Generation, Maintenance, and Identification of Germ-Free Zebrafish Models from Larvae to Juvenile Stages
Summary
This protocol outlines the primary steps for obtaining germ-free (GF) fish embryos and maintaining them from larvae until the juvenile stage, including sampling and detecting their sterile status. The use of GF models with infection is important for understanding the role of microbes in host health.
Abstract
Zebrafish serve as valuable models for research on growth, immunity, and gut microbiota due to their genomic similarities with mammals, transparent embryos developed in a relatively clean chorion environment, and extremely rapid development of larvae compared to rodent models. Germ-free (GF) zebrafish (Danio rerio) are crucial for evaluating pollutant toxicity and establishing human-like disease models related to microbial functions. In comparison to conventionally raised (CR) models (fish in common husbandry), GF zebrafish allow for more accurate manipulation of the host microbiota, aiding in determining the causal relationship between microorganisms and hosts. Consequently, they play a critical role in advancing our understanding of these relationships. However, GF zebrafish models are typically generated and researched during the early life stages (from embryos to larvae) due to limitations in immune function and nutrient absorption. This study optimizes the generation, maintenance, and identification of early GF zebrafish models without feeding and with long-term feeding using GF food (such as Artemia sp., brine shrimp). Throughout the process, daily sampling and culture were performed and identified through multiple detections, including plates and 16S rRNA sequencing. The aseptic rate, survival, and developmental indexes of GF zebrafish were recorded to ensure the quality and quantity of the generated models. Importantly, this study provides details on bacterial isolation and infection techniques for GF fish, enabling the efficient creation of GF fish models from larvae to juvenile stages with GF food support. By applying these procedures in biomedical research, scientists can better understand the relationships between intestinal bacterial functions and host health.
Introduction
The microbiota (i.e., Archaea, Bacteria, Eukarya, and viruses) play crucial roles in maintaining host health and contributing to the development of various diseases by influencing physiological and pathological processes through symbiotic interactions within the intestinal barrier, epithelial surface, and mucin functions in individuals1,2,3. The composition of the microbiota across different life stages, from infancy to juvenility, adulthood, and aging, as well as its presence in various locations such as nares, oral, skin, and gut sites, is dynamically shaped by diverse habitats and environments4. The intestinal microbiota in organisms is involved in nutrient absorption, immune response, pathogen invasion, metabolic regulation, etc5,6. Studies on patients have demonstrated that disruptions in gut microbiota are related to human obesity, sleep disorders, depression, inflammatory bowel disease (IBD), neurodegenerative diseases (Parkinson's, Alzheimer's), aging, and various cancers7,8,9. Furthermore, interactive pathways between gut microbiota and hosts involve inflammatory factors, neurotransmitters, metabolites, intestinal barrier, and oxidative stress, as observed in previous research using mice and fish models10,11.
Recently, multiple bacteria-related approaches or therapies, including potential probiotics and fecal microbiota transplantation (FMT), have been explored for these disorders in clinical and animal models. These explorations are based on discoveries related to the microbiota-gut-brain/liver/kidney axis, microbiota-derived products, and altered receptor activity12,13. However, the development, various functions, and mechanisms of the microbiota-host system are still incompletely understood and identified due to the complexity of the microbial community and the challenge of generating powerful human-like disease models.
To address these issues, germ-free (GF) animal models were urgently proposed in the mid-19th century and primarily developed during the 20th century. Subsequent refinements, including antibiotic-treated and gnotobiotic models, along with advancements in microbial detection and observation technologies, further perfected these models14,15,16. GF animals, created by erasing their own background and avoiding environmental microbes, offer an excellent strategy for exploring the interactions between microorganisms and their hosts17. Through the application of animal models and refined protocols, researchers have successfully replicated similar microbial compositions found in patients in GF mice and fish. Additionally, other GF animal models, such as dogs, chickens, and pigs, provide diverse options as research subjects18,19,20,21. This approach has enabled investigations into the potential therapeutic effects of commensal microbiomes on various diseases, including cancer immunotherapy in humans16,18. GF models offer more accurate insights into the characteristics and mechanisms of specific bacterial colonization, migration, multiplication, and interaction within hosts. This provides crucial novel insights into the occurrence and development of microbiota-related diseases22,23. The history of establishing and applying GF zebrafish in microbial research has evolved from the reports of Rawls et al. in 2004 and Bates et al. in 2006 to Melancon et al.'s protocol in 201716,24,25. However, the feasibility of adult or breeding GF models is still a prolonged process, accompanied by variable longevity, success rates, and health challenges.
Among various animal models, zebrafish (Danio rerio) stands out as a critical tool for both basic and biomedical research due to its advantageous similarity to human organs and genomics, short developmental cycle, high fecundity, and transparent embryos19,26. Zebrafish, serving as reliable human disease models, offer a visual representation of physiological and pathological processes in vivo, providing insights into the attractive features of host-microbe interactions. Notably, zebrafish exhibit distinct cell lineages, allowing imaging of intestinal physiology, microbial dynamics, gonads and reproductive development, maturation of the host immune system, behavior, and metabolism27. Zebrafish embryos develop within protective chorions until hatching, becoming larvae at 3 days post-fertilization (dpf). They actively hunt for food at 5 dpf and reach sexual maturity around 3 months post-fertilization (mpf)28. The first successful germ-free (GF) zebrafish, reported by Rawls et al.24, showed that larvae fed with autoclaved feed after yolk absorption exhibited tissue necrosis from 8 dpf and total death at 20 dpf. This indicated the effects of diet or the importance of considering exogenous nutrient supply in experiments involving long-term (>7 dpf) GF fish29. Subsequent studies improved the generation protocol of GF fish, employing sterile food and methods perfected in different fish models16.
However, most research on GF zebrafish models has focused on early life stages, involving bacterial infection at 5 dpf for 24 h to 48 h, with samples collected before 7 dpf at the conclusion of the experiments25,30,31. It's widely acknowledged that the microbiota in organisms, including humans and zebrafish, is colonized at the beginning of life and shaped during growth and development. The composition remains stable at adult stages, with the roles of microbiota in the host being crucial throughout life, especially in aging, neurodegenerative, metabolic-related obesity, and intestinal disease aspects3. Thus, perspectives from GF animals with longer survival can provide insights into the mechanisms of microbial roles in host organ development and functions, considering the immature immune and reproductive systems of fish larvae in early life. While bacterial strains in zebrafish intestines have been isolated and identified in previous studies, offering the potential for infecting GF animal models to select probiotics or research bacterial functions in the host19,25, the generation and application of GF fish models have primarily been restricted to early life stages. This limitation, attributed to the complex production process, high maintenance costs, and associated issues with food and immunity, hinders research efforts aimed at investigating the developmental and chronic effects of microbiota in the host.
