Assessing Rat Diaphragm Motor Unit Connectivity Outcome Measures as Quantitative Biomarkers of Phrenic Motor Neuron Degeneration and Compensation
Summary
In this study, we present an in vivo method for estimating motor unit number and size to quantify rat diaphragm motor unit connectivity. A step-by-step approach to these techniques is described.
Abstract
Loss of ventilatory muscle function is a consequence of motor neuron injury and neurodegeneration (e.g., cervical spinal cord injury and amyotrophic lateral sclerosis, respectively). Phrenic motor neurons are the final link between the central nervous system and muscle, and their respective motor units (groups of muscle fibers innervated by a single motor neuron) represent the smallest functional unit of the neuromuscular ventilatory system. Compound muscle action potential (CMAP), single motor unit potential (SMUP), and motor unit number estimation (MUNE) are established electrophysiological approaches that enable the longitudinal assessment of motor unit integrity in animal models over time but have mostly been applied to limb muscles. Therefore, the objectives of this study are to describe an approach in preclinical rodent studies that can be used longitudinally to quantify the phrenic MUNE, motor unit size (represented as SMUP), and CMAP, and then to demonstrate the utility of these approaches in a motor neuron loss model. Sensitive, objective, and translationally relevant biomarkers for neuronal injury, degeneration, and regeneration in motor neuron injury and diseases can significantly aid and accelerate experimental research discoveries to clinical testing.
Introduction
Phrenic motor neurons (MNs), extending from C3 to C6 myotome levels, form the final link from the central nervous system (CNS) to the diaphragm muscle1. Phrenic motor units (MUs) are comprised of a single spinal MN and its innervated diaphragm muscle fibers forming the smallest functional unit of the respiratory neuromuscular system. The ventilatory function requires adequate contraction of the diaphragm muscle achieved through coordinated activation of the phrenic MU pool2,3. Many neurological diseases, including amyotrophic lateral sclerosis (ALS), result in severe ventilatory impairment, ultimately contributing to the cause of death4.
Several electrophysiological approaches can be employed to evaluate and monitor the integrity of the motor unit (MU) pool in vivo. Compound muscle action potential (CMAP) reflects the summated depolarization of all muscle fibers in a specific muscle or muscle group after peripheral nerve stimulation and is sensitive to a range of neuromuscular conditions, including ALS5,6 and spinal muscular atrophy (SMA)7,8,9. A limitation of CMAP assessment is that collateral sprouting can lead to maintained CMAP amplitude and area even in the presence of MU loss10. To overcome this limitation, modifications have been made to the CMAP technique to evaluate both motor unit number and size11. Additionally, an in vivo study investigating the functional assessment of diaphragm CMAP by an electrophysiological system suggested that it may also be feasible to utilize the described diaphragm CMAP recording technique for motor unit number estimation12.
The incremental motor unit number estimation (MUNE) technique was initially introduced in the early 1970s by McComas et al. for the extensor digitorum brevis muscle in humans13. The incremental MUNE approach was a modification of the traditional CMAP recording technique during which a gradually increasing stimulation was delivered to record quantal, all-or-none submaximal increments as indices of single motor unit responses. The summed and averaged increments were used to calculate an estimate for the size of a single motor unit potential (SMUP). This calculated size was then divided into the CMAP amplitude to estimate the number of MUs innervating the muscle under examination11. MUNE demonstrates high sensitivity in detecting and monitoring motor unit loss, allowing for the identification of motor unit dysfunction before observable changes in measures such as CMAP amplitude or area14,15. In ALS patients, MUNE has proven to be exceptionally sensitive, serving as a prominent biomarker for disease onset, progression, and prognosis16,17.
Numerous adaptations of MUNE have been developed and widely used to assess MU function in conditions such as neurodegeneration, neural injury, and the natural aging process18,19,20,21. Since the initial description, various adaptations utilizing both electrophysiological responses and incremental force (mechanical) measurements have been employed in both human studies and animal models22. MUNE provides a non-invasive functional assessment of motor neuron connectivity with the muscle. Longitudinally applying MUNE enables the understanding of disease or induced phenotype progression and the evaluation of protective or regenerative effects of therapeutic interventions, both in clinical and preclinical settings. Regardless of the effectiveness of MUNE measures reproducibility and the clinical relevance of the technique for MU pools throughout most of the human body, efforts have largely focused on limb muscles in rodent muscles10,23,24,25.
Therefore, the objectives of this study were to describe an approach to obtaining compound muscle action potential (CMAP), SMUP, and phrenic motor unit number (MUNE) as in vivo assessments that can be used longitudinally in preclinical rodent studies to quantify the MUNE, motor unit size (represented as SMUP), and CMAP. Furthermore, we present representative data that highlights the loss of diaphragm MU number following intrapleural administration of a phrenic MN degenerative agent, cholera toxin B fragment conjugated to saporin (CTB-SAP).
