia is accomplished, following institutionally approved protocols, by exposing the subject to escalating concentrations of compressed CO2 gas. For instance, a displacement rate from 30% to 70% of the chamber volume per minute will be employed to prevent or reduce discomfort or distress. This is followed by ensuring the cessation of cardiovascular and respiratory movements through prolonged observation in room air for longer than 10 min. - Deploy the CSF from the syringe to a microcentrifuge tube and place it on ice.
- Spin the sample at 257 x g for 5 min at 4 °C, and gently remove the liquid (freeze the mouse CSF sample at -80 °C if needed for further analysis) without disturbing the cell pellet.
- Add 500 µL of sterile PBS and wash the cell pellet; repeat spin at 257 x g for 5 min at room temperature.
- Resuspend cells in HMC-conditioned media in a 96-well plate.
NOTE: CSF-CTCs that have been engrafted in vivo and successfully grown into LMD should be able to grow like normal cell cultures. Continue to expand by changing media every 3 days. Trypsinize and transfer cells to a larger cell culturing apparatus when the cells are confluent. These cells will become the in vivo PD-CSF-CTC cultures. In the current report, there was a 100% success rate with the CDX model, and have yet to generate a PDX M-LMD.
Representative Results
Understanding the requirements for successful CSF-CTCs growth ex vivo is an ongoing effort. To that end, it is believed that providing essential factors that mimic the CSF microenvironment is of key importance22. Human meningeal cells (HMCs) secrete a variety of growth factors into the CSF, including FGF-2, EGF, IGFBP2, and IGFBP6, and are known to support the growth of CTC cells12,13,14,23,24. Therefore, a human cytokine array analysis was performed on HMC-conditioned media to identify potentially important components required for CTC survival. Indeed, several growth factors were upregulated in the media cultured with HMCs (Figure 3A). For example, granulocyte-macrophage colony-stimulating factor (GM-CSF), VEGF-A, and IGFBPs (IGFBP2, 3, 4, and 6).
The CSF-cellular components from patients may consist of multiple cell types, such as CTCs, immune cells, and fibroblasts. Non-CTCs will eventually stop passaging overtime. Generally, cells that propagate successfully and remain in proliferation are cancer (M-LMD) cells. Validation of growing cells in culture is indeed M-LMD cells, which can be done by IF detection of MLANA expression and transcriptomic analyses, which have previously been shown7.
As a proof of concept to show the potential use and application of established in vitro and in vivo PD-CSF-CTC lines, single-cell RNA-sequencing (scRNA-seq) analysis was used, and the results revealed several genes that were enriched and retained from the uncultured patient CSF-CTCs7. Two of them include receptor tyrosine-protein kinase ErbB3 and IGF-1R, which have implications on melanoma progression and chemotherapy resistance25,26,27.
To test whether they played a role in CSF-CTC survival, a crystal violet proliferation assay was conducted on PD-CSF-CTCs treated with FDA-approved drugs tucatinib and ceritinib that target ErbB28 and IGF-1R7,29 respectively. Anti-IGFBP2 antibody was included as a positive control that should hinder the growth of PD-CSF-CTC cultures. The results showed that the absence of IGFBP2 or IGF-1R was effective in reducing the proliferation of PD-CSF-CTCs (Figure 3B). Given that MAPK signaling is downstream of IGF-1R, calcein-AM live cell staining and MTT cell survival assays were also performed in three M-LMD PD-CSF-CTC lines by treating them with ceritinib or the MAPK inhibitors, dabrafenib and trametinib or a combination of all three. The data demonstrated that the viability of all three cell lines was significantly reduced by ceritinib, whereas dabrafenib and trametinib had mixed effects (Figure 3C). The result from debrafenib and trametinib treatments was surprising. All three PD-CSF-CTC lines were derived from M-LMD patients that harbored a BRAFV600E mutation7. This may suggest an acquired chemo-resistance effect of CSF-CTCs, which is something to be investigated in the future.
