Basement Membrane Matrix Encapsulated Cell Aggregation for Investigating Murine Spleen Tissue Formation
Summary
This article describes a protocol for aggregating and encapsulating spleen cells within a semi-solid basement membrane matrix. Basement membrane matrix constructs can be used in three-dimensional culture for studying organoid development, or for in vivo transplantation and tissue regeneration studies.
Abstract
The spleen is an immune organ that plays a key role in blood-borne immune responses. The anatomical or functional loss of this tissue increases susceptibility to severe blood infections and sepsis. Auto-transplantation of spleen slices has been used clinically to replace lost tissue and restore immune function. However, the mechanism driving robust and immunologically functional spleen tissue regeneration has not been fully elucidated. Here, we aim to develop a method for aggregating and encapsulating spleen cells within a semi-solid matrix in order to investigate the cellular requirements for spleen tissue formation. Basement membrane matrix encapsulated cell constructs are amenable to both in vitro tissue culture of three-dimensional organoids as well as transplantation under the kidney capsule to directly assess in vivo tissue formation. By manipulating the input cells for aggregation and encapsulation, we demonstrate that graft-derived PDGFRβ+MAdCAM-1– neonatal stromal cells are required for spleen tissue regeneration under animal transplantation models.
Introduction
Traumatic rupture of the spleen and the appearance of multiple splenic nodules in the body was one of the first indications that spleen tissue harbored regenerative capacity1,2. Spleen auto-transplantations were later introduced into the clinic to preserve spleen tissue in patients requiring emergency splenectomy3. Yet, despite being a part of clinical practice for decades, very little is known about how the spleen regenerates. Animal transplantation models have provided insight into multiple parameters of spleen regeneration and immune function4,5. In particular, experimental modifications to the graft preparation method have allowed tissue regeneration to be studied in greater detail at the cellular and molecular level.
Transplantations involving whole spleen slices undergo a phase of mass necrosis before a new spleen structure is rebuilt6. The initial phase of graft necrosis suggests that the bulk of transplanted tissue largely consists of red and white blood cells and is unnecessary for spleen regeneration. This was investigated experimentally by excluding hematopoietic cells from spleen grafts before transplant under the mouse kidney capsule. Here, the non-leukocyte/non-erythrocyte fraction of the spleen, which includes stromal and endothelial cells, was shown to be sufficient to induce de novo tissue formation7. Spleen stromal tissue could be further processed into a single-cell suspension, enabling the use of cell sorting technologies to manipulate cellular graft composition. By selectively removing candidate cell types, two CD45–TER-119– stromal cell populations were identified that were indispensable for graft development: an endothelial-like CD31+CD105+MAdCAM-1+ cell population and a more broadly defined PDGFRβ+ mesenchymal cell population8.
The construction of grafts from spleen cells varies in terms of support materials and cell-loading processes. Tissue-engineered spleens have previously been prepared by loading splenic units onto a polyglycolic acid/poly-L lactic acid polymer scaffold5,9. Interestingly, spleen stromal cells absorbed into a collagen sponge failed to engraft, whereas stromal cells aggregated and loaded over a collagen sheet facilitated spleen regeneration8. The resuspension of spleen stromal cells inside a Matrigel matrix has also been demonstrated to induce cell aggregation under three-dimensional culture conditions10. However, this method has not been tested for use in transplantation models. The overall goal of the current protocol is to forcibly aggregate and encapsulate spleen stromal cells directly within the basement membrane matrix, which subsequently can be transferred to a three-dimensional in vitro tissue culture system or used as a vehicle for animal model transplantations (Supplementary Figure 1).
Protocol
All animal procedures were conducted according to experimental protocols approved by the University of Queensland Animal Ethics Committee (UQBR/079/19).
1. Tissue collection and stromal cell preparation
- Euthanize 0.5-1.5 day-old male and/or female BALB/c neonatal donor mice by induction of hypothermia by wrapping animals inside the tissue paper and placing them under crushed ice for >10 min.
NOTE: This protocol can be adapted to different mouse strains, ages, and the number of animals. - Prepare sterile surgical instruments.
- Lay the mouse in a right lateral position. Swab the skin with 80% ethanol and make a 1 cm incision with Iris surgical scissors to expose the peritoneal wall.