The survival rate, behavior, growth, maturation, and overall health of fish, especially in germ-free (GF) models, are significantly influenced by feeding practices, encompassing nutrition intake and absorption during the mouth-open period from early larvae to juveniles32,33. However, one of the challenges in GF fish husbandry is the scarcity of suitable sterile diets, limiting the effectiveness of nutritional support for sustaining the growth and survival of larvae. Resolving this issue is crucial to restoring the life of GF fish, considering their developmental defense mechanisms and weak digestion abilities due to the absence of an intestinal microbiome. In terms of food, live brine shrimp (Artemia sp.) emerges as the most suitable diet for mouth-open larvae to juvenile fish. It has been observed that fish fed with live brine shrimp exhibit higher growth and survival rates compared to those fed with cooked egg yolk or other natural and synthetic baits34. While early life models of GF fish can survive with yolk support and GF larvae models can be maintained with sterile feeding, generating long-term models from larvae to juveniles and reaching sexual maturity remains challenging. Additionally, flake or powder food is limited by unequal nutritional composition and can impact water quality. In contrast, live Artemia has advantages such as survival in both salt and freshwater, small size suitable for larvae to adults, ease of batching, and higher hatching quality35. Building upon previous methods16,24,30, we have simplified the complex treatment process and addressed the diet challenge by establishing easily incubated GF live Artemia sp. as sterile food for longer durations than early-life GF fish.
This study presents an optimized protocol covering (1) generation, (2) maintenance, (3) identification of sterile rate, and (4) maintenance and feeding to ensure the growth of germ-free (GF) zebrafish from embryos to larvae and juvenile stages. The results offer preliminary evidence on the hatching, survival, growth, and sterility of GF zebrafish, along with essential indices for GF Artemia sp. as sterile food. The detailed steps in model generation and preparation of sterile live foods provide crucial technical support for constructing and applying long-term GF fish models, as well as GF Artemia sp. in microbiota-host interaction research. The protocol addresses bacterial isolation, identification, and infection on GF fish models, outlining methods for bacterial fluorescence labeling and observing their colonization in fish intestines under a microscope. GF fish, gnotobiotic fish with bacterial infection, or transferred human microbiota models will undergo various detections to elucidate their functions and effects on host immunity, digestion, behavior, transcriptomic regulation, and metabolic aspects. In the long term, this protocol can be extended to different wild-type fish species, such as marine medaka, and potentially to other selected transgenic zebrafish lines correlated to specific tissues or diseases.
Protocol
The fish experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Chongqing and the Institutional Animal Care and Use Committee of Chongqing Medical University, China, as well as the standards for experimental animals issued by the State Bureau of Quality and Technical Supervision (Approval ID: GB14922-2001 to GBT14927-2001). Zebrafish (Danio rerio, wild type, AB strain) were sourced from the Institute of Hydrobiology, Chinese Academy of Sciences, and maintained in the laboratory following previously reported procedures36.
1. Adult zebrafish maintenance and embryo collection
NOTE: Drawing upon modifications from previous studies and reports16,37,38,39, the maintenance of adult zebrafish, larvae rearing, and practices for germ-free (GF) models in our laboratory were carried out following the steps outlined below. The ultra-pure flow system for fish culture was commercially obtained (see Table of Materials). The water, maintained with a balanced pH, salt, and alkali, undergoes filtration during cycling to ensure clean conditions.
- Maintain adult fish with the following standard procedure in the auto cycle system (see Table of Materials) with a photoperiod of the dark: light = 10 h: 14 h at 28 °C ± 0.5 °C and fed with fresh incubated Artemia sp. twice daily.
- Put the sexually mature adult zebrafish (male: female = 2:1) into tanks with fresh and clean water the night before.
- Let the fish spawn the next morning (approximately 8:30 a.m.) under regular light stimulation in the laboratory. After 1-2 h, transfer the fish to another tank.
- Collect embryos into water from the auto-cycling system as the rearing medium in a culture dish using a disposable Pasteur pipette.
2. Generation of GF zebrafish from embryos to larvae
NOTE: The procedure for generating germ-free (GF) fish is outlined in Figure 1, while the basic developmental indexes of conventional (CR) and GF models are presented in Supplementary Figure 1. Please follow the steps outlined below in a benchtop biological safety cabinet or laminar flow hood using a sterile aseptic technique in a clean room.
- Wash the embryos using the fish culture water and remove the feces impurities and dead or unfertilized eggs.
- Transfer the embryos at 2 hpf to sterilized plates (up to 480 embryos per sterile dish with a 10 cm diameter) filled with antibiotic germ-free zebrafish medium (AB-GZM, see Table of Materials) solution and culture at 28.5 °C for 6-8 h.
NOTE: AB-GZM is used to treat the embryos for several hours to eliminate the surface microorganisms, and GZM without antibiotics is used to culture GF fish in subsequence. Usually, the perfected culture time is 6 h). - Exclude eggs with white spots or a whitish appearance, and select those that are normally developed, displaying transparent and intact envelopes for further processing.
- Rinse the embryos using AB-GZM three times within 10 min.
- Soak the embryos in 0.2 g/L povidone-iodine solution (PVP-I) for 1 min (see Table of Materials).
NOTE: Higher concentration and longer than 2 min will influence the death and hatching rate of embryos. - Wash the embryos using germ-free zebrafish medium (GZM) three times within 10 min.
- Add embryos into sodium hypochlorite (NaClO) working solution, tightly cover the 50 mL microcentrifuge tubes, and bleach for 15 min. During this period, suspend the embryos in bleach solution by gently shaking them.
- Wash the embryos using GZM three times within 10 min.
- Transfer the embryos to sterile 6-well plates with 5 embryos and 10 mL of GZM per well. Culture them in an ultra-clean platform or incubator with a photoperiod of dark: light = 10 h: 14 h at 28 °C ± 0.5 °C.The culture density will influence the sterile rate.
- Renew the culture medium daily by removing the spent GZM, collecting it as samples for sterile status detection, and replenishing each well with the same volume of sterile GZM.
- Record the basic developmental indexes of GF embryos/larvae, including the survival rate, hatching rate, heartbeats, body length and weight, etc19.
- Transfer the GF fish to suitable containers (dishes, cell culture flasks, or glass bottles with covers) with expanded volume, providing sterile food throughout the growth process.
3. Maintenance of GF zebrafish from larvae to juvenile
- Renew the GZM without feeding before 7 dpf and daily detect the sterile states (see step 5).
- Feed zebrafish larvae from 8 dpf to juvenile stages with sterile egg yolk (10 µL prepared stock solution per well) once daily before changing GZM.
- Feed GF juveniles from 14 dpf with sterilized egg yolk mixed with GF Artemia sp. once daily (1-3 Artemia/larvae, see step 4), gradually increasing food mass with the growth and development of GF fish.
- Provide egg yolk until all fish can consume Artemia nauplii.
NOTE: GF Artemia should undergo sterility testing before use. Assess the number of Artemia nauplii left, observe pursuit and capturing progress, and examine the fish body with consumed food to indicate feeding success. - From 28 dpf to early adult stages, feed GF Artemia once daily (3-5 Artemia/larvae) and clean untapped or dead Artemia, and dead fish into the waste GZM as daily samples.
- During GF fish growth, change the 6-well plates after 7 dpf to sterile plates or cell culture flasks from 8 to 21 dpf, and then to sterile bottles after 21 dpf. Increase culture medium and food mass according to the number and weight of GF fish in each container.
- Renew GZM daily and check samples to ensure the success of GF models.
NOTE: Maintaining germ-free (GF) fish models until early adulthood and sexual maturity is challenging, with a low average survival rate at 30 dpf, typically less than 10%. This is primarily attributed to weak immunity, delayed development, and impaired intestinal function.
4. Preparation of GF Artemia nauplii as the sterile food for chronic GF fish models
NOTE: The cultivation and preparation method of germ-free (GF) Artemia is outlined in Figure 2. Note that all the following steps are conducted in a benchtop biological safety cabinet, using a sterile aseptic technique.