Protocol
All procedures were approved and conducted in compliance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Missouri. Experiments were performed on adult male Sprague-Dawley rats, aged 11 to 15 weeks. These rats were housed in pairs and kept under a 12:12 light-dark cycle, with access to standard commercial pelleted food and water available at all times.
1. Animal preparation and anesthesia delivery
- Wear suitable personal protection equipment while handling rats.
- Administer inhalation anesthetic with 3-5% isoflurane, ensuring proper induction. Once the rat is adequately anesthetized, place it in a supine position and maintain the anesthesia with 1-3% inhaled isoflurane. Check the sufficiency of the anesthesia depth by gently applying pressure to the hindlimb footpad using forceps to ensure there is no withdrawal response.
NOTE: Based on the rat's size and weight, monitor the depth of anesthesia, and adjust the isoflurane concentration accordingly. - Maintain the temperature of 37 °C using a thermostatic warming plate to prevent variations in temperature that can impact CMAP amplitude and latency.
- Apply a veterinary petroleum-based ointment to the eyes to prevent dryness. Monitor the depth of anesthesia by observing the respiration rate and checking for withdrawal responses upon applying pressure to the footpad with forceps.
- Remove hair from the lower third of the chest and neck to be studied using clippers. Monitor the respiration of the rat during the entire experiment.
NOTE: Following the CMAP and MUNE recordings and discontinuation of anesthesia, do not leave the rat unattended until it has regained sufficient consciousness. Do not return the animal to the home cage until fully recovered.
2. Electrode placement and setup
- Place a pair of 28 G monopolar needle electrodes to record the CMAP, SMUP, and MUNE as depicted in Figure 1.
- Place the active (E1) needle electrode subcutaneously over the mid clavicular line inferior to the last rib border, and the reference (E2) needle electrode subcutaneously in the angle between the xyphoid process and last sternocostal cartilage.
NOTE: The needle electrodes should not be inserted into the diaphragm muscle; instead, they should be positioned in the subcutaneous area.
- Place the active (E1) needle electrode subcutaneously over the mid clavicular line inferior to the last rib border, and the reference (E2) needle electrode subcutaneously in the angle between the xyphoid process and last sternocostal cartilage.
- For stimulation of the phrenic nerve at the carotid sheet, use a pair of 28 G monopolar needle electrodes as the cathode and anode for nerve stimulation and placed subcutaneously over the lateral neck between the anterior and middle scalene muscles, separated by approximately 1 cm. Ensure that the placement of the stimulating needles is at the level below the fourth cervical vertebrae (C4).
NOTE: Avoid inserting the stimulating electrodes too deep to avoid injury of the phrenic nerve or other structure. Figure 1 illustrates electrode placement. - For the ground electrode, place a disposable surface electrode on the tail.
3. Data acquisition
- Phrenic CMAP
- Record phrenic CMAP responses by applying monophasic cathodic square-wave pulses with a duration of 0.1 ms and intensity ranging from 60 to 100 mA to stimulate the phrenic nerve.
- Obtain CMAP responses while progressively increasing the stimulus intensity until the amplitude of the response ceases to show any further increase. To ensure supramaximal stimulation, raise the stimulus intensity to approximately 120% of the level used to elicit a maximal response, and record an additional response. If the CMAP size no longer increases, consider this response as the maximum CMAP.
NOTE: Delivering stimulation during exhalation is preferable to minimize concurrent muscle activity noise during CMAP recording. - Measure and document the peak-to-peak amplitudes of the CMAP in millivolts (mV) (Figure 2).
NOTE: CMAP amplitude can be assessed base-to-peak and peak-to-peak. Clinical electrodiagnostic systems are often defaulted to assess base-to-peak which is calculated from the isoelectric baseline to the initial negative peak.
- Average single motor unit potential (SMUP) size and MUNE calculation
- Calculate the average SMUP size using an incremental stimulation technique.
- To elicit incremental responses, administer submaximal stimulation with a duration of 0.1 ms at 1 Hz frequency, gradually increasing the intensity in 0.03 mA increments until a minimal all-or-none response is achieved. Acquire the initial response with a stimulus intensity ranging between 2 mA and 10 mA.
- If the initial response does not occur with a stimulus intensity between 2 mA and 10 mA, modify the position of the stimulating cathode, either bringing it closer or moving it farther from the phrenic nerve in the neck, to decrease or increase the necessary stimulus intensity, respectively.
- If the first incremental response is achieved with a stimulus intensity ranging from 2 mA to 10 mA, save the first response and acquire additional increments with progressively higher stimulus intensities, adjusting in increments of 0.03 mA, to achieve a total of 9 additional increments that fulfill the following criteria in step 3.2.2.
NOTE: Each SMUP is quantified by subtracting each increment from the preceding increment.