Next, as an example of how PD-CSF-CTCs can be utilized in vivo, murine-M-LMD models were established by intrathecally inoculated with varying numbers of PD-CSF-CTCs. The median survival times in mice were determined (Figure 3D). To visualize M-LMD progression, PD-CSF-CTC lines were tagged with a bioluminescent marker, such as the NL reporter system21, and captured by BLI (Figure 2C). The location of the LMD metastases was also demonstrated using immunohistochemistry with protein melan-A (MLANA)30 as a marker of the melanoma cells (Figure 3E). As a proof of concept to test therapeutic strategies against M-LMD in vivo, murine-M-LMD cohorts were given daily oral monotherapy of ceritinib or trametinib, or a combination of dabrafenib and trametinib or ceritinib and trametinib. The control (untreated) cohort received oral saline as a comparison. The results showed a significantly prolonged survival (Figure 3F) and delayed disease detection (Figure 3G) in the cohort that was treated with ceritinib and trametinib (untreated M-LMD median survival: 28.5 days vs. ceritinib and trametinib treated M-LMD median survival: 38.5 days; P value = 0.0052). These data underscore the potential usefulness of the developed M-LMD PD-CSF-CTC lines for conducting preclinical studies to determine the efficacy of novel therapeutics.
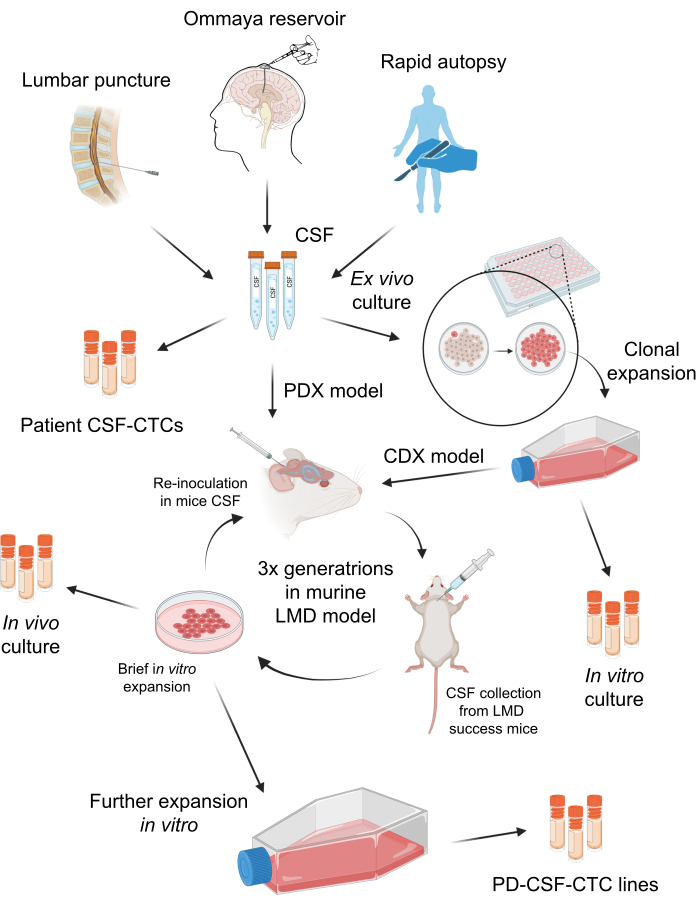
Figure 1: A schematic overview of the process of establishing patient-derived CSF-circulating tumor cells (PD-CSF-CTCs). CSF from patients can be sampled via lumbar puncture, Ommaya reservoir, or rapid autopsies. Through a series of in vitro and in vivo propagations, each step generates an intermediate CSF-CTC culture (i.e., patient CSF-CTCs, in vitro culture, in vivo culture) until establishing a PD-CSF-CTC line. Please click here to view a larger version of this figure.
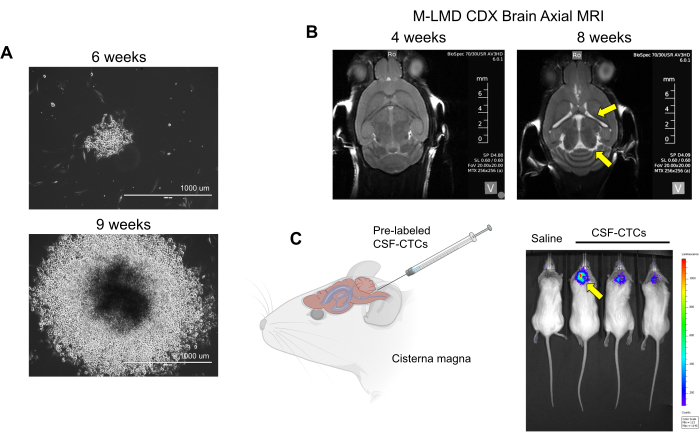
Figure 2: Examples of in vitro and in vivo culturing of CSF-CTCs derived from M-LMD patients. (A) Representative brightfield images showing the in vitro growth of an M-LMD CSF-CTC colony at 6 weeks and 9 weeks in HMC-conditioned media. Scale bar: 1000 µm. (B) MRI images at 4 weeks and 8 weeks after intrathecally inoculated with PD-CSF-CTCs; a successful establishment of a murine model of M-LMD. Yellow arrows point to enlarged ventricles and possible hydrocephaly in this M-LMD mouse. (C) Representative BLI visualization of M-LMD development in mice. The figure is adapted from Law et al.7. Please click here to view a larger version of this figure.
