Preparing a Mice Model of Severe Acute Pancreatitis via a Combination of Caerulein and Lipopolysaccharide Intraperitoneal Injection
Summary
Intraperitoneal drug administration is a safe and effective non-invasive approach for inducing pancreatic injury. This study compared five distinct intraperitoneal injection protocols on mice to induce varying degrees of pancreatic injury and established a model of severe pancreatic injury to investigate the pathological changes and treatment strategies for severe acute pancreatitis (SAP).
Abstract
The treatment of severe acute pancreatitis (SAP), with high mortality rates, poses a significant clinical challenge. Investigating the pathological changes associated with SAP using animal models can aid in identifying potential therapeutic targets and exploring novel treatment approaches. Previous studies primarily induced pancreatic injury through retrograde bile duct injection of sodium taviaurocholate, but the impact of surgical damage on the quality of the animal model remains unclear. In this study, we employed various frequencies of intraperitoneal Caerulein injections combined with different doses of LPS to induce pancreatic injury in C57BL/6J mice and compared the extent of injury across five intraperitoneal injection protocols. Regarding inducing acute pancreatitis in mice, an intraperitoneal injection protocol is proposed that results in a mortality rate as high as 80% within 5 days. Specifically, mice received ten daily intraperitoneal injections of Caerulein (50 µg/kg), followed by an injection of LPS (15 mg/kg) one hour after the last Caerulein administration. By adjusting the frequency and dosage of injected medications, one can manipulate the severity of pancreatic injury effectively. This model exhibits strong controllability and has a short replication cycle, making it feasible for completion by a single researcher without requiring expensive equipment. It conveniently and accurately simulates key disease characteristics observed in human SAP while demonstrating a high degree of reproducibility.
Introduction
Severe acute pancreatitis is characterized by rapid onset, rapid progression, and high mortality rates within the digestive system disease domain1. Its high fatality rate has always been a prominent focus of clinical research. Due to unpredictable changes in clinical conditions, heterogeneity of disease manifestations, and limited availability of human specimens, establishing animal models has become increasingly crucial for disease research.
Retrograde injection of sodium taurocholate into the common bile duct is commonly used to create a rat model of SAP2. By simulating pancreaticobiliary obstruction and inducing reflux of bile and pancreatic fluid, this modeling technique exhibits a high success rate in replicating SAP animal models. However, it should be noted that invasive surgery does have an impact on the animal model itself. Furthermore, this method is limited to larger animals, such as rats and dogs, which are primarily used as experimental subjects. Alternative techniques, including duodenal intubation3, direct duodenal puncture4, and direct puncture of the bile duct-pancreatic duct5, are frequently utilized for modeling purposes.
Intraperitoneal injection and dietary modeling methods offer non-invasive advantages that can be applied to animals of any size. The mouse model of SAP induced by feeding choline-deficient-ethionine (CDE)6 presents certain complications, such as poorly controllable hyperglycemia and hypocalcemia, making it unsuitable for evaluating new diagnostic and therapeutic approaches. On the other hand, intraperitoneal injection of Caerulein combined with L-arginine7 represents the most commonly employed method for inducing acute pancreatitis in mice. Specifically, repeated intraperitoneal administration of Caerulein-a cholecystokinin analog-provides a highly suitable approach for investigating various aspects related to this destructive disease, including pathogenesis, inflammation, and regeneration processes. Due to its structural similarity to cholecystokinin (CCK), Caerulein effectively stimulates gallbladder contraction and pancreatic enzyme secretion, leading to an imbalance in enzyme secretion followed by subsequent self-destruction8. Lipopolysaccharide (LPS), being ubiquitous and extensively studied as a pathogen-associated molecular pattern molecule, can be combined with Caerulein via intraperitoneal injection to establish an effective mice model of SAP. This combination rapidly triggers and releases a significant number of inflammatory cytokines, resulting in excessive local and systemic inflammation. Several studies have reported the induction of SAP models in mice through intraperitoneal injection of Caerulein combined with LPS. This may be attributed to the fact that intraperitoneal injection of Caerulein can cause pancreatic edema and hemorrhage in mice, while the addition of LPS can immediately induce pancreatic necrosis and exacerbate systemic inflammatory response, sepsis and even organ failure. Currently, there is variation in the dosage and frequency of intraperitoneal Caerulein injections as well as inconsistency in additional LPS dosage. Achieving consistency in mouse SAP models is challenging9,10,11,12; therefore, it is necessary to establish a standardized protocol for obtaining an ideal model. In this article, we describe a protocol for intraperitoneal injection in mice and investigate the optimal injection frequency and additional dosage of LPS.