- While measuring the incremental responses, make sure that each increment meets the following criteria:
- Make sure that the initial negative peak of the incremental responses aligns temporally with the negative peak of the maximal CMAP response.
NOTE: The slight movements observed due to background noise from respiratory cycles are inherent to the nature of the experiment. However, the consistent presence of SMUPs during live observation confirms their identity for that specific CMAP. - As the diaphragm is a dynamic muscle involved in respiration, each breathing cycle may induce baseline movement. Thus, verify the stability and absence of fractionation in each incremental response by confirming consistency across three duplicate responses.
NOTE: To distinguish low-amplitude evoked potentials, especially the first SMUP, from background noise due to respiratory activity, it is important to remain vigilant and observe for 3-4 respiratory cycles. Also ensure the alignment of peaks with those of the CMAP for accuracy. - Ensure that each increment is distinct and larger than the previous one. Therefore, visually distinguish incremental responses in real time, observing them as they overlay on the previously recorded increments.
NOTE: Multiple replicates of the stimulus at each amplitude might be conducted to ensure consistency in the increments and compliance with the predefined criteria. - After visually verifying each increment with the aforementioned criteria, confirm that the increment amplitude is at least 25 µV. If the increment is below 25 µV, discard the measurement and re-assess the response.
- Following the recording of 10 incremental responses, verify that the amplitude of each increment response is not greater than one-third of the combined amplitude of all 10 increments, representing the total amplitude of the final response. If this criterion is not satisfied, repeat the measurement of the 10 incremental responses.
NOTE: The threshold of one-third is based on the assumption that each incremental response represents the activation of a single motor unit. If the amplitude of any incremental response exceeds one-third of the combined amplitude of all ten increments, it suggests that the response may not be solely attributable to the activation of a single motor unit. Instead, it could be influenced by the recruitment of additional motor units or the presence of non-specific activity, such as electrical noise or artifacts22,26. - To estimate the average amplitude of the SMUPs, average the values of the 10 increments. Another method to measure average SMUP amplitude is to divide the entire amplitude of the final incremental response by the total number of increments11.
- Make sure that the initial negative peak of the incremental responses aligns temporally with the negative peak of the maximal CMAP response.
- Determine MUNE by dividing the maximum CMAP amplitude (peak-to-peak) by the average SMUP amplitude (peak-to-peak). In certain electrophysiological systems, SMUPs are recorded in microvolts (µV), while CMAP is usually expressed in millivolts (mV). If required, convert CMAP and SMUP measurements to the same units before calculating MUNE.
NOTE: Peak-to-peak CMAP, average SMUP, and MUNE are typically automatically calculated by clinic electromyography systems):
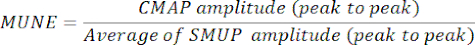
CMAP = Compound Muscle Action Potential
SMUP = Single Motor Unit Potential
MUNE = Motor Unit Number Estimation
- Calculate the average SMUP size using an incremental stimulation technique.
Representative Results
The CMAP, SMUP, and MUNE techniques outlined in this report enable the recording of neuromuscular function in the diaphragm muscle employing minimally invasive electrode placement (Figure 1). The parameters of amplitude and area can be employed to characterize the supramaximal CMAP size, providing an overall measure of muscle group output (Figure 2). However, in our current methods, we rely on amplitude to quantify both CMAP and SMUP sizes. CMAP, SMUP, and MUNE can be utilized to measure neuromuscular function in various rat models of neuromuscular disease. To demonstrate MU loss in the context of MN degeneration, rats underwent bilateral intrapleural injection with CTB-SAP (25 µg) to target phrenic MNs and extra CTB (25 µg), and control rats received unconjugated CTB (25 µg) and SAP (25 µg)1,27.
In Figure 3, findings in an adult control rat and an adult rat (12 weeks) seven days following intrapleural CTB-SAP injection are compared. Following intrapleural injection of CTB-SAP, MUNE is reduced at 60 estimated functional motor units compared with normal findings of 74 functional motor units in the control rat. However, the CMAP amplitude in the CTB-SAP rat (14.25 mV peak-to-peak) exhibited minimal changes compared to the control (14. 5 mV peak-to-peak) likely due to collateral sprouting. In Figure 4, diaphragm CMAP, SMUP, and MUNE were acquired bilaterally and averaged at baseline/preinjection (n = 14), as well as 7 (n = 14), 14 (n = 14), 21 (n = 6), and 28 (n = 14) days post injection. In Figure 4, no baseline differences between groups were found. MUNE showed a significant change for time (p < 0.05), CTB-SAP (p < 0.001), and interaction time x CTB-SAP (p < 0.01) with ~40% reduction of MUNE in CTB-SAP rats. The average SMUP of CTB-SAP rats demonstrated a significant change with time (p < 0.05) and CTB-SAP (p < 0.01), but no significant interaction with ~50-60% increase in SMUP amplitude. CMAP showed no significant change.
