Portable Paper-Based Immunoassay Combined with Smartphone Application for Colorimetric and Quantitative Detection of Dengue NS1 Antigen
Summary
Addressing urgent dengue diagnostic needs, here we introduce a smartphone app-integrated Dengue NS1 Paper-based Analytical Device (DEN-NS1-PAD) for quantifying Dengue NS1 antigen concentration in clinical serum/blood samples. This innovation enhances dengue management by aiding clinical decision-making in various healthcare settings, even resource-limited ones.
Abstract
Dengue virus (DENV) infection, which is transmitted by Aedes mosquitoes, is a major public health concern in tropical and subtropical countries. With an annual incidence of approximately 10 million cases and 20,000-25,000 deaths, particularly among children, there is an urgent need for practical diagnostic tools. The presence of dengue non-structural protein 1 (NS1) during early infection has been linked to cytokine release, vascular leakage, and endothelial dysfunction, making it a potential marker for severe dengue.
Paper-based immunoassays such as lateral flow assays (LFAs) and microfluidic paper-based analytical devices (PADs) have gained popularity as diagnostic tests due to their simplicity, rapidity, inexpensiveness, specificity, and ease of interpretation. However, conventional paper-based immunoassays for dengue NS1 detection typically rely on visual inspection, yielding only qualitative results. To address this limitation and enhance sensitivity, we proposed a highly portable NS1 dengue detection assay on a Paper-based Analytical Device (PAD), namely, DEN-NS1-PAD, that integrates a smartphone application as a colorimetric and quantitative reader. The development system enables direct quantification of NS1 concentrations in clinical samples.
Serum and blood samples obtained from patients were utilized to demonstrate the system prototype performance. The results were obtained immediately and can be employed for clinical assessment, both in well-equipped healthcare facilities and resource-limited settings. This innovative combination of a paper-based immunoassay with a smartphone application offers a promising approach for enhanced detection and quantification of dengue NS1 antigen. By augmenting sensitivity beyond the capabilities of the naked eye, this system holds great potential for improving clinical decision-making in dengue management, particularly in remote or underserved areas.
Introduction
Dengue virus (DENV) infection is the fastest-spreading mosquito-borne disease1, and more than 390 million people are infected with 96 million symptomatic infections, 2 million cases of severe disease, and more than 25,000 deaths per year occur in the world1,2. According to the World Health Organization (WHO), an estimated 3.9 billion people are at risk for dengue; ~70% live in Asia Pacific countries and mainly in Southeast Asia3. In 2019, the number of dengue cases reported to WHO was 4.2 million, and Thailand contributed at least 136,000 dengue cases and 144 death cases from dengue infection4. The dengue outbreak in Thailand occurs during the rainy season, from April to December, in both urban and rural areas, especially in the northeastern area.
DENV infections have different clinical manifestations ranging from subclinical symptoms, mild dengue fever (DF) to severe dengue hemorrhagic fever (DHF). The main characteristic of severe DHF condition is increased vascular permeability followed by shock and organ dysfunction1. Understanding the molecular pathway that can cause the vascular leak is very important in developing effective dengue treatments. Dengue non-structural protein 1 (NS1) is a secreted glycoprotein during early virus infection5,6, and it functions as a cofactor for viral RNA replication7. NS1 can trigger cytokine release and contribute to vascular leak by binding to toll-like receptor 4 (TLR4) and endothelial glycocalyx8,9. In vitro research has shown that NS1 interacts with endothelial cells and induces apoptosis. This condition can contribute to endothelial dysfunction and vascular leak10. NS1 antigen levels, correlated with serum Interleukin (IL)-10 levels, were increased significantly in patients with severe clinical disease11. Dengue NS1 also contributes to disease pathogenesis by inducing IL-10 and suppressing DENV-specific T-cell responses12,13. In addition, dengue NS1 protein was related to severe clinical disease, and the concentration of NS1 > 600 ng mL-1 in the first 3 days of illness was associated with the development of DHF14.
The persistence of the dengue NS1 antigen in patients with DHF could be used as a marker of severe dengue6. There are several methods to detect NS1 in clinical samples such as enzyme-linked immunosorbent assay (ELISA)and the rapid test15. The gold standard for measuring the concentration of NS1 proteins in a clinical setting is the ELISA method. However, the ELISA method is expensive and requires skilled personnel, and laboratory facilities16. Therefore, the development of technology for detecting and quantifying NS1 proteins in the point-of-care test (POCT) is still ongoing. In the last decade, paper-based immunoassays such as lateral flow assays (LFAs) and microfluidic paper-based analytical devices (µPADs) have become popular as diagnostic tests because of their simplicity, rapidity, inexpensiveness, and specificity17,18,19. In a paper-based immunoassay, several labels have been used to generate signals, such as gold nanoparticles (AuNPs)20, magnetic nanoparticles21,22, quantum dots23, and fluorescence materials24,25. AuNPs are the most common labels used in paper-based immunoassays due to their inexpensive cost of production, ease of manufacture, stability, and simple readout. Currently, lateral flow assays (LFAs) for dengue NS1 are famously used in the clinical setting26,27. However, conventional LFA label detection commonly uses the naked eye and only provides qualitative results.