- Make a second 0.5 cm incision above the spleen and use a pair of fine forceps to gently lift the tissue up. Use a pair of surgical scissors to cut the blood vessels and excise the tissue. Transfer the tissue into a Petri dish containing ice-cold Phosphate Buffered Saline (PBS). Remove any remaining connective tissue attached to the spleen.
NOTE: A stereomicroscope can assist fine motor movements required for manipulating surgical instruments. - To dissociate whole tissues, transfer neonatal spleens (pools of 10 or less) into the inner rim of an inverted 14 mL conical tube cap. Mechanically disrupt tissues with a pressing motion using the plastic back end of a 1 mL syringe plunger.
- Place and secure the cap tightly over a 14 mL conical tube containing 10 mL of cold PBS. Invert the tube 5x to wash all disrupted tissue from the cap into the tube.
- Repeat step 1.6 for any remaining tissue.
- Leave the tube on ice for 1 min to let the tissue settle to the bottom of the tube.
- Carefully discard the supernatant (containing hematopoietic cells) by passing the solution through a reversible 70 µm cell strainer.
- Recover the non-soluble stromal fraction by reversing the strainer and washing stromal tissue back into the 14 mL conical tube using freshly prepared 2 mL of supplemented Dulbecco's Modified Eagle Medium (sDMEM) containing 1 mg/mL Collagenase IV, 40 µg/mL DNase I and 2% Fetal Bovine Serum (FBS).
CAUTION: Collagenase IV and Collagenase D are hazardous substances and must be handled inside a biosafety cabinet with appropriate personal protective equipment. - To prepare a single cell suspension, enzymatically digest pooled tissue (up to 20) for 10 min at 37 °C with constant rotation.
- Add 4 mL of freshly prepared sDMEM containing 1 mg/mL Collagenase D, 40 µg/mL DNase I, and 2% FBS directly to the tube containing stromal tissue. Incubate for a further 10 min at 37 °C with constant rotation.
- Halt the digestion by adding 8 mL of ice-cold PBS. Collect cells by centrifuging at 200 x g for 5 min at 4 °C.
- Discard the supernatant, and wash cells twice by resuspending in 1 mL of cold PBS and centrifuging at 200 x g for 5 min at 4 °C. Keep cells on ice.
NOTE: Cells can be counted and adjusted to a desired concentration. This may require optimization for different cell populations. Using this protocol, 0.5 x 106– 1 x 106 stromal cells/spleen are typically recovered, depending on the age of donor mice. Cells can optionally be stained and FACS sorted at this stage to define cell composition.
2. Matrix encapsulation of cell aggregates
- Pre-chill sterile P200 pipette tips inside a -20 °C freezer.
- Cut flexible laboratory film (e.g., Parafilm) into 1 cm x 2 cm pieces and sterilize by submerging in 80% ethanol for 10 min, followed by PBS for 10 min. Laboratory film can be prepared in advance and stored sterile.
- Prepare 0.5 x 106-2.5 x 106 cells by centrifuging at 200 x g for 5 min at 4 °C. Carefully aspirate the supernatant, leaving approximately 20 µL of the remaining PBS volume. Keep the cell pellet on ice.
NOTE: The remaining PBS can be gently aspirated without disturbing the cell pellet to confirm the volume. - Aspirate 2 µL of ice-cold basement membrane matrix into a pre-chilled P200 pipette tip, using a P20 pipettor.
NOTE: A highly concentrated basement membrane matrix (e.g., Corning Matrigel Matrix High Concentration) assists in forming a strong solidified plug. - Gently twist to eject the pipette tip. Stretch a pre-sterilized strip of the laboratory film and place it over the end of the pipette tip, taking care not to pierce the film. Continue to wrap the tip in the film to seal the pipette tip. Carefully place the sealed tip directly on ice, taking care not to rupture the film.
- Resuspend the cell pellet in the remaining PBS and layer the solution gently over the basement membrane matrix. Keep the construct on ice.
- Prepare a centrifugation tube by placing a 1.2 mL cluster tube inside a 14 mL conical tube.
- Place the pipette tip inside the nested tube configuration and centrifuge at 400 x g and 4 °C for 5 min.
- Position the 14 mL conical tube in a vertical orientation and incubate at 37 °C for 15 min to allow the basement membrane matrix to solidify inside the pipette tip.
NOTE: The 14 mL conical tube can be placed on ice until required for downstream application.