Protocol
This protocol was reviewed and approved by the Ethics Committee at The First Affiliated Hospital of Anhui University of Science and Technology (Huainan, China) (Ethics Code: 2023-KY-905-001). The study followed the National Institutes of Health guidelines for the care and use of research rodents in all animal procedures. C57BL/6J adult mice weighing 20-30 g were used for the present study. The mice were housed in an animal laboratory for one week under controlled conditions (approximately 21 °C with a 12 h alternating day-night cycle). The mice had ad libitum access to food and water throughout. The details of the reagents and equipment used in the study are listed in the Table of Materials.
1. Animal preparation
- Assign 84 healthy C57BL/6J mice to six groups, including the control group, Pancreatic injury (PI) I, PI II, PI III, PI IV, and PI V.
- Prior to initiating the modeling procedure, label each group of mice with ear notches and allow them to fast for 12 h.
2. Preparation of induced drug diluent
- Dissolve the Caerulein (1 mg) into 1 mL of PBS and refrigerate at -20 °C.
- Measure and record the weight of the experimental mice 1 h before the intraperitoneal injection of the drug.
- Extract the total mass of Caerulein required at a ratio of 50 µg/kg, based on the total weight of all mice.
- Dilute the obtained Caerulein drug with PBS again.
NOTE: The total volume of PBS dilution should be equivalent to 5 times the total weight value of all mice.The same dilution method was used to obtain LPS diluent.
3. Intraperitoneal injection
NOTE: Intraperitoneal injections were administered to each group of mice according to the protocol outlined in Supplementary Table 1 to induce the model. An additional 10 mice were grouped and treated observe the 7-day survival rates.
- Grab and hold the mice in a way that the belly of the mice is facing up and the head is positioned lower than the tail to prevent damage to the organs when inserting the syringe.
- Disinfect the mice's abdomen using 75% alcohol cotton balls.
- Hold the syringe in the right hand (using a needle measuring ≤0.5/≥0.3) and insert the needle into the subcutaneous skin on either the left side of the abdominal white line.
NOTE:Change to the right side for the next injection. - Once the needle has reached the subcutaneous layer, move it forward approximately 3-5 mm, and then insert the syringe needle into the abdominal cavity at a 45° angle. At this point, one should feel some resistance.
- Keep the needle stationary and inject the substance slowly.
NOTE: Do not exceed 5 times the weight value of the mice as the volume of a single intraperitoneal injection. - After the injection, pull out the needle and gently massage and press the injection site with a sterile cotton swab to fully diffuse the drug into the mice's abdominal cavity.
- Use a new syringe to repeat with the next experimental mice.
4. Open-field behavioral ability testing
NOTE: 12 h after the last intraperitoneal injection, open-field behavioral ability testing was conducted to assess the total activity distance and immobility time of the mice.
- Place four identical white boxes directly beneath the camera.
- Connect the camera to the computer using a video cable.
- Ensure the experimental box is clean and free from any odors before starting the experiment.
- Position the animal in the middle of the grid, facing away from the experimenter, and allow it to adapt to the environment for 10 min.
- Start the video tracking software and click on the File menu to create a new experiment.
- Set up the monitoring for four simultaneous fields of view.
- Mark the length and width of the experimental object that can move within the box in the real-time monitoring screen (30 cm × 30 cm).
- Once the setup is complete, begin video surveillance and recording.
- Record the activity of the mice for 15 min.