- Rinse 0.25 g of Artemia cysts using 2.4 g/L sodium hypochlorite (NaClO, see Table of Materials) for 5 min.
- Filter the rinsed Artemia cysts with a sterilized ultra-dense filter (about 170 meshes in aperture) and wash with sterilized ultrapure water three times, each time for 1-2 min.
- Transfer the washed Artemia cysts into 100 mL of 2.5% sterilized saline water, mix, and package for aseptic hatching according to the steps mentioned below.
- Pack the 50 mL treated Artemia cysts mixture solution into a sterile bottle with a volume of 100 mL, seal it with film, and incubate in a shaking incubator at 150 rpm, 30 °C for 18 h to 24 h with light.
- Extract approximately 30 mL of hatching Artemia nauplii from the middle and lower layers using a disposable Pasteur pipette.
NOTE: Prevent floating eggs and empty shells from reaching the upper layer. The indexes of germ-free (GF) Artemia cysts and nauplii incubated for 24 h are presented in Figure 3. - Filter the Artemia with a sterilizing filter and wash them in a sterilizing beaker with sterilizing ultrapure water three times, about 30 s to 1 min for each time. Finally, add 4 mL of sterilized ultrapure water to prepare the Artemia mixed solution.
- Using a disposable Pasteur pipette, retrieve actively swimming Artemia from the upper layer, wash, and prepare the nauplii solution for feeding and aseptic identification.
5. Identification of GF fish models at the whole life stages
NOTE: Throughout the entire life stages of germ-free (GF) fish, key indexes and daily sample detections are crucial to ensure the success of the models. This step summarizes the common classification of samples and the usual methods for identification (Figure 4).
- Observe and count the survival status and rate of GF zebrafish larvae daily. In experiments calculating related rates, GF fish are cultured in 24- or 48-well plates with one fish per well.
- Survival rate = (number of live fish at the time of observation / total number of eggs) × 100%.
- Sterility rate = (number of sterile fish/number of surviving fish) × 100%.
NOTE: This protocol focuses on the sterile rate of live fish. The number of sterile fish is determined by counting successful GF fish after multiple checks among the live fish. Any dead fish or live fish GF models that have failed due to bacterial presence are eliminated during subsequent GF procedures.
- Collect the following samples of GF zebrafish larvae.
- Culture medium of GF zebrafish from each well or bottle container.
- Fish food, such as sterile egg yolk and GF Artemia.
- Waste medium, including fragments of egg membrane after hatching and food residue or feces during the feeding periods from larvae to juvenile fish.
- Dead fish samples to identify the presence of bacteria.
- Fish medium and agents used in embryo treatment as the sterile control.
- Samples from materials, operating platform, and incubator, etc.
- Detect samples collected daily using multiple methods.
- First, use the spread plate method and culture plates in an incubator at 30 °C.
- Coat with 20-50 µL of waste medium sample on the Trypticase Soy Agar (TSA) plate (see Table of Materials) under aerobic conditions.
- Coat with 20-50 µL of waste medium sample on the TSA plate under anaerobic conditions.
- Coat with 20-50 µL of waste medium sample on the blood TSA plates (see Table of Materials) under aerobic conditions.
- Coat with 20-50 µL of waste medium sample on the blood TSA plates under anaerobic conditions.
- Second, use the liquid culture method.
- Take 200 µL of sample into aerobic tubes with Brain Heart Infusion Broth (BHI) medium (see Table of Materials) and culture in the shaking incubator at 30 °C, 150-180 rpm.
- Add 200 µL of sample into anaerobic tubes with BHI medium and culture in the incubator at 30 °C.
- Add 200 µL of sample into aerobic tubes with Trypticase Soy Broth (TSB) medium, then culture in the shaking incubator at 30 °C, 150-180 rpm.
- Add 200 µL of sample into anaerobic tubes with TSB medium and culture in the incubator at 30 °C.
- Third, use molecular techniques-16s polymerase chain reaction (PCR) assay.
- Analyze the wastewater sample with two pairs of primers 27F and 1492R, as well as 63F and 1387R (Table 1).
NOTE: The PCR master mix was prepared according to Table 2 using a commercially available DNA polymerase kit (see Table of Materials), and the PCR machine was set up with the program specified in Table 3. - After the PCR program is completed, examine the products by 1% agarose gel electrophoresis40 at target bands of 1465 bp (using primers 27F and 1492R) or 1324 bp (using primers 63F and 1387R) to infer the sterility of samples.
- Analyze the wastewater sample with two pairs of primers 27F and 1492R, as well as 63F and 1387R (Table 1).
- First, use the spread plate method and culture plates in an incubator at 30 °C.
- Record and observe sterility tests, including plate and medium culture from 24 h to 3 days and 7 days, during which no colony on plates and no turbidity in liquid indicate the successful construction of GF models. Observe and record the plate and medium at 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, and 168 h.
6. Identification of GF Artemia samples before feeding on GF fish
NOTE: To detect the GF Artemia samples, which are indispensable as live food before feeding on GF fish (Figure 4), follow the steps below.
- Collect samples of GF Artemia models.
- Untreated Artemia cysts.
- Treated GF Artemia cysts.
- Hatched GF Artemia nauplii.
- Sterilized ultrapure water as a control.
- Detect GF Artemia with the following methods.
- Use the spread plate method to detect the samples by coating them on TSA plates and blood TSA plates under aerobic and anaerobic conditions. Incubate them at 30 °C.
- Use a liquid medium to detect the samples in aerobic and anaerobic TSB and BHI tubes. Culture in a shaker at 150-180 rpm, 30 °C.
- Use PCR assay to detect the presence of bacteria in the samples collected using 16s ribosomal RNA target genes primers 27F and 1492R, 63F and 1387R (Table 1). The volume and program are shown in Table 2 and Table 3.
- Record the results: Detect the PCR product by 1% agarose gel electrophoresis40 for target bands measuring 1465 bp (using primers 27F and 1492R) or 1324 bp (using primers 63F and 1387R). The results of plates and liquid medium can ensure the sterile status of GF Artemia before being used as GF fish food.
7. Isolation and identification of intestinal bacterial strains from zebrafish
NOTE: To explore intestinal functions in vitro and in vivo, it is necessary to isolate bacterial strains from zebrafish, which also provide the species source for potential probiotics screened by infection using GF animals (Figure 5). In this protocol, we describe the traditional dissection method to obtain intestinal contents, while the samples can also be collected directly from live anesthetized fish (data not shown).
- Choose healthy adult zebrafish and wash them with sterile water. Perform the following steps in the clean bench.
- Anesthetize the fish with 100 mg/L MS-222 for 5-10 min.
- Use 75% alcohol to treat the fish's body surface.
- Dissect the fish intestines and extrude the intestinal contents with TSB or BHI medium.
- Incubate the intestinal supernatant by applying TSA, blood plate, TSB, and BHI medium in aerobic and anaerobic conditions.
- Translate the bacteria to gain the signal strain and record the characteristics of different colonies.
- Store the bacteria within 30% glycerol at -80 °C.
- Then, identify the intestinal bacteria by genomic DNA extraction and PCR sequencing.