3. Three-dimensional organoid culture
- Carefully remove the laboratory film from the pipette tip.
- Insert a thin stainless steel wire plunger through the larger opening of the pipette tip.
- Expel the matrix plug until released through the tip into one well of a non-treated 6-well tissue culture plate containing 2 mL of sDMEM supplemented with 10% FBS, 1x Glutamax, 1x Non-essential Amino Acids (NEAA), 10 µM Rock Inhibitor, 10 U/mL Penicillin/Streptomycin, and 50 µM β-mercaptoethanol.
NOTE: The tissue culture vessel and corresponding media volume can be adjusted as required.
CAUTION: NEAA, Penicillin/Streptomycin, and β-mercaptoethanol are hazardous substances and must be handled inside a biosafety cabinet with appropriate personal protective equipment. - Culture organoids at 37 °C, 5% CO2, and 95% humidity for 4-12 weeks.
- Replace half the medium every 5 days.
NOTE: If organoids begin attaching to the tissue culture plate, transfer to a fresh well.
4. Kidney capsule transplantation
- Prepare sterile surgical instruments.
- Perform a subcapsular kidney transplantation of the basement membrane matrix plug:
- Anesthetize 8-week old BALB/c female recipient mouse with isoflurane following Institutional Animal Ethics Guidelines.
CAUTION: Isoflurane is a hazardous substance. Waste gas must be scavenged to prevent entry into the workspace environment. - Lay the mouse in a right lateral position.
- Shave hair from the surgery site and disinfect the skin following Institutional Guidelines.
NOTE: It is recommended that the surgical site be disinfected three times in a circular motion with alternating iodine-based or chlorhexidine-based and alcohol-based scrub. - Make a 2 cm incision in the skin perpendicular to the spine to expose the peritoneal wall.
NOTE: Fine surgical scissors or a scalpel blade can be used for skin incision. Users should follow Institutional Animal Ethics Guidelines or Standard Operating Procedures. - Make a smaller 0.5 cm incision in the peritoneal wall above the kidney.
NOTE: The incision should approximate the kidney width to ensure that the kidney remains exteriorized during transplantation. - Exteriorize the kidney through the peritoneal opening by applying downward pressure using the thumb and index finger. A pair of ring tweezers can be used to assist exteriorization. Ensure the kidney is moist throughout the procedure by regularly applying sterile PBS using a cotton swab.
- Under a stereomicroscope, pinch the perirenal fat of the kidney capsule with a pair of bent ultra-fine forceps at one pole of the kidney. Use a second pair of bent ultra-fine forceps to gently tear the kidney capsule membrane upwards in an opposing direction, creating a small opening.
- Carefully insert the prong of one forceps under the capsule membrane. Use a slow sweeping motion to separate the capsule membrane from the kidney parenchyma.
- Prepare the basement membrane matrix plug for transplantation by removing laboratory film from the pipette tip.
- Lift the kidney capsule using one prong of the forceps and insert the pipette tip through the opening, pushing it towards the opposing pole of the kidney.
- Insert a wire plunger into the pipette tip and expel the matrix plug whilst simultaneously withdrawing the pipette tip from the kidney.
- Moisten the kidney with PBS and re-internalize.
- Close the peritoneal wall with one 5-0 Vicryl suture and close the skin with two autoclips.
- Administer analgesic (Buprenorphine at 0.05-0.1 mg/kg subcutaneously or following Institutional Animal Ethics Guidelines).
CAUTION: Buprenorphine is a hazardous substance and must be handled with appropriate personal protective equipment.
- Anesthetize 8-week old BALB/c female recipient mouse with isoflurane following Institutional Animal Ethics Guidelines.
- Turn off the flow of isoflurane but keep the oxygen flow on to allow the mouse to breathe pure oxygen until it starts gaining consciousness.
- Return the mouse to its cage. Keep the animal warm during recovery by partially placing the cage on top of a heating pad or under a heat lamp, and monitor until the mouse fully recovers from anesthesia.
- Monitor post-surgical recovery for the first two weeks or as required by Institutional Guidelines.