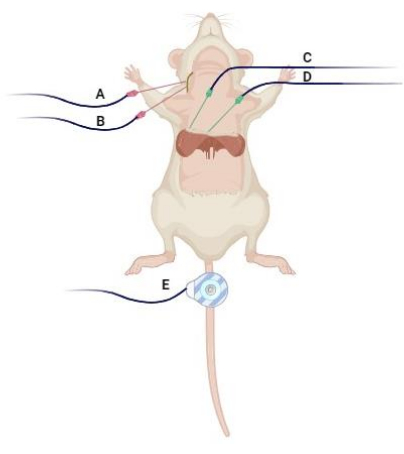
Figure 1: Electrode placement. The (A) stimulating cathode and (B) anode are subcutaneously inserted in the lateral neck between the anterior and middle scalene muscles approximately 1 cm apart. The (C) "active" electrode (E1) and (D) "reference" recording electrode (E2) are positioned over the midclavicular line inferior to the last rib border, following the angle between the xiphoid process and the last costosternal cartilage. Additionally, (E) a disposable disk electrode is situated on the tail as a ground to minimize artifacts. Created with BioRender.com. Please click here to view a larger version of this figure.
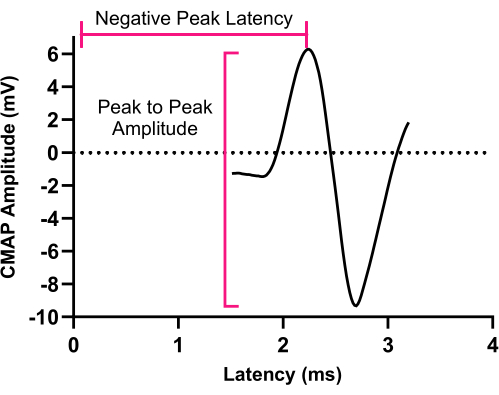
Figure 2: Compound muscle action potential. An illustration of a representative CMAP response recorded from the left hemidiaphragm muscle. CMAP peak to-peak amplitude is determined from negative peak voltage to positive peak voltage. Abbreviation: CMAP = Compound muscle action potential. Please click here to view a larger version of this figure.
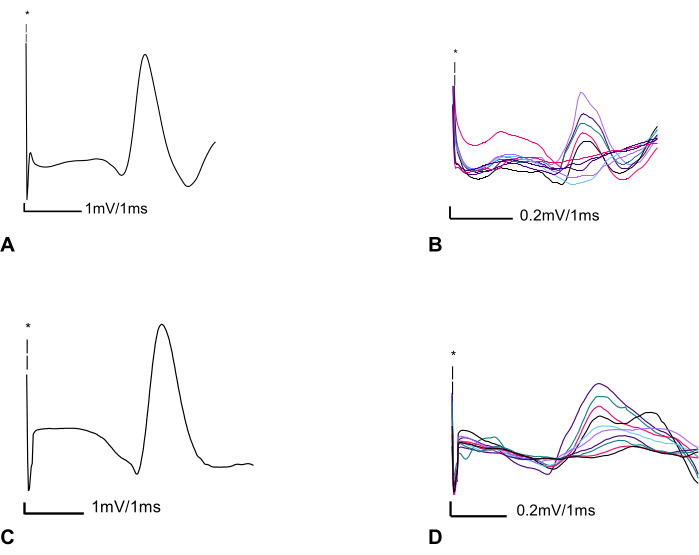
Figure 3: Two representative CMAP and MUNE recordings from a control rat, and an adult rat seven days after CTB-SAP intrapleural injection. (A) Left phrenic compound muscle action potential (CMAP) in a control adult rat (12 weeks of age) with peak-to-peak amplitude of 14.5 mV. Screen sensitivity = 1 mV/division and screen duration 1 ms/division. (B) Ten subsequent incremental responses with a total amplitude of 1.940 mV are divided by 10 to determine the average SMUP size (0.194 mV). Screen sensitivity = 0.2 mV/division and sweep speed of 1 ms/division. Calculated MUNE = 74 (MUNE=CMAP/average SMUP (14.5 mV/ 0.194 mV)) (C) Phrenic CMAP seven days following CTB-SAP injection showing almost no change in CMAP peak-to-peak amplitude 14.25 mV. Screen sensitivity = 1 mV/division and sweep speed of 1 ms/division. (D) Ten subsequent incremental responses with a total peak-to-peak amplitude of 2.360 mV divided by 10 to obtain an average SMUP size of 0.236 mV. Screen sensitivity = 0.2 mV/division and a sweep speed of 1 ms/division. Thus, the calculated MUNE was 60. Abbreviations: CMAP = compound muscle action potential; MUNE = motor unit number estimation; CTB-SAP = cholera toxin B fragment conjugated to saporin; SMUP = single motor unit potential. *Illustrates the artifact caused by electrical stimulation; it is important to note that due to the contrast between the amplitude of the electrical stimulus and CMAP, as well as incremental responses, we graphed only a portion of the stimulus. Blind-colored friendly colors have been utilized to enhance the visualization of the incremental responses for improved accessibility. Please click here to view a larger version of this figure.