NOTE: The main steps of bacterial genomic DNA extraction include liquid centrifugation, RNase A and Protease K dissociation, ethyl alcohol extraction, rinsing, and dissolution using commercially available kits following the manufacturer's instructions (see Table of Materials). - Analyze the 16S sequences using the National Center for Biotechnology Information (NCBI) and the Basic Local Alignment Search Tool (BLAST) with the Bacteria and Archaea database40,41(see Table of Materials).
- Pick the best-matched bacteria and characters to identify the isolated intestinal strains at the genus level.
- Detect the metabolic functions of bacterial strains using commercially available kits (see Table of Materials).
8. Flu-label, infection, and colonization of bacteria in GF zebrafish intestines
NOTE: Before infecting GF zebrafish, the isolated bacteria were modified with dyes and counted, making microbial colonization and migration visible continuously and in vivo (Figure 6).
- Choose potential beneficial bacteria or dominant strains with high abundance in the host to infect the GF fish models.
NOTE: The bacteria used in this study were Aeromonas sp. and Vibrio sp. (see Table of Materials), selected from the 8 genera that were isolated and identified from adult wild-type zebrafish in the laboratory42. - Label the fresh bacteria with CM-Dil dyes (see Table of Materials) and expose them to GF zebrafish at 5 dpf with a concentration of 106-108 Colony-Forming Units (CFUs)/mL.
- Observe the fluorescence under the fluorescent microscope with 3× magnification to indicate the colonization and distribution of bacteria in the gut of GF zebrafish larvae from 6 dpf and 14 dpf.
- Manually count the number of bacteria in GF fish by plate culture at each time point.
- Detect the colonies and sequences to ensure the infection was successful with the target bacterial strains.
- Collect samples of GF fish with bacterial infection for subsequent assays, including neuro-behavior, developmental indexes, histopathological analysis, and hormone measurements, etc43,44,45.
NOTE: The bacteria used in the infection experiment should be newly recovered and freshly cultured by the liquid medium.
Representative Results
The GF zebrafish models can be efficiently produced by utilizing the abundant eggs spawned by pairs of zebrafish, with the protocol optimized based on previous GF fish models35. A single 6-well plate can culture approximately 30-48 embryos/larvae, allowing for ample data collection and statistical analysis. After sterile treatment, the GF embryos are cultured in a clean incubator till hatching to larvae at 48-72 h, and changed GZM daily with the detection of collected samples, which is crucial to maintaining the germ-free state (Figure 1). After 7 dpf, the food of egg yolk and live Artemia should be prepared for larvae to juvenile stages. During the growth and development of fish, the volume, container, and density of GZM should be regulated or changed in time to avoid death and failure of sterile status. If the detection shows no bacterial pollution, the successful GF models can be maintained as chronic ones from juvenile to early adulthood, but the survival rate is much lower in practice. Finally, the sterile rate, survival rate, development index, behavior, histopathological analysis, and transcription profile of GF and CR fish models can be measured to explore the influence of microbiota and drug screen fields. In this study, the key developmental indexes were compared (Supplementary Figure 1), and the hatching rate of GF fish was 45.7% lower than CR fish, with 60% at 72 hpf. The heart rates of GF larvae at 7 dpf were significantly lower at 18.2 beats/10 s compared to CR fish with 22.6 beats/10 s, but there was no difference between the two groups at 14 dpf with rates of 23.5-23.8 beats/10 s. The survival rate of GF fish at 7 dpf was the same as for CR fish at 86.7%, but decreased to 60% at 14 dpf compared to 80% for CR fish. However, the survival rates of both GF and CR fish decreased to 25%-10% after the mouth-open period when they acquired feeding abilities or habits. The failure to maintain the sterile status of fish was one of the reasons for the lower survival rate. Therefore, feeding and nutrient maintenance from juvenile to early adulthood stages are critical challenges in fish culture.
Another key factor that affects the growth and development of fish is feeding. In this study, we explore multiple methods to obtain GF Artemia as live food for long-term GF zebrafish larvae to juveniles and adults (process shown in Figure 2). Due to the daily use of food for GF fish, the optimized protocol for GF Artemia should be easy, time-saving, and economical in practice. After comparing the active movement and death/immobility of nauplii observed by the microscope, characteristics of nauplii, unhatched eggs, and shell, the method is perfected. After daily incubation, the indexes of fresh GF Artemia obtained from bottles, including the hatching, survival and deformity rate, and the length and weight of nauplii, were observed and measured under the microscope (Figure 3). After treatment, the hatching rate of Artemia cysts was high, with an average of 88.15%, the survival of swimming nauplii was 77.83%, and the malformation rate was 1.87%. The body length of nauplii was 546.70 µm, and the body weight index was 3.80 mg/100, which are suitable for fish catching and feeding from the larvae month-open period to juvenile and adult stages.
The detection of GF fish and GF Artemia is also important for model maintenance. According to sample collection and multiple test methods, the results can provide the success of treated zebrafish embryos and Artemia cysts in the cultured plate and the liquid medium (Figure 4). The various samples collected daily should be detected as sterile in all test methods, which can then indicate the GF models at the time of collecting. The GF Artemia should be detected before feeding on GF fish, or the plate and medium incubator results should refer to the sterile state of fresh live nauplii. That is, cultivate GF Artemia from cysts on TSA plates or TSB or BHI liquid medium glass in shake incubation, which could be used for generating GF Artemia and sterile verification at the same time. The GF Artemia protocol can accommodate a limited number of larvae for feeding. During the feeding process, it was evident that the GF Artemia swam within the GZM and then was captured and consumed by the GF fish larvae.
The applications of GF fish models involve various fields in biology and medicine, allowing the investigation of microbial functions, growth and development, disease mechanisms, and probiotic or drug screening, among others46. In this study, for example, two major intestinal bacteria, Aeromonas sp. and Vibrio sp., were isolated from zebrafish. The colony characteristics and identification were performed, and multiple metabolic functions were measured in vitro using commercially available kits (Figure 5). These bacteria were isolated and identified with 16S sequencing, and the blast results were presented in a previous report42. The identified bacterial strains enable scientific research on the impact of single or combined microbiota on host health by infecting the GF fish models. The transparency of fish larvae allows the observation of fluorescence-labeled bacterial cells using CM-Dil dyes, revealing colonization and distribution in the intestines of GF fish (shown in Figure 6). After bacterial infection at 5 dpf, GF zebrafish larvae can be imaged with a fluorescence microscope and continuously observed from 6 to 14 dpf, providing insights into microbial functions combined with developmental indexes, histopathological changes, and molecular alterations47.
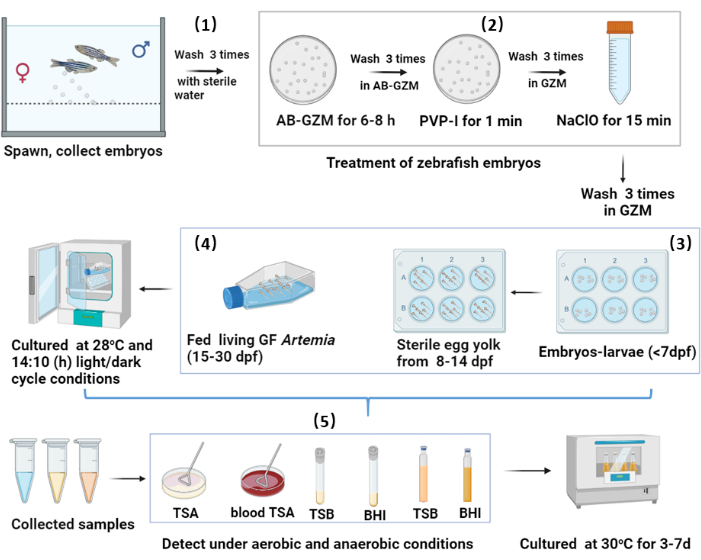
Figure 1: Protocol of GF zebrafish from embryos to larvae and juveniles. Key steps in the generation of GF zebrafish include: (1) Collection of embryos from wild-type zebrafish and placement in AB-GZM. (2) Rinse treatment with PVP-I and NaClO agents to ensure complete sterility. (3) Culture of GF embryos/larvae in 6-well plates with GZM, renewing the medium daily. (4) Regulation of culture conditions for optimal growth and development. (5) Sampling at various developmental stages, followed by sterility testing using plate and liquid medium methods. Please click here to view a larger version of this figure.