- After the testing, remove the animal from the grid and return it to its cage. Thoroughly clean the grid using a sterilant containing chlorine dioxide.
5. Collecting and testing the peripheral blood of mice
- Euthanize the mice (following institutionally approved protocol).
- Perform euthanasia on the experimental mice 36 h after the intraperitoneal injection. Measure and record the body weight of the mice prior to euthanasia.
NOTE: Administer 0.18 mL of 10% chloral hydrate intraperitoneally, ensuring no reaction to stimulation of the toe or tail. - With the left hand, secure the mice, and with the right hand, hold the scissors to trim the whiskers on one side of the mice.
- Gently press the skin around the eye to induce congestion and protrusion of the eyeball.
- Use curved forceps to grasp the eyeball and quickly remove it, collecting peripheral blood in a microcentrifuge tube.
NOTE: Two different types of microcentrifuge tubes were used, one containing anticoagulant and the other without. - Simultaneously, lightly press the mice's heart area with the middle finger of the left hand to increase the pumping speed of the heart.
- Perform euthanasia on the experimental mice 36 h after the intraperitoneal injection. Measure and record the body weight of the mice prior to euthanasia.
- Leave the peripheral blood without anticoagulant at room temperature for 30 min.
- Measure the concentrations of serum amylase (Amy) and lipase (Lip) using an automated biochemical analyzer through the enzyme rate method and enzyme circulation method (following the manufacturer's instructions).
- Determine the levels of inflammatory indicators such as Pro Calcitonin (PCT) in plasma using the chemiluminescence method using a commercial chemiluminescence imager (following the manufacturer's instructions).
- Use ELISA kits to measure HMGB-1, IL-6, and TNF-α levels in mice serum (following the manufacturer's instructions).
- Analyze the peripheral blood treated with anticoagulant using a fully automatic blood cell analyzer to assess the relevant parameters.
6. Collecting the pancreatic tissue and preparing a paraffin section
- Place the mice in a supine position and secure them on a foam plate.
- Shave and disinfect the area, then make an abdominal median incision and flip the small intestine tube to the right to fully expose the pancreas.
- Disconnect the duodenum and pyloric duct and locate the small intestine beneath the pancreas. Fully free the pancreatic tissue along the intestinal duct.
- Use toothless forceps to clamp the spleen and gently pull upwards.
NOTE: Do not directly touch the pancreatic tissue, and avoid using excessive force during the entire dissociation process. - Sharply dissect the posterior pancreatic ligament tissue up to the head of the pancreas and disconnect the bile duct and blood vessels.
- Remove the pancreatic tissue, pat dry the surface moisture with absorbent paper, weigh and record.
- Fix half of the pancreatic tissue with 4% paraformaldehyde and store the other half in a refrigerator at -80 °C.
- Prepare the pancreatic paraffin sections following the steps below.
- Clean the fixed pancreatic tissue with 75% alcohol and trim it to a size of approximately 0.5cm × 0.5cm.
- Immerse the tissue in 70% ethanol for 20 min, 80% ethanol for another 20 min, and 90% ethanol for 15 min.
- Treat the tissue twice with 95% ethanol for 15 min each time and then twice with 100% ethanol for 5 min each time.
- Subject the tissue to two rounds of 12 min and 5 min treatments with xylene solution for transparency.
- Soak the transparent tissue in a wax tank at 65 °C for 1 h.
- Embed the tissue in melted paraffin and allow it to cool. Obtain pancreatic paraffin sections with a slicer (thickness: 5 µm).
- Flatten the obtained paraffin slices in 45 °C water, mount them, and dry them (baking conditions: 40 °C for 14-16 h).
7. Hematoxylin and Eosin (H&E) staining
- Place the pancreatic paraffin sections in xylene I and II for 30 min, then in anhydrous, 95%, 85%, and 75% alcohol for 5 min each, and ultrapure water for 5 min.
- Stain the cell nucleus with hematoxylin for 160 s, then rinse with running water slowly.
- Apply a hydrochloric acid alcohol differentiation solution for 5-10 s, then rinse with running water quickly.