In the last decade, more than 5 billion smartphones have been widely used globally, and there is potential for developing portable detection28,29. Smartphones have multi-functional capacities such as built-in physical sensors, multi-core processors, digital cameras, USB ports, audio ports, wireless, and application software, making them suitable for use in various biosensor platforms30. In addition, wireless technologies allow data to be sent quickly and can be used for real-time and on-site monitoring31. Mudanyali et al. combined the paper-based immunoassay and smartphones to develop a portable, equipment-free, rapid, low-cost, and user-friendly POCT platform for malaria, tuberculosis, and HIV32. Ling et al. reported a lateral flow assay combined with a smartphone camera to detect alkaline phosphatase activity in milk quantitatively33. Hou et al. also developed a smartphone-based, dual-modality imaging system for quantitative signals from color or fluorescence in the lateral flow assay34. In addition, using the smartphone as a colorimetric and quantitative reader can improve the sensitivity while the naked eye cannot confidently report the presence of the target35.
Presenting a breakthrough in dengue diagnostics, the DEN-NS1-PAD36,37,38 (referred to as the device henceforth) offers a portable and efficient solution. Using wax-printed microfluidic paper-based technology, this device quantifies NS1 with high sensitivity and specificity through image processing. To further enhance its utility, we have developed a user-friendly smartphone app for colorimetric and quantitative reading. Clinical validation using patient samples from Thai hospitals underscores its immediate impact on real-time patient assessment. Our innovation marks a pivotal advancement in streamlined, point-of-care-dengue management, promising to revolutionize diagnostics in resource-limited healthcare landscapes.
Protocol
The Ethics Committee of the Institutional Review Board, Royal Thai Army Medical Department, Phramongkutklao Hospital, Bangkok, Thailand (IRBRTA 1218/2562) granted approval. In carrying out this study, we complied with all necessary ethical regulations.
1. Device fabrication of the paper-based Immunoassay
NOTE: The paper-based immunoassay device was fabricated following previously established methods36,37, and Thai patent request no. 19010081638.
- Design and pattern drawing: Design the paper analytical device (Figure 1A,B) with 18 PAD wax patterns on a computer.
NOTE: The design is specific and intended for A5-sized paper. The number of PADs is related to the size of the paper, as the user requires. - Print the designed pattern onto the cellulose paper using a wax printer (Table of Materials).
- Melt the wax-printed paper in a laboratory oven for 75 s at 150 °C. Subsequently, store it in a silica box until needed for the subsequent steps.
- Apply 0.5 µL of 0.025% poly-L-lysine (PLL) to both the test and control areas. Incubate at room temperature (RT) for 2 min in a silica box and then heat in the oven at 65 °C for 5 min.
- Apply 0.5 µL of 1 µg µL-1 of goat anti-mouse IgG antibody on the control area and 0.5 µL of 1 µg µL-1 of the capture antibody to the test area. Allow the drops to dry in a silica gel box at RT for 30 min.
- Apply 2 µL of the blocking buffer to the sample area, 3 µL to the conjugate area, and 2 µL to the detection area. Let the drops dry at RT in a silica gel box for 30 min.
- Apply 2 µL of gold nanoparticle-antibody complex (AuNPs-Ab) solution to the conjugate area and allow it to dry in a silica gel box at RT for 30 min.
2. Assembly of the paper-based Immunoassay
- Carefully remove the protective film on the reverse side of the adhesive plastic backing card to expose the adhesive.
- Align the treated cellulose paper with the adhesive plastic backing card and firmly press the two layers together.
NOTE: Avoid touching the hydrophilic field to minimize the risk of contamination or damage to the device. - Apply a plastic film to coat the paper and press them together.
- Cut the desired piece of devices using scissors from sheets of completely assembled devices.
- The DEN-NS1-PADs (Figure 1C) are now ready for use. For long-term stability, store them at 4 °C.
3. Preparation of the AuNPs-Ab conjugate
NOTE: The AuNPs-Ab was prepared as described previously by Prabowo et al.36.
- Combine 10 µL of 1 mg mL−1 anti-NS1 antibody in PBS, 1 mL of 40 nm AuNPs colloid, and 0.1 mL of 0.1 M borate buffer (pH 8.5).
- Rotate the mixture at 50 rpm for 60 min and incubate at RT.
- Apply 0.1 mL of 10 mg mL−1 BSA in BBS, rotate at 50 rpm, and incubate at RT for 15 min.
- Centrifuge the solution at 20,187 × g and 4 °C for 30 min.
- Carefully pipette and separate the supernatant from the precipitated AuNPs-Ab.
- Resuspend the AuNPs-Ab in 500 µL of BBS and disperse it using sonication.
- Repeat centrifugation at 20,187 × g and 4 °C for 30 min.
NOTE: Repeat the dispersion and centrifugation processes 3x. - Add 50 µL of the conjugate buffer to the suspension, making it ready for application onto the conjugate area.
4. Mobile application development
- Image processing and machine learning development
- Gather a dataset for a supervised image model by collecting over 900 auto-focusing images of DEN-NS1-PADs, capturing various conditions such as different concentrations, camera brands (12-13 megapixels), rotations (90° and 180°), and lighting settings. Aim for 30 images under each specific condition.
- Label the ground truth by Identifying and annotating two regions of interest as the test and control areas within the collected images for supervised learning.
- Design an algorithm to identify the background strip. Locate the centerline between the test and control regions, calculate its midpoint, and establish a square region proportional to the average size of the two main regions while maintaining the same rotational orientation.
- Create an image segmentation model using the dataset and ground truth labels from steps 4.1.1 and 4.1.2 to train an image segmentation model for identifying the regions of interest.
- Application algorithm
- Apply the trained image segmentation model to new images to locate the test, control, and background regions automatically.