Figure 2: Protocol of GF Artemia preparation as live food for GF fish. Key steps include: (1) Rinse treatment of Artemia cysts for complete sterility. (2) Preparation of GF cysts in the medium. (3) Incubation in a salt solution for 24 h to promote hatching. (4) Filtration of failed hatching and empty eggs to collect active nauplii. (5) Washing collected nauplii to remove debris, ensuring high-quality and contaminant-free live food. (6) Sterility testing of the GF Artemia before feeding to GF fish larvae. Please click here to view a larger version of this figure.
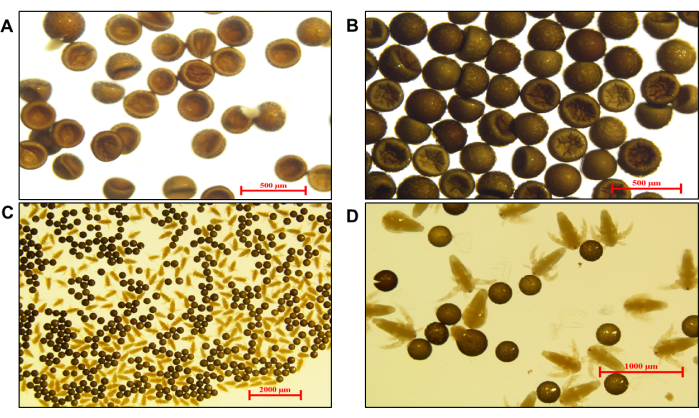
Figure 3: Indexes of GF Artemia cysts and nauplii incubated for 24 h. (A) Untreated Artemia cysts with impurity film (scale bar: 500 µm). (B) Treated Artemia cysts with a slightly convex surface after water absorption and a cleaner appearance (scale bar: 500 µm). (C) Microscopic examination of fresh incubated live GF Artemia nauplii (scale bar: 2000 µm) and (D) magnification with a scale bar of 1000 µm. Please click here to view a larger version of this figure.

Figure 4: Sterile detection of GF fish at each life stage and GF Artemia. (A) Sterile tests of CR and GF fish samples using TSB and BHI liquid medium, TSA, and blood plates under aerobic and anaerobic conditions. (B) Sterile tests of untreated Artemia cysts, GF Artemia cysts, and nauplii using TSB and BHI liquid medium, TSA, and blood plates under aerobic and anaerobic conditions. Please click here to view a larger version of this figure.
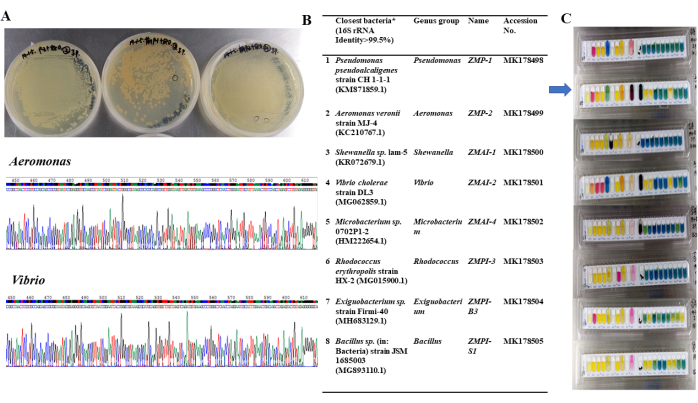
Figure 5: Isolation and identification of intestinal bacteria in zebrafish. (A) Bacterial strains, characteristics of colonies, 16S sequencing maps (examples: Aeromonas sp. and Vibrio sp.). (B) Genus and NCBI accessions. (C) Metabolic functions of bacteria isolated from zebrafish gut. Please click here to view a larger version of this figure.
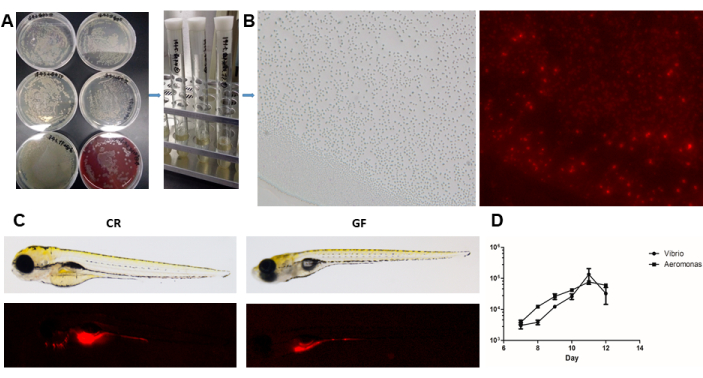
Figure 6: Infection and colonization of gut bacteria strains in GF zebrafish. (A) Recovery of isolated and identified bacterial strains for infection experiments. (B) Labeling of bacteria with fluorescent dyes and infection of GF zebrafish larvae at 5 dpf. Magnification: 1x. (C) Observation of the distribution and colonization of bacteria (Vibrio sp.) in gut tissues in vivo. Magnification: 3x. (D) Counting of CFUs of bacteria (Aeromonas sp. and Vibrio sp.) in each fish larva by daily sampling. Data presents mean ± SEM. Please click here to view a larger version of this figure.
| Primers | Sequences(5’-3’) | Product length |
| 27F | AGAGTTTGATCMTGGCTCAG | 1465 bp |
| 1492R | ACGGYTACCTTGTTACGACTT | |
| 63F | CAGGCCTAACACATGCAAGTC | 1324 bp |
| 1387R | GGGCGGWGTGTACAAGGC |
Table 1: Primers used for bacterial detection.
| Components | Units | Volume (µL) |
| Taq DNA polymerase | 2.5 U/µL | 0.1 |
| 10× Buffer(Mg2+) | — | 2.1 |
| dNTP Mixture | 2.5 mM | 1.6 |
| Primer 27F | 10 µM | 1 |
| Primer 1492R | 10 µM | 1 |
| DNA/RNA free H2O | — | 13.2 |
| Template DNA | — | 1 |
| Total volume | — | 20 |
Table 2: Final concentration and volume of components per reaction.
| Step | Temperature | Time | Cycles |
| Initial denaturation | 95 °C | 4 min | — |
| Denaturation | 94 °C | 1 min | 35 |
| Annealing | 55 °C | 1 min | |
| Extension | 72 °C | 1 min | |
| Final extension | 72 °C | 10 min | — |
| Hold | 4 °C | ∞ | — |
Table 3: PCR program for amplification of the 16S rRNA gene.