- Treat the sections with anhydrous alcohol for 5 min.
- Incubate the cytoplasm with eosin for 30 s, then rinse with running water.
- Immerse the sections in 75%, 85%, 95%, and 100% ethanol for 10 s each, then treat with xylene for 5 min to dehydrate.
- Finally, seal the sections with neutral resin and observe under a microscope.
- Perform the pathological scoring and standards13.
NOTE: Determine the severity of pancreatitis based on indicators such as edema, acinar necrosis, bleeding, hemorrhage and fat necrosis, and inflammatory and perivascular inflammation.Use the previously published implementation guidelines for scoring13.
8. Immunohistochemical staining
- Dewax the paraffin-embedded sections of pancreatic tissue in xylene and subsequently rehydrate them in a gradient of ethanol solutions.
- Carry out endogenous enzyme-blocking using 3% H2O2 for 20 min after a 30 min membrane rupture.
- Block the antibody with 5% bovine serum at room temperature for 30 min, then incubate overnight at 4 °C with a 1:1500 dilution of rabbit anti-HMGB1.
- Rinse the sections with PBS, then incubate them in corresponding secondary antibodies for 30 min at 37 °C. After that, carry out DAB color development, hematoxylin counterstaining, and seal with neutral gum.
9. TUNEL method for detecting apoptosis in pancreatic sections
- Dewax the pancreatic paraffin sections in xylene for 5 min, repeat twice and wash with gradient ethanol (100% for 5 min, 90% for 2 min, 70% for 2 min, and distilled water for 2 min).
- Wash away excess liquid surrounding the paraffin sections using PBS. Treat each sample with 100 µL of Proteinase K, ensuring complete coverage of the tissue. Incubate the samples at 37 °C for 20 min. Subsequently, soak the samples in PBS 3 times, each time for 5 min.
NOTE: The Proteinase K solution was prepared by diluting the original Proteinase K (200 µg/mL) solution with PBS at a ratio of 1:9 (volume), resulting in a final concentration of 20 µg/mL, excluding DNase. Thorough washing of Proteinase K is essential to avoid interference with subsequent labeling reactions. - Add a suitable amount of 3% H2O2 (diluted by PBS) onto the tissue to fully infiltrate it and incubate for 20 min. Rinse the tissue with PBS 3 times for 5 min each.
NOTE: The paraffin sections should be kept moist. To inactivate the endogenous peroxidases in the tissue, the incubation time should not be too long to prevent false positives due to DNA breakage caused by 3% H2O2. - Cover the entire area of the sample to be tested with 50 µL of Equilibration buffer and incubate for 10 min.
- Remove as much Equilibration buffer as possible. Then, add 56 µL of TdT Incubation buffer to each tissue sample and incubate for 1 h (at room temperature).
NOTE: The slide should not be allowed to dry, and exposure to light should be avoided. TdT Incubation buffer was prepared according to the manufacturer's instructions (Recombinant TdT enzyme: Biotin-dUTP Labeling Mix: Equilibration Buffer = 1 µL: 5 µL: 50 µL). - Wash the tissue samples with PBS immediately. Rinse them 4 times for 5 min each. Gently remove any excess PBS solution around the samples with filter paper.
- Add 100 µL of previously diluted Streptavidin-HRP reaction solution (Streptavidin-HRP: TBST = 1: 300) to each tissue sample for the Streptavidin-HRP reaction. Incubate for 30 min. Then, wash the samples with PBS and rinse them 3 times for 5 min each.
- Perform DAB staining by adding 50 µL of DAB to each paraffin section. Observe the staining under a microscope in real-time. After positive staining appears, immediately place the slides in a humid box. Stop the reaction by washing it with pure water.
- Immerse the slides in hematoxylin staining solution for 3-5 min, then rinse with pure water.
- Differentiate in hematoxylin differentiation solution for about 2 s, followed by immediate rinsing with pure water.
- Achieve blue coloration using hematoxylin rebluing solution for a few seconds, and rinse the slides clean with pure water.