Representative Results
Cell aggregation is important for promoting cell-to-cell contact and signaling. Encasing cell aggregates inside the basement membrane matrix supported both 3-dimensional cultures for in vitro tissue organoid formation and facilitated the mechanical delivery of cells into the kidney capsule for graft transplantation. To establish these constructs, the basement membrane matrix was first maintained in a fluidic state under ice-cold conditions. Cell aggregation was subsequently achieved by layering a concentrated cell suspension above and using centrifugal force to push cells through the high-density matrix. Optimization of the centrifugation speed (200-2000 x g) was required to achieve a mid-layer cell positioning (Figure 1A), which was also dependent on the cell number. Higher centrifugal forces (>500 x g) propelled cells to the very tip of the pipette (Figure 1B), and caution is required for smaller cell numbers (i.e., <5 x 105) that may be lost during the removal of the flexible laboratory film covering. Conversely, cells may not travel adequately through the basement membrane matrix if G-forces are too low (≤200 x g) (Figure 1C). Centrifugation speed for cell numbers <1 x 105 or >2.5 x 106 may need further optimization. Following centrifugation, cells were set inside the basement membrane matrix by warming the construct at 37 °C for 15 min. This solidification process enabled the matrix "plug" to be ejected entirely (Figure 1D) using a thin wire plunger.
In vitro spleen organoid formation
Basement membrane matrix plugs ejected into an appropriate tissue culture vessel reproducibly formed 3-dimensional organoid-like structures which could be maintained following standard tissue culture techniques and conditions (37 °C, 5% CO2, 95% humidity)11. In culture, the supporting basement membrane matrix substrate gradually dissipated between days 0 and 14 (Figure 2A, i-iii) and could no longer be observed by day 21, leaving an intact organoid-like spherical cell mass (Figure 2A, vi) measuring approximately 545 µm (s.d. = 120 µm, n = 6)11 in diameter. These organoid structures were supported in culture beyond 30 days but did not increase in size over time (Figure 2B). Spleen organoids were composed of CD45–TER-119– stromal, CD45–TER-119+ erythrocyte, and CD45+TER-119– lymphoid cells (Figure 2C), however, the frequency of each population was variable across individual organoids (n = 4; Table 1). To assess the general structure of spleen organoids, 50 µm thick tissue cryosections were prepared and visualized. Organoids were comprised of a non-hollow structure, with cells present across the entire diameter of the tissue (Figure 2D). Assessment of organoid sections at 7 µm single cell layer thickness revealed that cells were arranged in cord-like structures without a clear spatial orientation (Figure 2D), with areas void of nuclei between strings of cells. CD45 antibody staining verified the presence of nucleated hematopoietic cells which densely surrounded peripheral regions of the organoid (Figure 2E). In addition, CD45+ cells of distinct, spindle-shaped morphology were observed in more central organoid regions. A general absence of CD90.2 (T cell) and CD19 (B cell) antibody staining, but positive CD11b staining, demonstrated the specific presence of myeloid cells (Figure 2F). Non-hematopoietic CD105+ and CD31+ endothelial cells were also detected in multiple organoids11.
In vivo spleen graft transplantation
The encapsulation of aggregated spleen stromal cells within a semi-solid matrix facilitates animal transplantation studies. This technique was used to embed unfractionated or CD45–TER-119– FACS sorted neonatal spleen stromal cell preparations inside a basement membrane matrix plug, serving as a vehicle for transplantation under the mouse kidney capsule. In line with similar cell-aggregation protocols8, basement membrane matrix encapsulated cell constructs (MECCs) successfully regenerated spleen tissue in 4/5 independent animal transplantations, thereby confirming the viability of this graft construction technique. To test the utility of MECCs in defining cell types required for spleen regeneration, MECC grafts were constructed from neonatal spleen cells which were specifically depleted of PDGFRβ+MAdCAM-1+ or PDGFRβ+MAdCAM-1– stromal (CD45–) cells11. Grafts lacking PDGFRβ+MAdCAM-1+ cells retained the capacity for gross tissue regeneration (4/4 grafts; Figure 3A)11. Three out of four regenerated tissues exhibited normal spleen cell composition and structure, displaying central arterioles, white pulp follicles, segregated T and B cell compartments, follicular dendritic cells, marginal zone reticular cells, and marginal metallophilic macrophages, red pulp sinusoids, myeloid cells and macrophages (Figure 3B)11. In contrast, grafts that lacked PDGFRβ+MAdCAM-1– cells largely failed to regenerate spleen tissue (1/4 grafts)11. These data support the importance of graft-derived PDGFRβ+ cells in spleen tissue regeneration8, and pinpoint a specific requirement for PDGFRβ+MAdCAM-1– stromal cells.