- Use basic image processing techniques to obtain a single intensity value for each of the three regions of interest (test, control, and background).
- Transform the image into a 3D array representation (y, x channel) to access pixel values.
- Convert the image to grayscale by averaging RGB values and apply inversion with the formula (255-x).
- Normalize the values of the test and control area by subtracting the background area value.
- Use the preestablished calibration curve to calculate the concentration of NS1.
- Classify the results as positive or negative based on a cut-off value of 0.1103 derived from the normalized grayscale intensities37.
5. Calibration curve and sensitivities
- Prepare NS1 sample in human serum for calibration with concentrations of 0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 µg mL-1.
- Drop 50 µL of each concentration onto the sample area and perform measurements in triplicate.
- Allow the samples to wick completely into the device, which may take 20-30 min to obtain results.
- Capture images of the device using a digital camera or smartphone after 5 min of incubation.
- Analyze the test and control areas using ImageJ and a custom mobile application.
- Construct the calibration curve based on data from ImageJ and the mobile application.
- Calculate the limit of blank (LOB), the limit of detection (LOD), and the limit of quantification (LOQ) using equations (1-3) below:
LOB = Mean of the blank data + 1:645* ð (standard deviation of blank data) (1)
LOD = LOB +1:645*ð (standard deviation of the lowest concentration data) (2)
LOQ = Mean of blank data + 10*ð (standard deviation of blank data) (3)
6. Performing a paper-based Immunoassay with clinical samples
- Collect and process 300 µL of peripheral blood from 30 patients on the first day of hospitalization into purple top EDTA tubes, following good clinical practices.
- Centrifuge the blood at 2,884 × g and 4 °C for 20 min.
- Transfer the liquid component (plasma) into a clean polypropylene tube using a pipette.
- Store the plasma in the freezer immediately at −20 °C for subsequent analysis.
- Apply 20 µL of plasma to the sample area on top of the device. Then, add 30 µL of wash buffer (0.05% v/v Tween 20 in 1x phosphate buffered saline).
- Allow the sample to wick completely into the device, which may take 20-30 min to obtain the results.
- Capture images of the device using a digital camera or smartphone after 5 min of incubation at room temperature.
- Analyze the test and control areas using ImageJ and a custom mobile application.
7. Quantification with mobile application
NOTE: The intensity of paper-based immunoassay is analyzed in the mobile application (Figure 2).
- Open the developed mobile application on the smartphone.
- Select Use camera or Upload from Gallery to choose or upload the data source. Do this through camera capturing or by selecting an image from the device's gallery.
- Navigate to the analytic section and touch the Analyze button on the screen.
- Wait for the application to analyze the data and display the results.
Representative Results
Selecting a fabrication method is pivotal to ensure reproducible assay performances in paper-based immunoassay devices. In our study, we explored various manufacturing processes and materials in the context of demonstrating a paper-based immunoassay. Our chosen method utilizes a wax printing system to create hydrophobic barriers within paper-based microfluidic devices. This approach stands out due to its simplicity, speed, and consistent results. Of note, it offers the advantage of avoiding the use of photoresist chemicals, which have the potential to interfere with protein adsorption and increase the hydrophobicity of cellulose paper. Furthermore, wax printing ensures consistent dimensions of fluidic channels, contributing to repeatable assay performance.
Following the formation of hydrophobic barriers, the necessary reagents for the immunoassay were applied to the cellulose paper surface. With electrostatic adsorption, PLL assisted in biomolecule immobilization by interacting with both the positive charge of amine functional groups and the negatively charged antibody. This step facilitates the modification and immobilization of antibodies and the application of label-antibody conjugates during the fabrication processes. Importantly, this step can be conducted in parallel. The assembly of the paper immunoassay devices (DEN-NS1-PAD, as shown in Figure 1A) is completed by stacking the modified paper onto an adhesive plastic backing card and laminating it with a plastic film.
The main purpose of this study is to develop a user-friendly method utilizing a smartphone to measure NS1 concentrations. This approach could be used as a point-of-care testing (POCT) device in both home and clinical settings. Given the wide range of NS1 concentrations in patient serum, simple linear models were employed based on the results of these experiments. For each NS1 concentration, a dataset comprising three test devices was prepared. Photos of the devices were captured using a smartphone under standard settings and optimal lighting conditions, eliminating the need for a dark box. The test zone of the PADs contains mouse dengue NS1 monoclonal antibody, while the control zone features goat anti-mouse IgG antibody. With a sandwich assay format, higher NS1 concentrations in samples correspond to increased intensity of red color in the test zone. In contrast, the color intensity in the control zone remains relatively constant. Figure 1B showcases unprocessed smartphone images, which provide an advantageous visual observation without requiring specialized equipment.
Using a dedicated mobile application, we normalized the intensities and computed simple linear models for spiked NS1 concentrations in serum samples-the coefficient correlation (r2) obtained from the mobile application. The coefficient correlation (r2) obtained from the mobile application was 0.92 (Figure 3), aligning with expectations. This smartphone-based approach outperformed naked-eye observation, significantly enhancing sensitivity by 178%. Additionally, the limit of blank (LoB), limit of detection (LoD), and limit of quantification (LoQ) were calculated for the normalized intensities, as presented in Table 1.