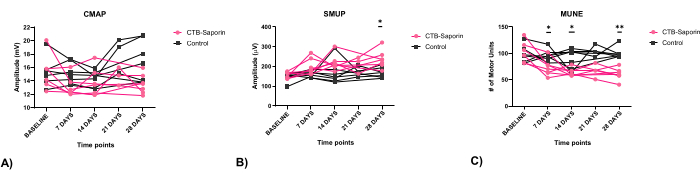
Figure 4: Electrophysiological assessment of CMAP, SMUP, and MUNE following CTB-SAP. No baseline differences between groups were found (seven 11-week-old male rats in both CTB-SAP and control groups). (A) CMAP showed no significant change. (B) The average SMUP of CTB-SAP rats showed a significant change over time (p < 0.05) and with CTB-SAP (p < 0.01), with no significant interaction, indicating a 50-60% increase in SMUP amplitude. (C) Seven days after CTB-SAP injection, the mean MUNE significantly decreased by 27.07%, from 102.58 ± 20.54 to 72.43 ± 15.59 estimated functional motor units (Mean ± SD). This reduction persisted, with an additional 11.75% decline at 28 days (MUNE: 60.64 ± 11.33, p < 0.01). All measurements at baseline, 7, 14, and 28 days after injection were obtained from 14 rats, with n = 7 rats injected with conjugated CTB-SAP and n = 7 rats receiving unconjugated CTB and SAP as the control group. Six rats were used on day 21. CMAP, SMUP, and MUNE data are presented as mean, and comparisons were performed using two-way ANOVA. Asterisks indicate differences at various time points: *p < 0.05, and **p < 0.01. Abbreviations: CMAP = compound muscle action potential; MUNE = motor unit number estimation; CTB-SAP = cholera toxin B fragment conjugated to saporin; SMUP = single motor unit potential. Please click here to view a larger version of this figure.
Discussion
In MN degenerative diseases, such as ALS, it is crucial to assess the MUs involved in ventilation28. Despite the occurrence of respiratory MN degeneration in ALS patients, the specific onset and progression of MN death remain incompletely understood29,30,31. Recognizing the significance of this aspect, various models, both genetic-based (e.g., SOD12,32) and non-genetic-based (e.g., CTB-SAP intrapleural injection3,27,33), have been employed to emulate respiratory impairment in animal models of neurodegenerative diseases. Accordingly, identifying a biomarker for diagnosing, monitoring, and assessing potential treatment effects is advantageous for these models. Additionally, biomarkers can facilitate the clinical translation of preclinical research findings.
To quantify the number of innervating MNs in rodent degenerative models, different labeling techniques, including retrograde tracers and adenoviruses, have been employed25,34,35,36. Previously, immunohistochemistry has been utilized to label phrenic motor neurons in the anterior horn of the cervical spinal cord2,3,33. Labeling techniques, though valuable for MN evaluations, have limitations in assessing the functionality of MUs and are not suitable for longitudinal assessments37. Objective electrophysiological assessments are crucial for successful cross-species translation, especially considering pronounced structural differences in the functional and anatomical features of the motor systems between humans and rodents38,39. Incorporating neurobiological differences presents a challenge in translating findings from rodent models to patients. MUNE overcomes these obstacles by serving as an objective electrophysiological measure of MN function, allowing the evaluation of functional MU connectivity as a biomarker in MN degenerative model experiments3,40.
CMAP, SMUP, and MUNE are commonly employed in research studies and for evaluating patients with neuromuscular disorders9,10,11. These potential biomarkers are minimally invasive, enabling longitudinal assessment of function within the same individual over time. Importantly, while they do not directly gauge the activation or recruitment of the MU by cortical MNs, they do provide a clinically relevant estimation of the MN integrity and its functional counterpart, the MU. Rodent models of MN degenerative diseases are crucial for comprehending the physiopathologic mechanisms underlying human diseases and for the preclinical advance of effective treatments. Developing outcome measures and biomarkers that are translatable across species can streamline and expedite the translation of promising preclinical findings to clinical trials11. Given the relative complexity of the measures, we have adapted and refined these techniques from rat limbs to the diaphragm muscle. This translation has been presented in a visual format to facilitate broader utilization and implementation in rat studies.