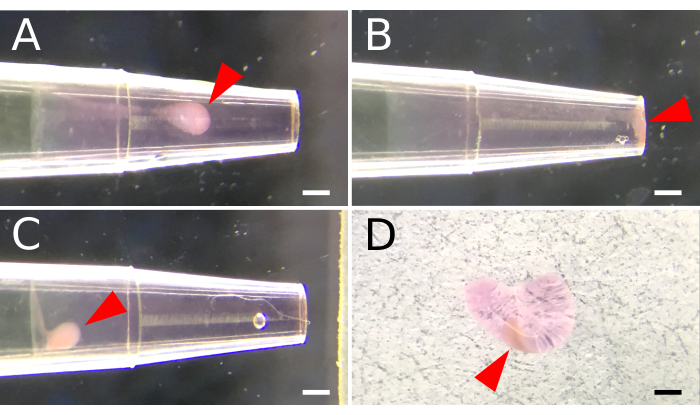
Figure 1. Basement membrane matrix encapsulation of aggregated spleen stromal cells. A 200 µL pipette tip is used to facilitate cell aggregation inside a basement membrane matrix. A fluidic matrix layer is first established by aspirating 2 µL of ice-cold basement membrane matrix into a pre-chilled pipette tip. A cell suspension is deposited above the matrix layer, and centrifugal force is applied to mobilize and aggregate cells within the basement membrane matrix. (A) Settings for centrifuge speed are optimized to position cell aggregates midway along the matrix layer. (B) Excessive centrifugal speed results in cells that aggregate at the end of the pipette. (C) Insufficient speed prohibits cell movement through the matrix. (D) A semi-solid matrix plug is ejected from the pipette tip following incubation at 37 °C for 15 minutes. Arrowheads indicate the placement of cell aggregates within the matrix. Scale Bar, 100 µm. Please click here to view a larger version of this figure.
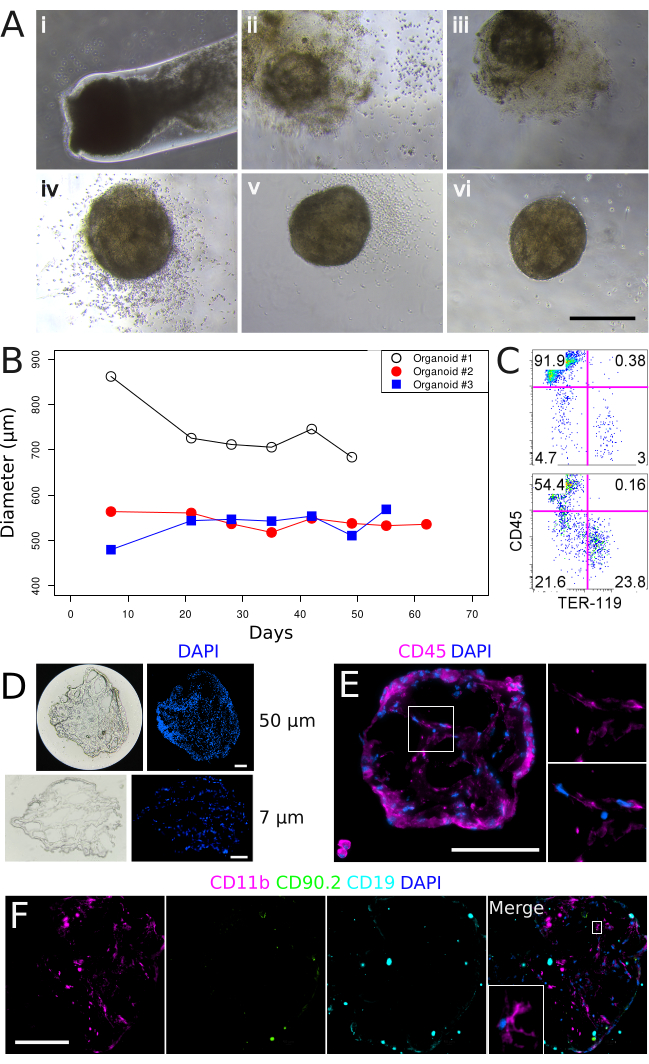
Figure 2. In vitro culture of basement membrane matrix-encapsulated spleen aggregates and the formation of 3-dimensional organoid structures. (A) Spleen organoid development over a 35-day time course. Panels (i-vi) correspond to Days 0, 7, 14, 21, 28, and 35 in culture. Images were captured on a Nikon TS2 Inverted Phase Contrast Microscope. Scale bar, 500 µm. (B) Spleen organoid diameter over extended culture periods. Each line represents an individual organoid. (C) Flow cytometry characterization of stromal (CD45–TER-119–), lymphoid (CD45+TER-119–) and erythroid (CD45–TER-119+) cell populations