Real-world clinical samples were utilized to demonstrate the practical functionality of DEN-NS1-PADs in clinical settings. The paper-based immunoassay produced qualitative color readouts within 20-30 min, allowing visual determination of negative or positive outcomes. Serum samples from patients suspected of dengue were subjected to the device. Figure 4 illustrates and compares the results obtained from both the device and a commercial rapid diagnostic test (RDT). Table 2 summarizes the outcomes of visual readings and the smartphone-based system. The commercial RDT and the device yielded similar results, with seven positive and 23 negative outcomes from visual reading. In contrast, the smartphone-based reader system exclusively applied to the device reported nine positive and 21 negative outcomes from clinical samples.
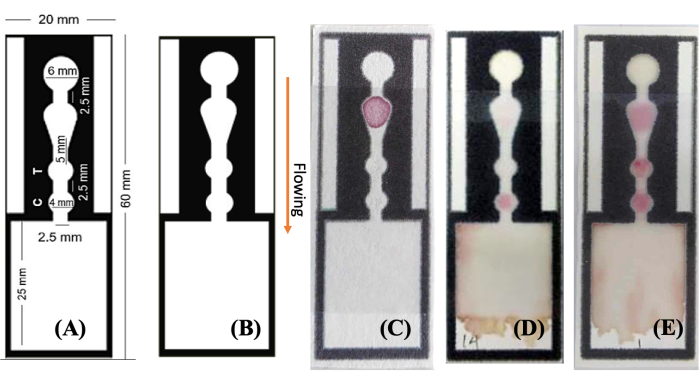
Figure 1: Images of designed and fabricated DEN-NS1-PAD. (A,B) A single channel from the wax-patterned hydrophobic barrier is designed and depicted in three states (C) before and (D,E) after introducing the sample solution to the designated area and showing the (D) negative and (E) positive results, respectively. The sample solution wicks through the channel (see arrow labeled), interacting with the components at key locations-AuNPs-Ab at the conjugate area, anti-NS1 antibody at the test area (indicative of a positive dengue NS1 result), and anti-mouse IgG at the control area. The results are readily observable by the naked eye and can be quantified by image processing using a flatbed scanner or smartphone camera. Abbreviation: AuNPs-Ab = gold nanoparticles-antibody conjugate. Please click here to view a larger version of this figure.
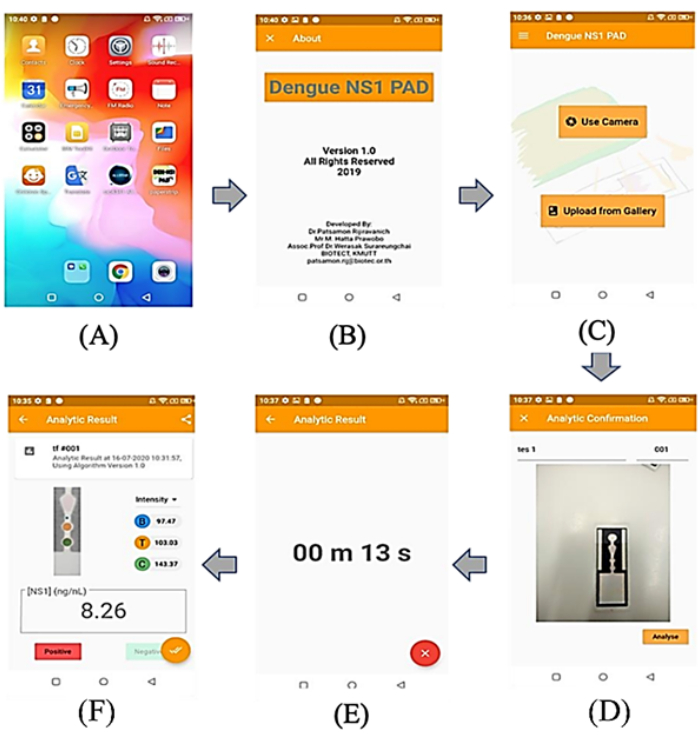
Figure 2: Screenshots of phone's screen from mobile app. (A) User screen of the Android application running on the mobile phone device, (B) display of application screen, (C) main menu of the application that the users can select to use camera or upload image from gallery, (D) display of a related image for testing, (E) display of the countdown time to analyze, (F) display of test results, including the intensity of the test and control zone, the decision of infection (positive/negative), and the concentration of NS1 in the sample. Please click here to view a larger version of this figure.
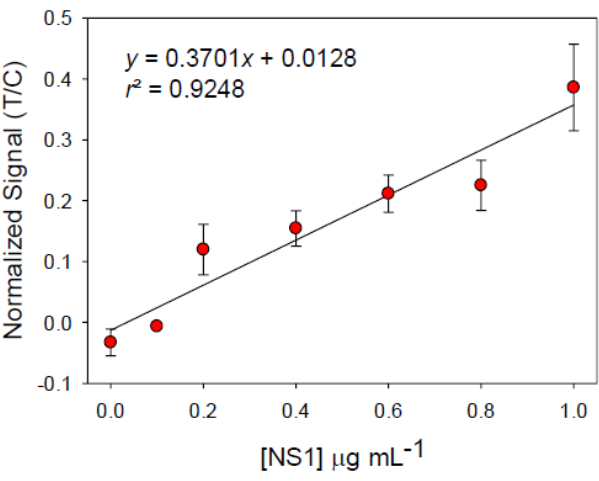
Figure 3: Linear calibration curve for NS1 detection in serum. The device was used and images interpreted by processing via an application based on smartphone data. The error bars show ±1 standard deviation, n = 3. Abbreviations: T = test area; C = control area. Please click here to view a larger version of this figure.