From our experience, the clinical electrodiagnostic systems are well-suited for the studies outlined in this context. This suitability stems from enhanced ergonomics in the interface between the examiner and the electrodiagnostic system, facilitating convenient control. In our laboratory group, we utilize a two-channel system featuring two non-switched amplifier channels. These channels are equipped with a 24-bit Analog to Digital Converter and operate at a sampling rate of 48 kHz per channel. The hardware gain is adjustable within a range from 10nV to 100 mV per division. We employed a low-frequency filter with a range spanning from 0.2 Hz to 5 kHz, and the high-frequency filter settings covered a range from 30 Hz to 10 kHz. Additionally, we utilized a constant-current stimulator with adjustable intensity ranging from 0 to 100 mA and duration from 0.02 to 1 ms. The majority of clinical systems offer comparable features that are suitable for recording CMAP, SMUP, and MUNE responses. These systems can be adjusted accordingly to ensure accurate recording of the desired data. For instance, CMAP amplitude can be assessed base-to-peak and peak-to-peak. Clinical electrodiagnostic systems are often defaulted to assess base-to-peak which is calculated from the isoelectric baseline to the initial negative peak.
The process of obtaining CMAP, SMUP, and MUNE responses from the diaphragm involves several crucial steps. Consistent and accurate electrode placement, along with adequate electrode depth, is essential for reliably measuring amplitudes and minimizing background noises during recording. Incorrect placement of stimulating electrodes can lead to unintended consequences. Placing stimulating electrodes too posteriorly can result in exaggerated forelimb muscle contractions to brachial plexus excitation, while higher than C4 level placement can make stimulation of the phrenic nerve more difficult. Deeper placement carries the risk of injuring the carotid artery or evoking the vagus nerve, potentially causing cardiac conductivity issues. Placing recording electrodes higher or lower than the last rib, measurements from intercostal or rectus abdominis stimulation may occur, respectively. To avoid recording from other muscles, notice that the diaphragm negative peak of CMAP latency is typically 1.5-3.5 ms, and its characteristic shape is shown in Figure 2. Our observations indicated that needle electrodes yield more consistent CMAP, SMUP, and MUNE recordings compared to disc-shaped electrodes for diaphragm muscle. This enhanced consistency is attributed to the dome shape of the diaphragm muscle and the presence of other muscles in proximity, such as the intercostal and rectus abdominis muscles. Additionally, flexible placement of the needle electrodes is another advantageous factor. Regarding electrical stimulation intensity, we utilized a range of 60 to 100 mA to stimulate the phrenic nerve, which exceeds the reported ranges for other motor systems. This difference arises from the deeper location of the phrenic nerve in the neck compared to the more superficial locations of the sciatic nerve and brachial plexus in the hindlimb and forelimb, respectively.
Obtaining the average SMUP poses greater technical challenges compared to CMAP. The smaller response size, in the microvolt range rather than millivolts, makes the impact of background noise more pronounced. To mitigate background noise, consider optimizing electrode placement by adjusting the ground electrode, cathode, and anode. Additionally, ensure there is minimal interference from nearby electrical devices in the experimental setup. A Faraday cage, commonly utilized in intracellular electrophysiology applications, is not necessary for this setup. The visual identification of individual SMUP responses is a challenging skill that requires practice for consistent and repeatable results. As the diaphragm is a dynamic muscle, the baseline can shift with each ventilation cycle. Being attentive to the SMUP increments ensuring alignment with the previous SMUP is a critical factor in overcoming this challenge. Additionally, ensuring that the SMUPs initiate within the latency timeframe of the maximal CMAP is important for accurate recordings.
Another challenge of diaphragm recording is the asymmetric nature of this muscle, characterized by varying thicknesses from left to right and influenced by the pressure exerted by the liver on the right side41. To address this, we conducted electrophysiological measurements separately on the left and right hemidiaphragms. Following that, we calculated the averages for CMAP, SMUP, and MUNE responses separately.
Since the CMAP response reflects the collective depolarization of muscle fibers in a specific muscle, any pathology affecting the phrenic MN to the diaphragm muscle fiber can result in a reduction in CMAP size. Hence, calculating the average CMAP from bilateral hemidiaphragms offers an excellent assessment of the overall functional status of the respiratory neuromuscular system. Compensatory changes, such as collateral sprouting, can lead to the preservation of CMAP size even in the presence of MN or motor axonal loss11. Recording individual increments allows for the estimation of the average output of single MUs (SMUP size), presenting more detailed insights into the functional status of MUs of the respiratory system. Hence, the MUNE technique becomes essential for evaluating the phrenic MNs or axons' input to the diaphragm. Applying these biomarkers in preclinical experiments of therapies for studying MN diseases holds the potential to improve the translation to clinical trials.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by a Spinal Cord Injury/Disease Research Program Grant from the Missouri Spinal Cord Injury/Disease Research Program (NLN and WDA).