after 53 days in culture. Upper panel: Control tissue prepared from neonatal spleen stroma. Lower panel: Spleen organoid, Day 52. (D) Organoid tissue gross morphology at 50 µm (upper panels) and 7 µm (lower panels) section thicknesses after 22 days in culture. (E) Localization and morphology of CD45+ hematopoietic cells after 34 days in culture. Magnified region is shown in right panels. Sections are 7 µm. (F) Assessment of myeloid (CD11b), T (CD90.2) and B (CD19) cells after 34 days in culture. Magnified region is shown in inset. Sections are 7 µm. Images were captured using a Nikon Eclipse Ti2-E Live Cell Microscope. Scale bars (D, E, F), 100 µm. Please click here to view a larger version of this figure.
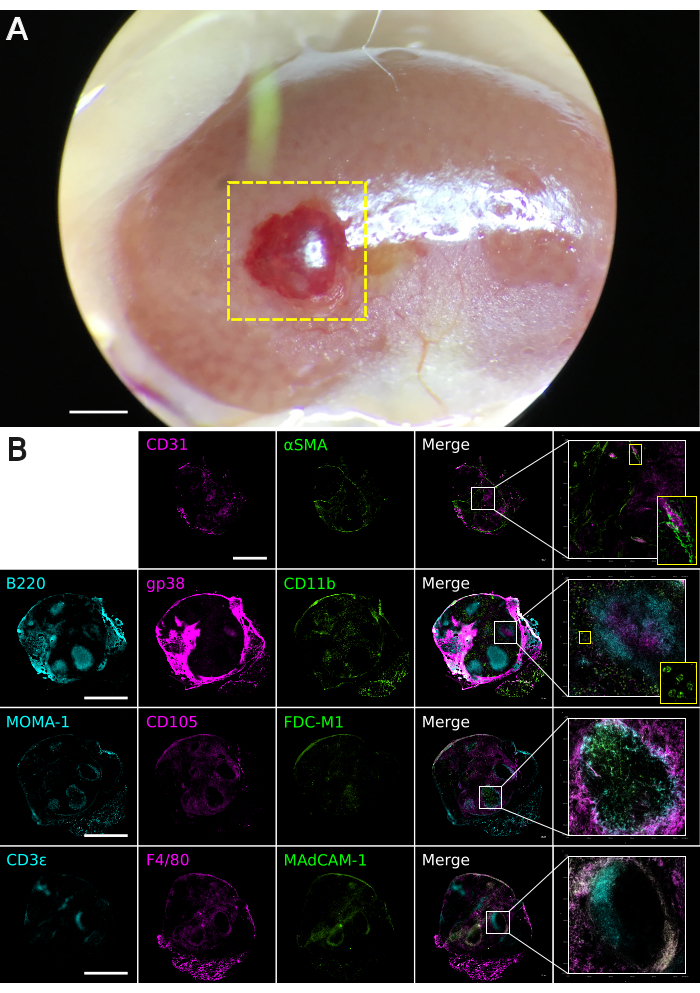
Figure 3. Basement membrane matrix graft tissue analysis. Grafts were constructed from neonatal spleen stroma depleted of PDGFRβ+MAdCAM-1+ cells (n = 4). Additional data available online11. (A) Macroscopic appearance of a regenerated spleen graft at 4 weeks post-transplantation under the kidney capsule. The boxed yellow area indicates regenerated spleen tissue. The image was captured using a Leica M60 Stereomicroscope equipped with a Snap zoom adapter and an Apple iPhone 6S. Scale bar, 1000 µm. (B) Composite multi-color immunofluorescence images of 30 µm-thickness graft tissues cryosectioned on the coronal plane. Antibody staining was performed with indicated markers to visualize spleen tissue micro-architecture. Images were captured using a Nikon Eclipse Ti2-E Live Cell Microscope. Boxed areas show regions of higher magnification. Scale bar, 1000 µm. Please click here to view a larger version of this figure.