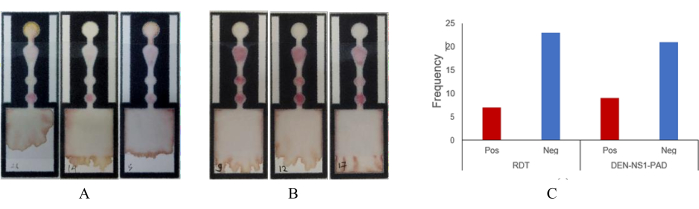
Figure 4: Image of DEN-NS1-PAD from the clinical sample assay. Serum (50 µL) was used in a paper-based immunoassay. (A) Negative result example, (B) positive results, (C) overall results and comparison of RDT versus paper-based immunoassay. Abbreviations: RDT = Rapid Diagnostic test; Pos = positive; Neg = negative. Please click here to view a larger version of this figure.
| Parameter | Naked eyes | Mobile App |
| Limit of Blank (LoB) | – | 43.15 ng mL-1 |
| Limit of Detection (LoD) | 200 ng mL-1 | 112.19 ng mL-1 |
| Limit of Quantification (LoQ) | – | 373.58 ng mL-1 |
Table 1: LoB, LoD, and LoQ from ImageJ and mobile application on data calibration standard NS1 in serum. Abbreviations: LoB = limit of blank; LoD = limit of detection; LoQ = limit of quantification.
| Patient No. | Naked Eyes | Smartphone App | ImageJ | |
| RDT | Paper-based Immunoassay | |||
| 1 | – | – | – | – |
| 2 | – | – | – | – |
| 3 | – | – | – | – |
| 4 | – | – | – | – |
| 5 | – | – | – | – |
| 6 | – | – | – | – |
| 7 | – | – | – | – |
| 8 | – | – | – | – |
| 9 | + | + | + | + |
| 10 | – | – | + | + |
| 11 | – | – | – | – |
| 12 | + | + | + | + |
| 13 | – | – | – | – |
| 14 | – | – | – | – |
| 15 | – | – | – | – |
| 16 | + | + | + | + |
| 17 | + | + | + | + |
| 18 | – | – | – | – |
| 19 | – | – | – | – |
| 20 | – | – | – | – |
| 21 | + | + | + | + |
| 22 | + | + | + | + |
| 23 | – | – | – | – |
| 24 | + | + | + | + |
| 25 | – | – | – | – |
| 26 | – | – | – | – |
| 27 | – | – | – | – |
| 28 | – | – | – | – |
| 29 | – | – | – | – |
| 30 | – | – | + | + |
Table 2: Comparison of visual reading and smartphone-based reader system results for serum samples. (+) and (-) indicate positive and negative interpretations of the results, respectively.
Discussion
One of the important design parameters for a smartphone-based reader system is the ability to provide reproducible imaging processing of samples. In this study, for simplicity and convenience, the images were captured from three different smartphone brands with 12-13 MP cameras without using an imaging box or accessories. Variable conditions of image capturing, such as the resolution of the camera, image capturing time, lighting conditions, and environment, can influence the color intensity of the test and control spots on the device. The impact of different image capture times on the lighting and drying of PAD on the signal intensities of the devices was minimized by using the normalized signal intensities, which remained consistent across images captured at various times36. Background signal subtraction emerged as a strategy to enhance the accuracy of color intensity measurements, effectively mitigating the influence of lighting conditions. Our finding aligns with previous research highlighting the efficacy of baseline or background subtraction techniques in minimizing environmental effects39,40.
The ongoing debate surrounding the superiority of using an imaging box or an accessory-free method has implications for image processing39,41. An imaging box can enhance the robustness of imaging processing results by minimizing variations in imaging conditions41,42,43. In this study, we utilized a mobile application based on cloud machine learning for image processing. This approach leverages machine learning within a cloud-based platform to classify, extract, and enrich image data automatically. The application effectively identified and processed the region of interest within images, encompassing the background, test, and control zones. This step was pivotal in differentiating between these zones38. Prior research suggests that image processing involving machine learning yields superior classification between outcomes for control and test samples43,44,45. In this study, employing the fixed location and background subtraction generated a consistent background signal from the cellulose µPADs, enhancing the consistency and classification accuracy of readings provided by the mobile application40,43.
Concerning the consistent performances in demonstrating a paper-based immunoassay, the fabrication method plays an important role. In a previous study36, several optimization conditions for the design and fabrication method of DEN-NS1-PAD, such as concentrations of PLL, blocking substance, and NS1 antibody, were studied. The device successfully tested NS1 in buffer, cell culture, and human serum both qualitatively and quantitatively.
Matrix effects stemming from serum components can interfere with the detection limit of the device when testing serum samples. However, the results indicate that the developed immunoassay could successfully detect NS1 concentrations in serum samples, producing outcomes comparable to those of the commercial RDT (Table 2). The apparent results using visual inspection and the mobile application (Figure 4) were observed, underscoring the assay's effectiveness for serum testing. While the higher viscosity of serum might affect analysis times, it does not hinder result interpretation36. Utilizing a mobile application can provide more positive results for sample assays because using a mobile application significantly improves the sensitivity of sample testing46. It is worth noting that further comparisons with molecular assays such as RT-PCR15,47,48 for dengue NS1 are needed to determine potential false-positive or false-negative results.