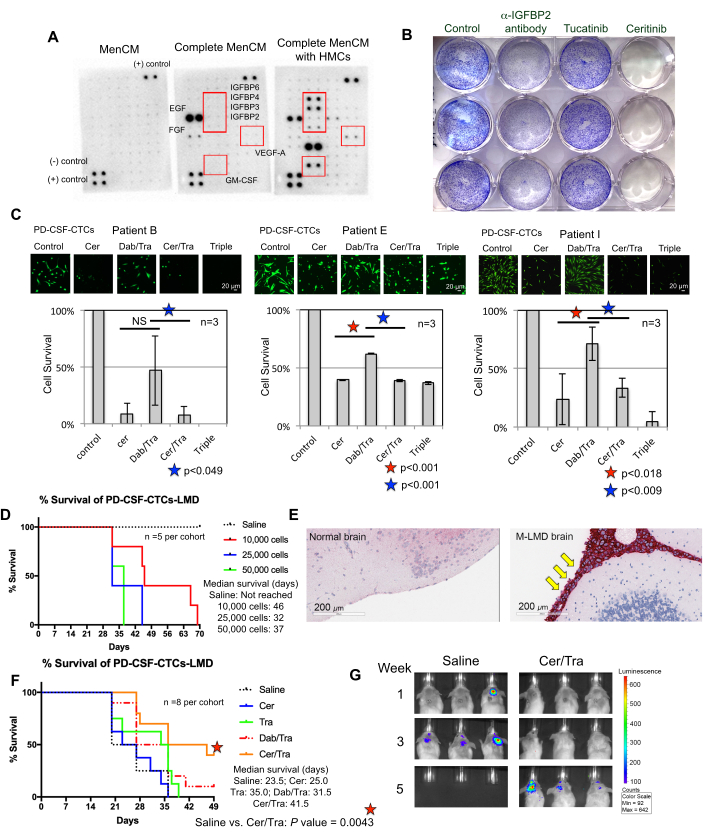
Figure 3: PD-CSF-CTC lines are used in various preclinical experiments to study M-LMD. (A) A human cytokine array showing an increase of different secreted growth factors (i.e., IGFBPs, VEGF-A, and GM-CSF) in culture media (MenCM) in the presence of human meningeal cells (HMCs). (B) A scanned image of a crystal violet cell proliferation assay to determine the efficacy of anti-IGFBP2 antibody, tucatinib, and ceritinib against one of the PD-CSF-CTC lines. The control condition was given vehicle treatment. The experiment was performed in triplicate. (C) Cell survival assay of three different established PD-CSF-CTC lines (from patients 09, 12, and 16) in vitro. Cells were treated with either ceritinib (cer), a combination of dabrafenib (dab) + trametinib (tra), cer + tra, or all three. Cells were collected at 72 h after treatment. Calcein-AM staining was used to visualize cell viability, and an MTT assay was used to determine cell survival. A paired sample t-test was used for statistical analysis. Scale bars: 20 µm. (D) A survival curve of a murine M-LMD model. NSG mice were inoculated intrathecally (via the cisterna magna) with one of the PD-CSF-CTC lines at 10,000, 20,000, and 50,000 cells. The median survival of M-LMD mice was determined. (E) IHC detection for MLANA, a marker for melanoma, in the brain sections of M-LMD mice. Positive MLANA was found in the meninges (stained in red; pointed by yellow arrows), whereas the normal (healthy) brain did not show cancer growth (negative for MLANA). Scale bars: 200 µm. (F) A representative efficacy experiment of murine M-LMD cohorts given either daily oral saline, cer, tra, dab/tra or cer/tra. Survival of mice was determined. The log-rank (Mantel-Cox) test was used for statistical analysis. (G) Representative BLI images of M-LMD progression in 5 weeks, comparing control (saline) treated vs. cer/tra treated murine M-LMD cohorts. Panel (C) of the figure is adapted from Law et al.7. Please click here to view a larger version of this figure.