| Organoid No. | Stromal cells | Erythrocytes | Lymphocytes |
| 1 | 62 | 7.7 | 31 |
| 2 | 22 | 24 | 55 |
| 3 | 3.6 | 0.1 | 96 |
| 4 | 10 | 76 | 12 |
Table 1. Percentage of stromal, erythroid and lymphoid cell populations amongst individual spleen organoids.
Supplementary figure 1. Schematic overview of the protocol highlighting the major steps in generating matrix embedded cell constructs for in vitro and in vivo studies. Please click here to download this File.
Discussion
The aggregation of neonatal spleen cells inside a semi-solid medium represents a viable method for generating spleen constructs. Similar basement membrane matrix-based protocols have been used to initiate three-dimensional spleen cultures10. Here, we demonstrate that spleen constructs are equally amenable to in vitro organoid culture systems as well as to in vivo transplantation models. Of note, the transplantation of in vitro cultured spleen organoids has not yet been tested but it would be of interest to determine the capacity for full or partial spleen tissue regeneration. This protocol also inherits cell aggregation techniques described in previous spleen transplantation studies8. These studies involved loading cell aggregates over a collagen sheet followed by transplantation under the kidney capsule. Encasement of cell aggregates inside the basement membrane matrix offers a distinct advantage in terms of graft orientation, where variation associated with collagen sheet-side down versus cell aggregate-side down transplantation is eliminated.
The methodology for preparing basement membrane matrix constructs is relatively straightforward. The use of high-concentration basement membrane matrix ensures that cells are initially supported in an aggregated state, before the matrix dissipates over several days. In our experience, there is minimal mixing and dilution of the basement membrane matrix following careful layering (Step 2.6) and centrifugation (Step 2.8) of a PBS-suspended cell solution. Most optimizations center around positioning the cell pellet within the matrix plug (Figure 1). This can be addressed by testing several variables such as centrifugation speed, time, basement membrane matrix volume composition, PBS-suspended cell solution volume, and pipette tip size. Cell number and characteristics (size, density) may also influence settings, and each cell preparation should be optimized individually.
Several limitations are important to highlight. Basement membrane matrix can be a temperature-sensitive product, and care should be taken to work fast to keep reagents cold. This prevents premature matrix solidification and ensures proper encapsulation of the cells. While all relevant materials are stored, prepared, or maintained in a cold environment, manual handling steps (e.g., ejecting the pipette tip, sealing with flexible laboratory film [Step 2.5]) may variably introduce external heat from the user, changing the matrix viscosity and influencing cell migration characteristics during centrifugation. Reproducibility within and between experiments may, therefore, be difficult to achieve consistently.
The second limitation is the requirement for user experience and proficiency. Certain manual techniques may require extended training to perform efficiently and successfully. For example, sealing the pipette tip with laboratory film (Step 2.5) may initially result in low success rates, but this will inevitably improve with practice. Competence with animal micro-surgical techniques is also obtained through repetition. For transplantations, care must be taken, especially when inserting the pipette tip underneath the kidney capsule (Step 4.2.10), inserting the wire plunger to eject the plug (Step 4.2.11), and withdrawing the tip from the kidney. It is important that the plug is ejected gently and steadily whilst the pipette tip is simultaneously withdrawn. Excess movement may result in the kidney capsule becoming perforated or the parenchyma becoming damaged, leading to excessive bleeding.
In summary, a process for encapsulating cells within a semi-solid matrix has been described here with a focus on understanding spleen tissue formation. This system could equally be applied to a wide range of cell types with the potential for multiple in vitro or in vivo downstream applications.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the National Health and Medical Research Council of Australia (#GNT1078247).