A notable limitation of this study arises when considering a more complex sample matrix such as blood. The components present in blood could indeed interfere with the detection limit of the device. In such cases, employing additional absorbent pads to enhance running buffer absorption represents a potential solution. This modification can aid blood coagulation, leveraging cellulose's hemostyptic properties by influencing blood platelets49. Another approach involves applying 4% (w/v) saline drops to the sample pad to enhance blood coagulation. Previous research has demonstrated that salts such as calcium chloride and sodium chloride induce red blood cell (RBC) coagulation50,51. Na+ can destabilize the suspension of RBCs in the blood by suppressing the electric double layer on the RBC surface and reducing the charge repulsion between RBCs. In addition, a high concentration of salt also induces blood aggregation52. Moreover, the counter ion valency charge of the Na+ suppresses the thickness of the charged double layer of RBCs, leading to the aggregation of the deflated RBCs. The addition of 4% (w/v) saline solution (NaCl) facilitates plasma separation on cellulose paper51. However, careful optimization of the saline concentration is necessary to avoid inducing undesirable effects on blood aggregation and gold nanoparticle aggregation53,54.
Commercial wax printers provided an ideal combination of cost and simplicity of prototyping. As these printers were discontinued in 2016, alternative fabrication methods were required such as inkjet printing55, screen printing56, and photolithography57. An office toner printer is a good candidate for the fabrication of µPAD. The polyester resin in the toner creates hydrophobic patterns at 200 °C for 60 min with various designs58.
Antibody NS1 serotype two, as specified by the company, was used in this research. However, we found that this antibody also interacts with all serotypes36,37. The sensitivities for detection of DENV-4 are lower (87.5%) compared with other serotypes. The sensitivity of DENV-1 and DENV-2 is approximately 88.89%, and for DENV-3 is 100%37. These findings align with earlier research, which also reported lower sensitivity of RDT to DENV-4 compared to other serotypes59. The overall sensitivity of the device is ~88.89%, with a specificity of approximately 86.67%. It is noteworthy that the actual sensitivity of DEN-NS1-PAD may surpass that of commercial RDT. However, RDT demonstrates a positive predictive value (PPV) of 84.62% and an accuracy of 87.67%. Notably, the DEN-NS1-PAD performed better in detecting dengue infection in the first 5-6 days, whereas RDT is effective only in the first 5 days37.
In summary, combining a portable paper-based immunoassay (DEN-NS1-PAD) with a smartphone application holds great promise for dengue NS1 measurement. The mobile application significantly enhances sensitivity and efficiency in quantifying NS1 in serum samples compared to observation with the naked eye. The mobile application's benefits include reduced analysis time, user-friendliness, and compatibility with diverse smartphone devices, positions, and lighting conditions. However, further enhancement is needed to improve the sensitivity and performance. Meanwhile, modification of the paper-based immunoassay is necessary to improve its performance when dealing with blood samples. Further, comprehensive evaluations are required of the DEN-NS1-PAD for detecting dengue using a more significant number of primary infection serotypes and selected samples collected from various patients (children and adults).
Disclosures
The authors have nothing to disclose.
Acknowledgements
M.H.P. gratefully acknowledges the scholarship research fund from Universitas Islam Indonesia (UII). The authors extend their gratitude to Mr. Nutchanon Ninyawee for his valuable expertise and assistance throughout the development of the mobile application and his contributions to the manuscript. Furthermore, the authors appreciate the financial support provided by Thailand Science Research and Innovation (TSRI), Basic Research Fund: Fiscal year 2023 (project no. FRB660073/0164) under Program Smart Healthcare of King Mongkut's University of Technology Thonburi.
Materials
| Materials | |||
| 0.1 M phosphate-buffered saline (PBS, pH 7.2) | |||
| BBS containing 0.1% Tween 20, 10% sucrose, and 1% casein | the conjugate area treatment and blocking buffer | ||
| Borate buffered saline (BBS) (25 mM sodium borate and 150 mM sodium chloride at pH 8.2) supplemented with 1% BSA | the washing buffer during the conjugation process AuNPs with the antibody | ||
| Boric acid | Merck | 10043-35-3 | |
| Bovine serum albumin fraction V (BSA) | PAA Lab GmbH (Germany) | K41-001 | |
| Casein | Merck | 9005-46-3 | |
| Chromatography paper Grade 2 | GE Healthcare | 3002-911 | |
| Clear laminate film | 3M (Stationery shops) | ||
| Disodium hydrogen phosphate | Merck | 7558-79-4 | |
| Double tape side | Stationery shops | ||
| Goat anti-mouse IgG antibody | MyBiosource (USA) | MBS435013 | |
| Gold nanoparticles (40 nm) | Serve Science Co., Ltd. (Thailand) | ||
| Human IgG polyclonal antibody | Merck | AG711-M | |
| Mouse dengue NS1 monoclonal antibody | MyBiosource (USA) | MBS834415 | |
| Mouse dengue NS1 monoclonal antibody | MyBiosource (USA) | MBS834236 | |
| NS1 serotype 2 antigens | MyBiosource (USA) | MBS 568697 | |
| PBS 1X containing 0.1% Tween 20 was used as t | elution buffer | ||
| Plastic backing card 10×30 cm | Pacific Biotech Co., Ltd. (Thailand) | ||
| Poly-L-lysine (PLL) | Sigma Aldrich | P4832 | |
| Potassium Chloride | Merck | 104936 | |
| Potassium monophosphate | Merck | 104877 | |
| Sodium Chloride | Merck | 7647-14-5 | |
| Sodium tetraborate | Sigma Aldrich | 1303-96-4 | |
| Sucrose | Merck | 57-50-1 | |
| Tween 20 | Sigma Aldrich | 9005-64-5 | |
| Instruments | |||
| CytationTM 5 multimode reader | BioTek | ||
| Mobile phones | Huawei Y7, iPhone 11, Samsung a20 | ||
| Photo scanner | Epson Perfection V30 | ||
| Oven | Memmert | ||
| Wax printer | Xerox ColorQube 8880-PS | ||
| Software | |||
| Could AutoML Vision Object Detection documentation | Google Cloud | ||
| ImageJ | National Institute of Health, Bethesda, MD, USA | ||
| Inkscape 0.91 Software |
References
- Cattarino, L., Rodriguez-Barraquer, I., Imai, N., Cummings, D. A. T., Ferguson, N. M. Mapping global variation in dengue transmission intensity. Science Translational Medicine. 12 (528), 1-11 (2020).