Materials
| 2 mL Glass Syringe | Kent Scientific Corporation | SOMNO-2ML | |
| 50 mL, Model 705 RN syringe | Hamilton Company | 7637-01 | Utilized to conduct intrapleural injection |
| Autoclavable 26 G needles (26S RN 9.52 mm 40°) | Hamilton Company | 7804-04 | Utilized to conduct intrapleural injection |
| Cholera toxin B-subunit (CTB) | MilliporeSigma | C9903 | Utilized for intrapleural injection to label surviving motor neurons |
| Cholera toxin B-subunit conjugated to saporin (CTB-SAP) | Advanced Targeting Systems | IT-14 | Utilized for intrapleural injection to cause motor neuron death |
| Detachable Cable | Technomed | 202845-0000 | to connect the recorder electrode to the electrodiagnostic machine |
| Disposable 2" x 2" disc electrode with leads | Cadwell | 302290-000 | ground electrode |
| disposable monopolar needles 28 G | Technomed | 202270-000 | cathode and anode stimulating electrodes- recording electrodes |
| EMG needle cable (Amp/stim switch box) | Cadwell | 190266-200 | to connect monopolar electrodes to electrodiagnostic stimulator |
| Helping Hands alligator clip with iron base | Radio Shack | 64-079 | Maintaining recording electrode placement |
| Isoflurane (250 mL bottle) | Piramal Healthcare | ||
| monoject curved tip irrigating syringe | Covidien | 81412012 | utilized for application of electrode gel |
| PhysioSuite Physiological Monitoring System with RightTemp Homeothermic Warming | Kent Scientific Corporation | PS-RT | Includes infrared warming pad, rectal probe, and pad temperature probe |
| Pro trimmer Pet Grooming Kit | Oster | 078577-010-003 | clippers for hair removal |
| Saporin (SAP) | Advanced Targeting Systems | PR-01 | Utilized for intrapleural injection (control agent when injected by itself) |
| Sierra Summit EMG system | Cadwell Industries, Inc., Kennewick, WA | portable electrodiagnostic system | |
| SomnoSuite Low-Flow Digital Anesthesia System | Kent Scientific Corporation | SOMNO | Includes anti-spill, anti-vapor bottle top adapter; Y adapter tubing; charcoal scavenging filter |
| Sprague-Dawley rat | Envigo colony 208a, Indianapolis, IN | ||
| Veterinarian petroleum-based ophthalmic ointment | Puralube | 26870 | applied during anesthesia to avoid corneal injury |
References
- Mantilla, C. B., Zhan, W. -. Z., Sieck, G. C. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods. 182 (2), 244-249 (2009).
- Nichols, N. L., Satriotomo, I., Harrigan, D. J., Mitchell, G. S. Acute intermittent hypoxia induced phrenic long-term facilitation despite increased sod1 expression in a rat model of als. Exp Neurol. 273, 138-150 (2015).
- Nichols, N. L., Craig, T. A., Tanner, M. A. Phrenic long-term facilitation following intrapleural ctb-sap-induced respiratory motor neuron death. Respir Physiol Neurobiol. 256, 43-49 (2018).
- Kiernan, M. C., et al. Amyotrophic lateral sclerosis. Lancet. 377 (9769), 942-955 (2011).
- Boërio, D., Kalmar, B., Greensmith, L., Bostock, H. Excitability properties of mouse motor axons in the mutant sod1g93a model of amyotrophic lateral sclerosis. Muscle Nerve. 41 (6), 774-784 (2010).
- Shibuya, K., et al. Motor cortical function determines prognosis in sporadic als. Neurology. 87 (5), 513-520 (2016).
- Lewelt, A., et al. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle Nerve. 42 (5), 703-708 (2010).
- Mcgovern, V. L., et al. Smn expression is required in motor neurons to rescue electrophysiological deficits in the smnδ7 mouse model of sma. Hum Mol Genet. 24 (19), 5524-5541 (2015).
- Arnold, W. D., et al. Electrophysiological biomarkers in spinal muscular atrophy: Preclinical proof of concept. Ann Clin Transl Neurol. 1 (1), 34-44 (2014).
- Harrigan, M. E., et al. Assessing rat forelimb and hindlimb motor unit connectivity as objective and robust biomarkers of spinal motor neuron function. Sci Rep. 9 (1), 16699 (2019).
- Arnold, W. D., et al. Electrophysiological motor unit number estimation (mune) measuring compound muscle action potential (cmap) in mouse hindlimb muscles. J. Vis. Exp: JoVE. (103), e52899 (2015).
- Martin, M., Li, K., Wright, M. C., Lepore, A. C. Functional and morphological assessment of diaphragm innervation by phrenic motor neurons. J. Vis. Exp: JoVE. (99), e52605 (2015).
- Mccomas, A., Fawcett, P. R. W., Campbell, M., Sica, R. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 34 (2), 121-131 (1971).
- Felice, K. J. A longitudinal study comparing thenar motor unit number estimates to other quantitative tests in patients with amyotrophic lateral sclerosis. Muscle Nerve. 20 (2), 179-185 (1997).