Table 1: Summary of clinical CSF-CTCs obtained for ex vivo culture in M-LMD patients. A summary table of 11 M-LMD patients, which their CSF-CTCs have been attempted to propagate. The patients in the Table were previously characterized in Law et al.7. Please click here to download this Table.
Discussion
M-LMD is a devastating, universally fatal disease, and there is an urgent need to find better treatment strategies. One of the major barriers to studying M-LMD is the inability to acquire enough CSF-CTCs to perform molecular and functional studies1,7. Though there are existing methods to culture CTCs from peripheral blood and CSF of other cancer types, such as breast and ovarian cancers11,31,32, these CTC propagation methods are usually short-term, and there has been no reported success in culturing CSF-CTCs from melanoma. In addition, the current methodologies for propagating CTCs exist in short-term ex vivo settings and have yet to yield an in vivo LMD model derived from patient LMD cells. Here, a novel protocol is presented to culture these cells in vitro and in vivo, leading to unique patient-derived cell lines. Currently, of 11 M-LMD patients in the study, there was an approximately 60% (7 of 11) chance of success in propagating M-LMD CSF-CTCs in vitro, and while this was lowered to ~20% (2 of 11) in vivo using the CDX method7.
It is clear that in vitro conditions do not recapitulate the CSF microenvironment. However, proteomic approaches have previously been performed to study protein components in the CSF and provided some insights as to key factors that were required for CTC growth ex vivo3. For example, it was identified that one of the major pathways promoting CTC survival in M-LMD patients was associated with heightened IGF-related activities3,7. Further, studies have shown that the leptomeninges secretes a variety of cytokines/growth factors into the CSF, including FGF-2, EGF, GM-CSF, and proteins related to IGF-signaling12. Indeed, this was recapitulated in the media cultured with HMCs, supporting a potential role for these growth factors in promoting CSF-CTC growth.
A major advantage in generating a PDX (or CDX) model is the ability to gain deeper insights into the pathology of disease, something that in vitro conditions lack. Ideally, a PDX approach is preferred since the CSF-CTCs would be directly from patients without ex vivo culturing. Initially, attempts were made to create M-LMD using this approach, but they have not been successful thus far. The difficulty in generating PDX mice is possibly associated with the abundance and integrity of the starting material (i.e., very few viable CTCs in patient CSF at routine collection in the clinic). This may explain why we had superior success growing CTCs from CSF collected at autopsy7. To increase the probability of in vivo propagation, this protocol was modified to provide an alternate CDX approach. CSF-CTCs can be first expanded in vitro (step 3) to generate PD-CSF-CTC lines that have long-term and greater growth potential. These cells are then inoculated in mice to create M-LMD. Though the current method generated a limited number of in vivo CDX M-LMD (~ 20%) models, this might reflect the transcriptional diversity of CSF-CTCs, the complexity of the CSF microenvironment, and the difficulty in culturing these cells in general. We posit that future development of a humanized mouse model may enhance the engraftment success rate given the importance of the microenvironment in supporting cancer cell viability33.
A limitation of the CDX approach is that only certain clones were selected from patient samples, and genetic drift of cancer cells through ex vivo culturing may no longer reflect the transcriptional profile of the original source. However, it has been reported that despite in vitro culturing, PD-CSF-CTC lines retained approximately 97% similarity of gene expression to isolated, non-cultured patient CSF-CTCs7. In that study, scRNA-seq analyses revealed overlapping enriched gene signatures between non-cultured, in vitro PD-CSF-CTCs and in vivo PD-CSF-CTCs, such as SOX9, ErbB3, and IGF-1R7, suggesting these may be potential therapeutic targets. Additionally, these commonly enriched genes are involved in biological pathways associated with transcriptional regulation and metabolism7. Collectively, this highlights the translational value of PD-CSF-CTC cultures for better understanding the biology of M-LMD, identifying targetable molecular mechanisms and pathways driving the disease, and designing rational therapies in future studies.