Supplementary Figure 1: The critical developmental indexes of GF and CR zebrafish models. (A) The hatching rate of CR and GF zebrafish at 72 hpf, and (B) heart beats/10 s of CR and GF fish at 7 dpf and 14 dpf. The survival rates (%) of CR and GF fish decreased from 7 days and 14 days with 86%, 60%-80% to 10%-25% at 30 dpf. Please click here to download this File.
Discussion
Critical steps within the protocols of GF fish and GF food preparation
During the generation of GF fish models, several critical steps were involved, including the preparation of sterile materials, sterilization of embryos, daily renewal of GZM, collection of various samples, and the sterile examination of each sample using multiple methods. Among these steps, the initial treatment of embryos is fundamental and decisive for the success of GF models. Controlling agents, their concentrations, and treatment times are crucial for achieving optimal sterilization rates and hatching success. Careful attention is necessary when handling GF fish embryos to larvae, involving daily medium changes and ensuring that materials and platforms are thoroughly sterilized using ultraviolet light or autoclaving when removing plates from the incubator. Monitoring temperature conditions and operating time during winter is essential to prevent larval death caused by lower temperatures in clean benches.
Sterile egg yolk and GF Artemia should undergo detection before being fed to fish after 7 dpf to ensure their sterile state for long-term GF models. During the feeding period, cleaning food residues is important as part of the samples collected for detection when renewing the waste medium. The container, volume of GZM, and food mass should be adjusted along with the growth and development stages to prevent GF fish loss due to limited surroundings and water quality issues. Regular monitoring and adjustments in these parameters contribute to the successful maintenance of GF fish models.
Modifications and significance of these protocols
The simplified three-step process for generating GF zebrafish embryos, involving optimized agents, concentrations, and exposure times of AB-GZM, PVP-I, and NaClO solutions, ensures a high sterile rate and healthy development of models. Additionally, the sterile detection methods for daily samples have been perfected to include plates and liquid medium cultures under aerobic and anaerobic conditions, along with molecular techniques using common bacterial primers. Further experiences in testing for bacterial and fungal contaminants aim to optimize isolator contaminants and sterile testing during GF animal techniques48.
The specific protocol for GF Artemia nauplii as live food for fish models has been investigated in our study, and a patent was granted to PPJ and DSP by the China National Intellectual Property Administration (No. ZL202210482684.8). Long-term GF fish models are consistently constrained by inadequate and unsuitable nutrition, exacerbating weaknesses in gut functions related to digestion and absorption. As a credible food source for medaka and zebrafish models at different life stages, GF Artemia sp. should be easy to prepare with a simple procedure, short incubation time, and real-time sterile detection for daily use. In this method, normal Artemia cysts are rinsed in a sufficient concentration of NaClO to be aseptic and then incubated in a film-coated bottle, as well as on a plate and in a liquid medium for detection. This supports freshly hatching nauplii for GF fish feeding. The sterile rate and hatching rate of Artemia, as well as the body length, weight, and activity of, are excellent, indicating available applications in practice.
The significance of these modifications in GF fish and GF food protocols benefits researchers in generating models at embryos, larvae, juvenile stages, and even early adult and adult stages in marine and freshwater modeµspecies. Long-term GF animals will provide permission as in vivo models in research on drug screening and microbial functions during host growth and development, especially in the maturity of immunity and related intestinal cell lines. For example, zebrafish larvae only possess innate immunity in the first week, then develop the adaptive immunity system during 7-21 dpf, along with thymus maturity31,49. Moreover, GF fish models can provide the possibility to analyze bacteria-bacteria and microbiota-host interactions with live observation of dynamic colonization and migration processes in organisms50,51.
Limitations of the method
Culturing GF fish from juveniles to early adults (28 dpf-2.5 mpf) and sexually mature adults (>2.5 mpf) remains challenging due to increased mortality and the risk of bacterial contamination during model maintenance. These limitations primarily arise from the absence of suitable autoculture systems for fish living in a GF environment. These systems should include clean air, water, and a food system to effectively maintain the animals and their environments, ensuring complete isolation from external microorganisms. Unlike other GF animal models such as mice and chickens52, obtaining GF zebrafish through anatomical operations from the CR parents is challenging due to their aquatic habits and natural fertilization style.
Treating adult animals as completely devoid of intestinal microbiota is also challenging. While antibiotic treatment may induce a state similar to specific pathogen-free (SPF) animals, characterized by a reduced microbiota detected in fecal samples, achieving true GF adult fish models remains difficult. The challenges involved in establishing and maintaining GF models, especially during the transition from juvenile to long-term states, are significant. The importance of GF offspring, such as GF F1 or F2 eggs from adult GF fish, in microbial research is acknowledged. However, overcoming challenges in establishing and maintaining long-term adult GF fish models requires substantial time.
It's worth noting that gnotobiotic zebrafish with specific bacterial colonization has been developed, showing chronic survival based on GF models. Nevertheless, it remains uncertain whether these models can serve as viable alternatives to GF models in bacterial infection and biomedical research.
Future applications of GF fish models
In this study, we have refined the protocol for generating GF zebrafish models, progressing from embryos to juveniles. This advancement holds significant applications in developmental biology, immunology, human disease research, and organogenesis. Through mass isolation and identification, the study explores the functions, mechanisms, and gut-derived metabolites of single bacterial strains. The investigation involves infecting GF zebrafish models after the vent opens stage47,53. Additionally, labeled bacteria with fluorescence facilitate the observation of colonization and distribution in live GF larvae, free from other microbial influences54. Similar GF fish models, such as marine or freshwater medaka, can be created using comparable methods with various agents or medium changes.
To probe human disease mechanisms, the study employs FMT methods, transferring samples from patients or donors to healthy animals or GF models55. Unlike conventionally reared (CR) animals, GF models offer robust evidence of microbial functions, impacts, or responses in the host, particularly when combined with bacteriophages to regulate target bacteria in the microbiota46.
In summary, GF zebrafish serve as powerful models for assessing bacterial safety, drug effectiveness, pollutant toxicity, or compound interference, owing to their sensitive and pure intestinal environment. Future exploration of GF fish applications should encompass various fields, with a need to create adult GF fish models for a deeper understanding of microbial functions across different life stages.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We sincerely thank the support from Chongqing Medical University Talent Project (No. R4014 to DSP and R4020 to PPJ), National Natural Science Foundation of China (NSFC, No.32200386 to PPJ), Chongqing Postdoctoral Innovation Mentor Studio (X7928 DSP), and Program of China-Sri Lanka Joint Center for Water Technology Research and Demonstration by Chinese Academy of Sciences (CAS)/China-Sri Lanka Joint Center for Education and Research by CAS.