- World Health Organization (WHO). . Treatment, prevention and control global strategy for dengue prevention and control. , 1-34 (2012).
- . WHO Dengue and severe dengue Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (2020)
- Department of Disease Control Ministry of Health Thailand. . Weekly Disease Forecast Dengue. , (2020).
- Malavige, G. N., Ogg, G. S. Pathogenesis of vascular leak in dengue virus infection. Immunology. 151 (3), 261-269 (2017).
- Paranavitane, S. A., et al. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infectious Diseases. 14 (1), 570 (2014).
- Muller, D. A., Young, P. R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Research. 98 (2), 192-208 (2013).
- Modhiran, N., et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Science Translational Medicine. 7 (304), 304ra102 (2015).
- Glasner, D. R., et al. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLOS Pathogens. 13 (11), e1006673 (2017).
- Lin, C. -. F., et al. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. Journal of Medical Virology. 69 (1), 82-90 (2003).
- Adikari, T. N., et al. Dengue NS1 antigen contributes to disease severity by inducing interleukin (IL)-10 by monocytes. Clinical and Experimental Immunology. 184 (1), 90-100 (2016).
- Malavige, G. N., et al. Suppression of virus specific immune responses by IL-10 in acute dengue infection. PLoS Neglected Tropical Diseases. 7 (9), e2409 (2013).
- Malavige, G. N., et al. Serum IL-10 as a marker of severe dengue infection. BMC Infectious Diseases. 13 (1), 341 (2013).
- Libraty, D. H., et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. The Journal of Infectious Diseases. 186 (8), 1165-1168 (2002).
- World Health Organization (WHO) and the Special Programme for Research and Tropical Diseases (TDR). . Dengue: guidelines for diagnosis, treatment, prevention and control — New edition. , (2009).
- Axelrod, T., Eltzov, E., Marks, R. S. Capture-layer lateral flow immunoassay: a new platform validated in the detection and quantification of dengue NS1. ACS Omega. 5 (18), 10433-10440 (2020).
- Kim, S. -. W., Cho, I. -. H., Lim, G. -. S., Park, G. -. N., Paek, S. -. H. Biochemical-immunological hybrid biosensor based on two-dimensional chromatography for on-site sepsis diagnosis. Biosensors and Bioelectronics. 98, 7-14 (2017).
- Fu, Q., et al. Development of a novel dual-functional lateral-flow sensor for on-site detection of small molecule analytes. Sensors and Actuators B: Chemical. 203, 683-689 (2014).
- Dzantiev, B. B., Byzova, N. A., Urusov, A. E., Zherdev, A. V. Immunochromatographic methods in food analysis. TrAC Trends in Analytical Chemistry. 55, 81-93 (2014).
- Hu, J., et al. Advances in paper-based point-of-care diagnostics. Biosensors and Bioelectronics. 54 (4), 585-597 (2014).
- Zhong, Y., et al. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchimica Acta. 183 (6), 1989-1994 (2016).
- Figueredo, F., Garcia, P. T., Cortón, E., Coltro, W. K. T. Enhanced analytical performance of paper microfluidic devices by using Fe 3 O 4 nanoparticles, MWCNT, and graphene oxide. ACS Applied Materials & Interfaces. 8 (1), 11-15 (2016).
- Bahadır, E. B., Sezgintürk, M. K. Lateral flow assays: Principles, designs and labels. TrAC – Trends in Analytical Chemistry. 82, 286-306 (2016).
- He, M., Liu, Z. Paper-based micro fluidic device with upconversion fluorescence assay. Analytical Chemistry. 85, 11691-11694 (2013).
- Derikvand, F., Yin, D. L. T., Barrett, R., Brumer, H. Cellulose-based biosensors for esterase detection. Analytical Chemistry. 88 (6), 2989-2993 (2016).
- Kumar, S., Bhushan, P., Krishna, V., Bhattacharya, S. Tapered lateral flow immunoassay-based point-of-care diagnostic device for ultrasensitive colorimetric detection of dengue NS1. Biomicrofluidics. 12 (3), 034104 (2018).
- Sinawang, P. D., Rai, V., Ionescu, R. E., Marks, R. S. Electrochemical lateral flow immunosensor for detection and quantification of dengue NS1 protein. Biosensors and Bioelectronics. 77, 400-408 (2016).
- Zhang, D., Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosensors and Bioelectronics. 75, 273-284 (2016).
- Preechaburana, P., Suska, A., Filippini, D. Biosensing with cell phones. Trends in Biotechnology. 32 (7), 351-355 (2014).
- Laksanasopin, T., et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Science Translational Medicine. 7 (273), 273re1 (2015).
- Kim, J., et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sensors. 1 (8), 1011-1019 (2016).