Though the current methodology remains imperfect, as there is no way to predetermine the status and viability of CSF-CTCs in M-LMD patients, several observations have been made that would increase the likelihood of success since the CTCs are low in number and quite fragile. These critical steps include coordinating with the clinic to have CSF samples placed on ice as soon as they are drawn and have them quickly transported to the lab so as to maintain cellular integrity. Subsequently, CSF-CTCs should be processed immediately, either by plating them in culture or cryopreserving the cells.
Overall, culturing and expanding CSF-CTCs was a trial-and-error process, but the establishment of this protocol to generate patient-derived M-LMD cells will give researchers the resources required to perform experiments with patient samples, which could not have been done previously. A major goal moving forward is to utilize M-LMD PD-CSF-CTCs to conduct molecular characterization, high throughput drug screening, and in vivo drug efficacy studies to design rational therapies to treat M-LMD. It is believed that this approach will lead to treatment strategies that will greatly reduce the morbidity and mortality associated with this currently fatal aspect of advanced metastatic melanoma.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank patients and families for their extraordinary generosity in donating tissue for this scientific study. This work was supported by grants from the National Institutes of Health grants P50 CA168536, R21 CA256289, R21 CA216756 (to KSMS and PAF) K99 CA226679 (to IS). Moffitt Foundation Research Acceleration Fund (to BC and PAF), Moffitt Chemical Biology & Molecular Medicine Program (PAF and DD), Moffitt Foundation (PAF). The Molecular Genomics, Tissue, and Bioinformatics & Biostatistics Shared Resource Cores at Moffitt is supported in part by the National Cancer Institute through a Cancer Center Support Grant (P30-CA076292) and the Moffitt Foundation.
Materials
| 1 mL syringe 27 – 29 G needles | Any vendor | n/a | 0.1 mm Sterile Filtered |
| 1.5 mL Eppendorf tubes | Any vendor | ||
| 15 ml and 50 mL polystyrene centrifuge tubes | Any vendor | n/a | |
| 6 - 8 weeks females NOD SCID gamma (NSG) mice | Charles River | Males optional | |
| Buprenorphine Sustained-Release (Bup-SR) | Zoopharm | DEA controlled | |
| Fetal bovine serum (FBS) | ScienCell | #0010 | |
| Gas inhalation anestehsia system | VeteEquip | #901812 | COMPAC5 |
| Hamilton microliter syringes | Hamilton | 10, 25, 50, and 100ml | 30 G for cisterna magna injection |
| Human basic fibroblast growth factor (bFGF) | Milipore Sigma (or any vender) | #F0291 | |
| Human epidermal growth factor (EGF) | Milipore Sigma (or any vender) | #SRP3027 | |
| Human meningeal cells (HMCs) isolated from human leptomeninges | ScienCell | #1400 | |
| IVIS 200 imaging system | Caliper Life Sciences | n/a | |
| Magnifying glass with light | Any vendor | n/a | |
| Meningeal Cell Culture Media (MenCM) | ScienCell | #1401 | |
| Meningeal cell growth supplement (MCGS) | ScienCell | #1452 | |
| MRI imaging | Bruker | BioSpec series | Optional |
| P1000, P200, P20 pipettes/ pipette tips | |||
| penicillin-streptomycin Antibiotic solution | ScienCell (or any vender) | #0503 | |
| Phosphate buffered saline (PBS) | Any vendor | n/a | 0.1 mm Sterile Filtered |
| Rodent Surgical Instruments (Scissors, Forceps) | Roboz Surgical Instrument (or any vendor) | ||
| Screw cap cryo tubes | |||
| Sterile blue paper/ drape covering | Any vendor | n/a | n/a |
| Sterile cotton sticks | Any vendor | n/a | |
| Tissue culture plates/flasks (96-well, 24-well, 12-well, 6-well, T175 etc.) |
References
- Glitza, I. C., et al. Leptomeningeal disease in melanoma patients: An update to treatment, challenges, and future directions. Pigment Cell Melanoma Res. 33 (4), 527-541 (2020).
- Khaled, M. L., Tarhini, A. A., Forsyth, P. A., Smalley, I., Pina, Y. Leptomeningeal disease (LMD) in patients with melanoma metastases. Cancers (Basel). 15 (6), 1884 (2023).
- Smalley, I., et al. Proteomic analysis of CSF from patients with leptomeningeal melanoma metastases identifies signatures associated with disease progression and therapeutic resistance). Clin Cancer Res. 26 (9), 2163-2175 (2020).
- Larkin, J., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 381, 1535-1546 (2019).