Materials
| 96 Well Polypropylene 1.2 mL Cluster Tubes | Corning | CLS4401 | For placing inside a 14 ml conical tube |
| B-mercaptoethanol | Gibco | 21985023 | Stock 55 mM, use at 50 uM |
| Collagenase D | Roche | 11088858001 | |
| Collagenase IV | Sigma-Aldrich | C5138 | From Clostridium histolyticum |
| Deoxyribonuclease I (DNase I) | Sigma-Aldrich | D4513 | Deoxyribonuclease I from bovine pancreas,Type II-S, lyophilized powder, Protein ≥80 %, ≥2,000 units/mg protein |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Sigma-Aldrich | D5796 | 4500 mg/L Glucose, L-Glutamine, and Sodium Bicarbonate, without Sodium Pyruvate, Liquid. Sterile Filtered. |
| Dulbecco's Phosphate Buffered Saline (PBS) | Sigma-Aldrich | D8537 | Without calcium chloride and magnesium chloride, sterile-filtered |
| Eclipse 200 μl Pipette Tips | Labcon | 1030-260-000 | Bevel Point |
| Fetal Bovine Serum (FBS) | Gibco | 26140-079 | Lot# 1382243 |
| GlutaMAX | Gibco | 35050061 | Stock 100X, use at 1X |
| Matrigel | Corning | 354263 | Matrigel matrix basement membrane High Concentration, Lot# 7330186 |
| MEM Non-essential Amino Acids | Gibco | 11140076 | Stock 100X, use at 1X |
| Penicillin/Streptomycin | Gibco | 15140122 | Stock 10,000 units/ml Penicillin, 10,000 ug/ml Streptomycin |
| Reversible Cell Strainer | STEMCELL Technologies | 27216 | 70 μm |
| Ring Tweezers | NAPOX | A-26 | Ring size: 3 mm |
| Rock Inhibitor (Y-27632) | MedChemExpres | HY-10071 | |
| Thermofisher Heraeus Megafuge 40R Centrifuge | Thermofisher | Acceleration and deceleration speeds were set to 8 | |
| Ultra Fine Tweezers | EMS | 78340-51S | Style 51S. Antimagnetic/anti-acid SA low carbon austenitic steel tweezers are corrosion resistant. Anti-glare satin finish. |
| Vicryl 5/0 Suture Ligapak Reel | Ethicon | J283G | |
| Wiretrol II Long Wire Plunger | Drummond | 5-000-2002-L | Stainless Steel Plunger, 25 & 50 μL/WRTL II, Long |
| Wound Clip Applier | MikRon | 427630 | |
| Wound Clips | MikRon | 427631 | 9 mm |
References
- Jarcho, S., Andersen, D. Traumatic autotransplantation of splenic tissue. American Journal of Pathology. 15 (5), 527-546 (1939).
- Storsteen, K. A., ReMine, W. Rupture of the spleen with splenic implants: splenosis. Annals of Surgery. 137 (4), 551-557 (1953).
- Mizrahi, S. Posttraumatic autotransplantation of spleen tissue. Archives of Surgery. 124 (7), 863 (1989).
- Miko, I., et al. Spleen autotransplantation. Morphological and functional follow-up after spleen autotransplantation in mice: A research summary. Microsurgery. 27 (4), 312-316 (2007).
- Grikscheit, T. C., et al. Tissue-engineered spleen protects against overwhelming Pneumococcal sepsis in a rodent model. Journal of Surgical Research. 149 (2), 214-218 (2008).
- Pabst, R., Westermann, J., Rothkotter, H. Immunoarchitecture of regenerated splenic and lymph node transplants. International Review of Cytology. 128, 215-260 (1991).
- Tan, J. K. H., Watanabe, T. Murine spleen tissue regeneration from neonatal spleen capsule requires lymphotoxin priming of stromal cells. The Journal of Immunology. 193 (3), 1194-1203 (2014).
- Tan, J. K. H., Watanabe, T. Stromal cell subsets directing neonatal spleen regeneration. Scientific Reports. 7 (1), 40401 (2017).
- Gee, K., et al. Spleen organoid units generate functional human and mouse tissue-engineered spleen in a murine model. Tissue Engineering Part A. 26 (7-8), 411-418 (2020).
- Ueno, Y., et al. Transcription factor Tlx1 marks a subset of lymphoid tissue organizer-like mesenchymal progenitor cells in the neonatal spleen. Scientific Reports. 9 (1), 20408 (2019).
- . Online data repository: Matrigel encapsulated cell aggregation for investigating murine spleen tissue formation Available from: https://osf.io/ehrbw/?view_only=7d6a8e05c84144d3a12d36ffe7f94f01 (2023)