- Vucic, S., Rutkove, S. B. Neurophysiological biomarkers in amyotrophic lateral sclerosis. Curr Opin Neurol. 31 (5), 640-647 (2018).
- Carleton, S., Brown, W. Changes in motor unit populations in motor neurone disease. J Neurol Neurosurg Psychiatry. 42 (1), 42-51 (1979).
- Yuen, E. C., Olney, R. K. Longitudinal study of fiber density and motor unit number estimate in patients with amyotrophic lateral sclerosis. Neurology. 49 (2), 573-578 (1997).
- Gooch, C. L., et al. Motor unit number estimation: A technology and literature review. Muscle Nerve. 50 (6), 884-893 (2014).
- Henderson, R. D., Ridall, P. G., Hutchinson, N. M., Pettitt, A. N., Mccombe, P. A. Bayesian statistical mune method. Muscle Nerve. 36 (2), 206-213 (2007).
- Shefner, J., et al. Multipoint incremental motor unit number estimation as an outcome measure in als. Neurology. 77 (3), 235-241 (2011).
- Stein, R. B., Yang, J. F. Methods for estimating the number of motor units in human muscles. Ann Neurol. 28 (4), 487-495 (1990).
- Shefner, J. M. Motor unit number estimation in human neurological diseases and animal models. Clin Neurophysiol. 112 (6), 955-964 (2001).
- Ahad, M., Rutkove, S. Correlation between muscle electrical impedance data and standard neurophysiologic parameters after experimental neurogenic injury. Physiol Meas. 31 (11), 1437 (2010).
- Kasselman, L. J., Shefner, J. M., Rutkove, S. B. Motor unit number estimation in the rat tail using a modified multipoint stimulation technique. Muscle Nerve. 40 (1), 115-121 (2009).
- Ngo, S., et al. The relationship between bayesian motor unit number estimation and histological measurements of motor neurons in wild-type and sod1g93a mice. Clin Neurophysiol. 123 (10), 2080-2091 (2012).
- Feasby, T., Brown, W. Variation of motor unit size in the human extensor digitorum brevis and thenar muscles. J Neurol Neurosurg Psychiatry. 37 (8), 916-926 (1974).
- Nichols, N. L., Vinit, S., Bauernschmidt, L., Mitchell, G. S. Respiratory function after selective respiratory motor neuron death from intrapleural ctb-saporin injections. Exp Neurol. 267, 18-29 (2015).
- Nichols, N. L., et al. Ventilatory control in als. Respir Physiol Neurobiol. 189 (2), 429-437 (2013).
- Cifra, A., Nani, F., Nistri, A. Respiratory motoneurons and pathological conditions: Lessons from hypoglossal motoneurons challenged by excitotoxic or oxidative stress. Respir Physiol Neurobiol. 179 (1), 89-96 (2011).
- Kobayashi, Z., et al. Fals with gly72ser mutation in sod1 gene: Report of a family including the first autopsy case. J Neurol Sci. 300 (1), 9-13 (2011).
- Su, M., Wakabayashi, K., Tanno, Y., Inuzuka, T., Takahashi, H. An autopsy case of amyotrophic lateral sclerosis with concomitant alzheimer’s and incidental lewy body diseases. No to shinkei= Brain and nerve. 48 (10), 931-936 (1996).
- Lladó, J., et al. Degeneration of respiratory motor neurons in the sod1 g93a transgenic rat model of als. Neurobiol Dis. 21 (1), 110-118 (2006).
- Borkowski, L. F., Smith, C. L., Keilholz, A. N., Nichols, N. L. Divergent receptor utilization is necessary for phrenic long-term facilitation over the course of motor neuron loss following ctb-sap intrapleural injections. J Neurophysiol. 126 (3), 709-722 (2021).
- Nicolopoulos-Stournaras, S., Iles, J. F. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 217 (1), 75-85 (1983).
- Tosolini, A. P., Morris, R. Targeting motor end plates for delivery of adenoviruses: An approach to maximize uptake and transduction of spinal cord motor neurons. Sci Rep. 6 (1), 33058 (2016).
- Mchanwell, S., Biscoe, T. The localization of motoneurons supplying the hindlimb muscles of the mouse. Phil. Trans. R. , 477-508 (1981).
- Nair, J., et al. Histological identification of phrenic afferent projections to the spinal cord. Respir Physiol Neurobiol. 236, 57-68 (2017).
- Courtine, G., et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans. Nat Med. 13 (5), 561-566 (2007).
- Friedli, L., et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci Transl Med. 7 (302), 134 (2015).
- Arnold, R., et al. Nerve excitability in the rat forelimb: A technique to improve translational utility. J Neurosci Methods. 275, 19-24 (2017).
- Boriek, A., Rodarte, J., Reid, M. Shape and tension distribution of the passive rat diaphragm. Am J Physiol Regul Integr Comp Physiol. 280, R33-R41 (2001).

.