- Boire, A., et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 168 (6), 1101-1113 (2017).
- Chi, Y., et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. 369 (6501), 276-282 (2020).
- Law, V., et al. A preclinical model of patient-derived cerebrospinal fluid circulating tumor cells for experimental therapeutics in leptomeningeal disease from melanoma. Neuro Oncol. 24 (10), 1673-1686 (2022).
- Carmona-Ule, N., et al. Short-term ex vivo culture of CTCs from advance breast cancer patients: Clinical implications. Cancers (Basel). 13 (11), 2668 (2021).
- Zhang, L., et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 5 (180), (2013).
- Yu, M., et al. Cancer therapy: Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 345 (6193), 216-220 (2014).
- Mohamed, B. M., et al. Ex vivo expansion of circulating tumour cells (CTCs). Sci Rep. 13 (1), 3704 (2023).
- Decimo, I., Fumagalli, G., Berton, V., Krampera, M., Bifari, F. Meninges: From protective membrane to stem cell niche. Am J Stem Cells. 1 (2), 92-105 (2012).
- Mercier, F., Hatton, G. I. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: Roles in stem cell proliferation and morphological plasticity. J Comp Neurol. 431 (1), 88-104 (2001).
- Stylianopoulou, F., Herbert, J., Soares, M. B., Efstratiadis, A. Expression of the insulin-like growth factor II gene in the choroid plexus and the leptomeninges of the adult rat central nervous system. Proc Natl Acad Sci U S A. 85 (1), 141-145 (1988).
- Nordqvist, A. C., Mathiesen, T. Expression of IGF-II, IGFBP-2, -5, and -6 in meningiomas with different brain invasiveness. J Neurooncol. 5, 19-26 (2002).
- Zumkeller, W., Westphal, M. The IGF/IGFBP system in CNS malignancy. Mol Pathol. 54 (4), 227-229 (2001).
- Wang, L., et al. Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin Oncol. 43 (4), 464-475 (2016).
- Liu, Y., et al. Patient-derived xenograft models in cancer therapy: technologies and applications. Signal Transduct Target Ther. 8, 160 (2023).
- Chen, J., et al. The development and improvement of immunodeficient mice and humanized immune system mouse models. Front Immunol. 13, 1007579 (2022).
- Law, V., et al. A Murine Ommaya xenograft model to study direct-targeted therapy of leptomeningeal disease. J Vis Exp. (167), e62033 (2021).
- Stacer, A. C., et al. NanoLuc reporter for dual luciferase imaging in living animals. Mol Imaging. 12 (7), 1-13 (2013).
- Luo, Y. T., et al. The viable circulating tumor cells with cancer stem cells feature, where is the way out. J Exp Clin Cancer Res. 37, 38 (2018).
- Stumm, R., Kolodziej, A., Schulz, S., Kohtz, J. D., Hollt, V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J Comp Neurol. 502 (3), 382-399 (2007).
- Aviezer, D., et al. basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 79 (6), 1005-1013 (1994).
- Tiwary, S., et al. ERBB3 is required for metastasis formation of melanoma cells. Oncogenesis. 3 (7), e110 (2014).
- Sun, X., et al. miR-7 reverses the resistance to BRAFi in melanoma by targeting EGFR/IGF-1R/CRAF and inhibiting the MAPK and PI3K/AKT signaling pathways. Oncotarget. 7 (33), 53558-53570 (2016).
- Satyamoorthy, K., Li, G., Vaidya, B., Patel, D., Herlyn, M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. 61 (19), 7318-7324 (2001).
- Lin, N. U., et al. Tucatinib vs Placebo, both in combination with Trastuzumab and Capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: Updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 9 (2), 197-205 (2023).
- Russo, A., et al. Ceritinib-induced regression of an insulin-like growth factor-driven neuroepithelial brain tumor. Int J Mol Sci. 20 (17), 4267 (2019).
- Ohsie, S. J., Sarantopoulos, G. P., Cochran, A. J., Binder, S. W. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 35 (5), 433-444 (2008).
- Kulasinghe, A., et al. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget. 7 (37), 60101-60109 (2016).
- Li, X., et al. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget. 9 (2), 2705-2714 (2018).
- Lelliott, E. J., et al. A novel immunogenic mouse model of melanoma for the preclinical assessment of combination targeted and immune-based therapy. Sci Rep. 9 (1), 1225 (2019).

.