Materials
| AB-GZM | Amphotericin:Solarbio; kanamycin:Solarbio; Ampicillin:Solarbio. | Amphotericin:CAS:1397-89-3; kanamycin:CAS: 25380-94-0; Ampicillin:CAS: 69-52-313. |
49.6 mL GZM, 50 µL amphotericin stock solution (250 µg/mL), 25 µL kanamycin stock solution (10 mg/mL), and 250 µL ampicillin stock solution (20 mg/mL). |
| 1.5 mL, 15 mL, 50 mL EP tubes | biosharp | BS-15-M | To collect samples, and hold agents |
| 2.4 g/L NaClO | XILONG SCIENTIFIC Co., Ltd. | CAS: 7681-52-9 | Diluted with 8% sodium hypochlorite aqueous solution. |
| 6-well plates, 24-, 48- well plates | LABSELECT | 11112 | To culture fish |
| Aeronomas | NCBI database | No.MK178499 | 2019-JPP-ESN |
| Anaerobic TSA plates | tryptone:Oxoid ; soy peptone:Solarbio ;NaCl:Biosharp; agar powder:BioFroxx. |
tryptone:LP0042B; soy peptone:Cat#S9500; NaCl:BS112; agar powder:9002-18-0. |
The TSA plates were prepared with 400 mL medium containing 6 g tryptone, 2 g soy peptone, 2 g NaCl, and 6 g agar powder under the anaerobic system. |
| Anaerobic work station | GENE SCIENCE | E200G | Bacterial isolation, sterile testing |
| Analysis | GraphPad Prism 5 | v6.07 | To analysis the data |
| API 20 E kits | BioMerieux SA, France | No.1005915090 | Ref 20100 Kits to detect bacterial metabolism |
| Artemia (Brine shrimp) | Shangjia Aquarium Co., Ltd. | Aquamaster brand | Artemia cysts, and brine shrimp eggs |
| Auto cycle system for fish culture | Ningbo Hairui Technology Co., Ltd | No Cat | Maintain the fish |
| Autoclave | Zeal Way | G154DWS | Prepare the materials |
| BHI Aerobic | Coolaber | Cat#PM0640 | BHI medium was prepared, wherein 100 mL medium included 3.7 g BHI powder. |
| BHI Anaerobic | Coolaber | Cat#PM0640 | BHI medium was prepared and divided into anaerobic tubes under the anaerobic system. |
| Biochemical incubator | LongYue Co., Ltd | SPX | For fish and plates |
| Biosafety cabinet | Haier | HR40-IIA2 | Sterile treatment and testing |
| Bleaching agent of 0.02 g/L NaClO | XILONG SCIENTIFIC Co., Ltd. | CAS: 7681-52-9 | Working solution with sodium hypochlorite (NaClO) concentration: Diluted with 8% sodium hypochlorite aqueous solution or 166.6 uL 6% sodium hypochlorite with 500 mL distilled water. |
| Blood plates | sheep blood:Solarbio | Cat. NO. TX0030 | Sterile-defibrinated sheep blood was added into TSA to prepare 5% blood plates. |
| Cell culture flask | Corning | 430639 | To culture fish |
| CM-Dil dyes | Molecular Probes | Cat#C7000 | To label the bacteria |
| Constant temperature shaking incubator | Peiving Co., Ltd | HZQ-X100 | Bacterial culture |
| Database | NCBI | Bacteria and Archaea database | Link: Archaea FTP: ftp://ftp.ncbi.nlm.nih.gov/refseq/TargetedLoci/Archaea/ Bacteria FTP: ftp://ftp.ncbi.nlm.nih.gov/refseq/TargetedLoci/Bacteria/ |
| Disposable Pasteur pipette | biosharp | bs-xh-03l | Used to change water, and transfer eggs |
| Disposable petri dish | biosharp | BS-90-D | To culture fish |
| DNA kits | Solaribio | Cat#D1600 | Bacterial genomic DNA extraction kits |
| Electric pipette | SCILOGEX | Levo me | Change water |
| Exiguobacterium | NCBI database | No.MK178504 | 2019-JPP-ESN |
| GZM | Sea salt:LANDEBAO Co., Ltd. | No Cat | Composed of 1 L of water and 1.5 mL of sea salt solution (40 g/L), autoclaved. The content of sea salt in the GZM solution was 60 mg/L. |
| Laboratory pure water system | Hitech Co., Ltd | Prima-S15 | Prepare the agents |
| Microscope | Nikon | SMZ18 | With fluorescent light to observe fish larvae |
| PCR kits | TIANGEN | Cat#ET101 | Taq DNA Polymerase kit |
| Pipette | LABSELECT | sp-013-10 | Change water |
| Povidone iodine (PVP-I) | Aladdin | Lot#H1217005 | Aqueous solution povidone iodine 0.4 g/L pure water. |
| Timing converter | PinYi Co., Ltd | AL-06 | To regulate the light |
| TSA plates | tryptone:Oxoid ; soy peptone:Solarbio ;NaCl:Biosharp; agar powder:BioFroxx. |
tryptone:LP0042B; soy peptone:Cat#S9500; NaCl:BS112; agar powder:9002-18-0. |
TSA plates were prepared with 400 mL medium containing 6 g tryptone, 2 g soy peptone, 2 g NaCl, 6 g agar powder. |
| TSB Aerobic | tryptone:Oxoid ; soy peptone:Solarbio ;NaCl:Biosharp; |
tryptone:LP0042B; soy peptone:Cat#S9500; NaCl:BS112; |
TSB medium was prepared, wherein 400 mL medium included 6 g tryptone, 2 g soy peptone, and 2 g NaCl. |
| TSB Anaerobic | tryptone:Oxoid ; soy peptone:Solarbio ;NaCl:Biosharp; |
tryptone:LP0042B; soy peptone:Cat#S9500; NaCl:BS112; |
TSB medium was prepared and divided into the anaerobic tubes under the anaerobic system. |
| Ultra-clean workbench | Airtech | SW-CJ-2FD | Sterile treatment and testing |
| Ultra-pure flow system for fish culture | Marine Biological Equipment company | No Cat | Produce water for fish |
| Vibrio | NCBI database | No.MK178501 | 2019-JPP-ESN |
References
- Sieber, M., Traulsen, A., Schulenburg, H., Douglas, A. E. On the evolutionary origins of host-microbe associations. Proc Natl Acad Sci U S A. 118 (9), e2016487118 (2021).
- Sommer, F., Backhed, F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 11 (4), 227-238 (2013).
- Kim, S., Jazwinski, S. M. The gut microbiota and healthy aging: A mini-review. Gerontology. 64 (6), 513-520 (2018).
- Milani, C., et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 81 (4), e00036 (2017).
- De Vos, W. M., Tilg, H., Van Hul, M., Cani, P. D. Gut microbiome and health: Mechanistic insights. Gut. 71 (5), 1020-1032 (2022).
- Shi, N., Li, N., Duan, X., Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 4 (1), 14 (2017).
- Liu, B. N., Liu, X. T., Liang, Z. H., Wang, J. H. Gut microbiota in obesity. World J Gastroenterol. 27 (25), 3837-3850 (2021).
- Aron-Wisnewsky, J., Warmbrunn, M. V., Nieuwdorp, M., Clément, K. Metabolism and metabolic disorders and the microbiome: The intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology. 160 (2), 573-599 (2021).
- Chen, Y. W., Zhou, J. H., Wang, L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. 11, 625913 (2021).
- Hao, W. Z., Li, X. J., Zhang, P. W., Chen, J. X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 284, 112691 (2020).
- Nadal, A. L., et al. gut immunity: Using the zebrafish model to understand fish health. Front Immunol. 11, 114 (2020).
- Asadi, A., et al. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. 36 (5), e24420 (2022).
- Mlynarska, E., et al. The role of the microbiome-brain-gut axis in the pathogenesis of depressive disorder. Nutrients. 14 (9), 1921 (2022).
- Yu, Y. J., Raka, F., Adeli, K. The role of the gut microbiota in lipid and lipoprotein metabolism. J Clin Med. 8 (12), 2227 (2019).