- Mudanyali, O., et al. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab on a Chip. 12 (15), 2678 (2012).
- Yu, L., Shi, Z., Fang, C., Zhang, Y., Liu, Y., Li, C. Disposable lateral flow-through strip for smartphone-camera to quantitatively detect alkaline phosphatase activity in milk. Biosensors and Bioelectronics. 69, 307-315 (2015).
- Hou, Y., et al. Smartphone-based dual-modality imaging system for quantitative detection of color or fluorescent lateral flow immunochromatographic strips. Nanoscale Research Letters. 12 (1), 291 (2017).
- You, D. J., Park, T. S., Yoon, J. -. Y. Cell-phone-based measurement of TSH using Mie scatter optimized lateral flow assays. Biosensors and Bioelectronics. 40 (1), 180-185 (2013).
- Prabowo, M. H., Chatchen, S., Rijiravanich, P. Dengue NS1 detection in pediatric serum using microfluidic paper-based analytical devices. Analytical and Bioanalytical Chemistry. 412, 2915-2925 (2020).
- Prabowo, M. H., et al. Clinical evaluation of a developed paper-based Dengue NS1 rapid diagnostic test for febrile illness patients. International Journal of Infectious Diseases. 107, 271-277 (2021).
- Prabowo, M. H., et al. Preparation and detection method for the diagnostic device of dengue NS1 detection in serum, cell medium, and buffer. Thai Patent. , (2019).
- Kong, T., et al. Accessory-free quantitative smartphone imaging of colorimetric paper-based assays. Lab on a Chip. 19 (11), 1991-1999 (2019).
- Jung, Y., Heo, Y., Lee, J. J., Deering, A., Bae, E. Smartphone-based lateral flow imaging system for detection of food-borne bacteria E. coli O157:H7. Journal of Microbiological Methods. 168, 105800 (2020).
- Chen, G., et al. Improved analytical performance of smartphone-based colorimetric analysis by using a power-free imaging box. Sensors and Actuators B: Chemical. 281, 253-261 (2019).
- Kim, H., et al. Smartphone-based low light detection for bioluminescence application. Scientific Reports. 7 (1), 40203 (2017).
- Kim, H., Awofeso, O., Choi, S., Jung, Y., Bae, E. Colorimetric analysis of saliva-alcohol test strips by smartphone-based instruments using machine-learning algorithms. Applied Optics. 56 (1), 84 (2017).
- Qin, Q., et al. Algorithms for immunochromatographic assay: review and impact on future application. The Analyst. 144 (19), 5659-5676 (2019).
- Yan, W., et al. Machine learning approach to enhance the performance of MNP-labeled lateral flow immunoassay. Nano-Micro Letters. 11 (1), 7 (2019).
- Srisa-Art, M., Boehle, K. E., Geiss, B. J., Henry, C. S. Highly sensitive detection of Salmonella typhimurium using a colorimetric paper-based analytical device coupled with immunomagnetic separation. Analytical Chemistry. 90 (1), 1035-1043 (2018).
- Santiago, G. A., et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nature Communications. 9 (1), 1391 (2018).
- Lanciotti, R. S., Calisher, C. H., Gubler, D. J., Chang, G. J., Vorndam, A. V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of Clinical Microbiology. 30 (3), 545-551 (1992).
- Yang, X., et al. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomaterials Science. 5 (12), 2357-2368 (2017).
- Li, H., Han, D., Pauletti, G. M., Steckl, A. J. Blood coagulation screening using a paper-based microfluidic lateral flow device. Lab Chip. 14 (20), 4035-4041 (2014).
- Nilghaz, A., Shen, W. Low-cost blood plasma separation method using salt functionalized paper. RSC Advances. 5 (66), 53172-53179 (2015).
- Ataullakhanov, F. I., Pohilko, A. V., Sinauridze, E. I., Volkova, R. I. Calcium threshold in human plasma clotting kinetics. Thrombosis Research. 75 (4), 383-394 (1994).
- Pamies, R., et al. Aggregation behaviour of gold nanoparticles in saline aqueous media. Journal of Nanoparticle Research. 16 (4), 2376 (2014).
- Christau, S., Moeller, T., Genzer, J., Koehler, R., Von Klitzing, R. Salt-induced aggregation of negatively charged gold nanoparticles confined in a polymer brush matrix. Macromolecules. 50 (18), 7333-7343 (2017).
- Abe, K., Kotera, K., Suzuki, K., Citterio, D. Inkjet-printed paperfluidic immuno-chemical sensing device. Analytical and Bioanalytical Chemistry. 398 (2), 885-893 (2010).
- Sameenoi, Y., Nongkai, P. N., Nouanthavong, S., Henry, C. S., Nacapricha, D. One-step polymer screen-printing for microfluidic paper-based analytical device (µPAD) fabrication. The Analyst. 139 (24), 6580-6588 (2014).
- Mora, M. F., et al. Patterning and modeling three-dimensional microfluidic devices fabricated on a single sheet of paper. Analytical Chemistry. 91 (13), 8298-8303 (2019).
- Ng, J. S., Hashimoto, M. Fabrication of paper microfluidic devices using a toner laser printer. RSC Advances. 10 (50), 29797-29807 (2020).
- Pal, S., et al. Multicountry prospective clinical evaluation of two enzyme-linked immunosorbent assays and two rapid diagnostic tests for diagnosing dengue fever. Journal of Clinical Microbiology. 53 (4), 1092-1102 (2015).