- Al-Asmakh, M., Zadjali, F. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol. 25 (10), 1583-1588 (2015).
- Melancon, E., et al. Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods Cell Biol. 138, 61-100 (2017).
- Bhattarai, Y., Kashyap, P. C. Germ-free mice model for studying host-microbial interactions. Methods Mol Biol. 1438, 123-135 (2016).
- Wang, X. N., Wu, C. W., Wei, H. Humanized germ-free mice for investigating the intervention effect of commensal microbiome on cancer immunotherapy. Antioxid Redox Signal. 37 (16), 1291-1302 (2022).
- Jia, P. P., et al. Role of germ-free animal models in understanding interactions of gut microbiota to host and environmental health: A special reference to zebrafish. Environ Pollut. 279, 116925 (2021).
- Gootenberg, D. B., Turnbaugh, P. J. Companion animals symposium: Humanized animal models of the microbiome. J Anim Sci. 89 (5), 1531-1537 (2011).
- Hintze, K. J., et al. Broad scope method for creating humanized animal models for animal health and disease research through antibiotic treatment and human fecal transfer. Gut Microbes. 5 (2), 183-191 (2014).
- Kamareddine, L., Najjar, H., Sohail, M. U., Abdulkader, H., Al-Asmakh, M. The microbiota and gut-related disorders: Insights from animal models. Cells. 9 (11), 2401 (2020).
- Rogala, A. R., Oka, A., Sartor, R. B. Strategies to dissect host-microbial immune interactions that determine mucosal homeostasis vs. Intestinal inflammation in gnotobiotic mice. Front Immunol. 11, 214 (2020).
- Rawls, J. F., Samuel, B. S., Gordon, J. I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A. 101 (13), 4596-4601 (2004).
- Bates, J. M., et al. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 297 (2), 374-386 (2006).
- Choi, T. Y., Choi, T. I., Lee, Y. R., Choe, S. K., Kim, C. H. Zebrafish as an animal model for biomedical research. Exp Mol Med. 53 (3), 310-317 (2021).
- Xia, H., et al. Zebrafish: An efficient vertebrate model for understanding role of gut microbiota. Mol Med. 28 (1), 161 (2022).
- Parichy, D. M., Elizondo, M. R., Mills, M. G., Gordon, T. N., Engeszer, R. E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev Dyn. 238 (12), 2975-3015 (2009).
- Pham, L. N., Kanther, M., Semova, I., Rawls, J. F. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc. 3 (12), 1862-1875 (2008).
- Shan, Y., et al. Immersion infection of germ-free zebrafish with listeria monocytogenes induces transient expression of innate immune response genes. Front Microbiol. 6, 373 (2015).
- Arias-Jayo, N., Alonso-Saez, L., Ramirez-Garcia, A., Pardo, M. A. Zebrafish axenic larvae colonization with human intestinal microbiota. Zebrafish. 15 (2), 96-106 (2018).
- Singleman, C., Holtzman, N. G. Growth and maturation in the zebrafish, danio rerio: A staging tool for teaching and research. Zebrafish. 11 (4), 396-406 (2014).
- Clift, D., Richendrfer, H., Thorn, R. J., Colwill, R. M., Creton, R. High-throughput analysis of behavior in zebrafish larvae: Effects of feeding. Zebrafish. 11 (5), 455-461 (2014).
- Nascimento, M. D., Schorer, M., Dos Santos, J. C. E., Rocha, M. S. A., Pedreira, M. M. Live and frozen Artemia nauplii for catfish (steindachner, 1876) larvae in different salinities. Trop Anim Health Prod. 52 (2), 653-659 (2020).
- Jia, P. P., et al. Breaking the mold: The first report on germ-free adult marine medaka (oryzias melastigma) models. bioRxiv. , (2023).
- Avdesh, A., et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. JoVE. (69), e4196 (2012).
- Wilson, C. Aspects of larval rearing. ILAR J. 53 (2), 169-178 (2012).
- Aleström, P. a. -. O., et al. Zebrafish: Housing and husbandry recommendations. Lab Anim. 54 (3), 213-224 (2020).
- Brand, M., Granato, M., Nüsslein-Volhard, C. Keeping and raising zebrafish. Zebrafish. , (2002).
- Nursyirwani, N., et al. Phenotype and genotype of lactic acid bacteria (lab) isolated from the tiger grouper Epinephelus fuscoguttatus alimentary tract. F1000Res. 6, 1984 (2017).
- Karolenko, C., Desilva, U., Muriana, P. M. Microbial profiling of biltong processing using culture-dependent and culture-independent microbiome analysis. Foods. 12 (4), 844 (2023).
- Jia, P. P., et al. Chronic exposure to graphene oxide (go) induced inflammation and differentially disturbed the intestinal microbiota in zebrafish. Environ Sci Nano. 6 (8), 2452-2469 (2019).
- Sun, B. L., et al. Probiotic supplementation mitigates the developmental toxicity of perfluorobutanesulfonate in zebrafish larvae. Sci Total Environ. 799, 149458 (2021).
- Qian, H. F., Liu, G. F., Lu, T., Sun, L. W. Developmental neurotoxicity of microcystis aeruginosa in the early life stages of zebrafish. Ecotoxicol Environ Saf. 151, 35-41 (2018).
- Bertotto, L. B., Catron, T. R., Tal, T. Exploring interactions between xenobiotics, microbiota, and neurotoxicity in zebrafish. Neurotoxicology. 76, 235-244 (2020).
- Jia, P. P., et al. Bacteriophage-based techniques for elucidating the function of zebrafish gut microbiota. Appl Microbiol Biotechnol. 107 (7-8), 2039-2059 (2023).
- Xin, G. Y., et al. Gut bacteria vibrio sp. And aeromonas sp. Trigger the expression levels of proinflammatory cytokine: First evidence from the germ-free zebrafish. Fish Shellfish Immunol. 106, 518-525 (2020).
- Dremova, O., et al. Sterility testing of germ-free mouse colonies. Front Immunol. 14, 1275109 (2023).
- Lam, S. H., Chua, H. L., Gong, Z., Lam, T. J., Sin, Y. M. Development and maturation of the immune system in zebrafish, danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 28 (1), 9-28 (2004).
- Rolig, A. S., Parthasarathy, R., Burns, A. R., Bohannan, B. J. M., Guillemin, K. Individual members of the microbiota disproportionately modulate host innate immune responses. Cell Host Microbe. 18 (5), 613-620 (2015).
- Stressmann, F. A., et al. Mining zebrafish microbiota reveals key community-level resistance against fish pathogen infection. ISME J. 15 (3), 702-719 (2021).
- Guitton, E., et al. Production of germ-free fast-growing broilers from a commercial line for microbiota studies. JoVE. (160), e61148 (2020).
- Rea, V., Bell, I., Ball, T., Van Raay, T. Gut-derived metabolites influence neurodevelopmental gene expression and wnt signaling events in a germ-free zebrafish model. Microbiome. 10 (1), 132 (2022).
- Russo, P., et al. Zebrafish gut colonization by mcherry-labelled lactic acid bacteria. Appl Microbiol Biotechnol. 99 (8), 3479-3490 (2015).
- Rawls, J. F., Mahowald, M. A., Ley, R. E., Gordon, J. I. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 127 (2), 423-433 (2006).