NOTE: Conduct microscopic examination after nuclear staining. If the staining is too dark, return the slides to the differentiation solution. If the staining is too light, restart the staining process from the nuclear staining step. - Dehydrate the samples with 4 rounds of fresh anhydrous ethanol for 5 min each. Then, soak them in butanol for 5 min and in xylene for 5 min. Finally, use fresh xylene for another 5 min.
- Mount the slides using neutral gum. Allow them to air-dry naturally or dry them in a 60 °C oven.
- Perform histological examination using a white light microscope. Apoptotic nuclei will appear brown.
- Calculate the corresponding apoptotic index (AI).
NOTE: Observe the slides in a double-blind manner. In TUNEL-positive slides, randomly select 5 positive areas under high magnification (400x), and count at least 100 acinar cells in each area to determine the percentage of positive cells. AI = (total number of apoptotic cells/total number of cells) × 100%.
10. Flow cytometry
- Obtain fresh pancreatic tissues and thoroughly wash them after perfusing with PBS.
- Prepare a 0.5% collagenase IV digestion solution and use sterile tissue scissors to adequately digest the pancreatic tissues.
- Add a 5% BSA digestion termination solution depending on the condition of the pancreatic fragments and liquid turbidity, and centrifuge the mixture at low temperature for 5 min (~300 x g, 4 °C).
- Discard the supernatant to terminate the digestion.
- Prepare a cell culture medium containing phenyl methane sulfonyl fluoride (PMSF) and 2.5% fetal bovine serum cell suspension to resuspend the pancreatic cells.
- Filter the pancreatic acinar cell suspensions through a 200-mesh nylon mesh to obtain cell suspensions through cell filtering.
- Centrifuge the pancreatic acinar cell suspension at low temperature (follow the conditions as mentioned in step 10.3).
- Discard the supernatant.
- Wash the cells with pre-chilled PBS.
- Gently resuspend the cells in pre-chilled 1x binding buffer through centrifugal resuspension.
- Label the pancreatic acinar cells with Annexin V-FITC/PI according to the manufacturer's instructions after adjusting the cell concentration.
- Use flow cytometry within 1 h to detect apoptosis in pancreatic acinar cells.
11. Western blot detection of Caspase-3 and HMGB-1
- Extract the pancreatic protein.
- Extract 50 mg of pancreatic tissue and cut it into small fragments.
- Add 1 mL of RIPA lysis buffer (containing PMSF and phosphorylated protease inhibitor).
- Homogenize the pancreatic tissue on ice for 5 min using an electric grinder.
- Incubate the homogenized tissue on ice with gentle shaking for 2 h.
- Centrifuge the homogenate at 4 °C for 10 min at 4,500 x g.
- Collect the supernatant solution and store it at -80 °C.
- Perform protein quantification.
- Dilute a certain volume of BSA standard sample (25 mg/mL) to a concentration of 0.5 mg/mL.
- Prepare a specific amount of BCA working solution by mixing 50 volumes of BCA solution A with 1 volume of BCA solution B, ensuring thorough and even mixing.
- Place the BSA standard sample into the standard sample holes on a 96-well plate in the order of 0, 1, 2, 4, 8, 12, 16, and 20 µL. Balance the volume of each hole with distilled water, resulting in a total volume of 20 µL.
- Add 2 µL of samples into the sample holes, then sequentially add 18 µL of distilled water for dilution (1:9).
- Fill each well with 200 µL of BCA working solution and allow it to stand at 37 °C for approximately 20-30 min.
- Measure the absorbance at a wavelength of 562 nm. Calculate the protein concentration of the samples based on the standard curve.
- Add a specific volume of RIPA cracking solution and diluting buffer to dilute the samples until the concentration reaches 5-10 µg/µL, which is considered appropriate.
- Store the samples at -20 °C after protein denaturation (100 °C; 10 min).
- Perform the Western blotting.
- Make preparations for SDS-PAGE.
- Install the glue-making molds and inspect their sealing performance.
- Add TEMED to the 10% separation adhesive and mix evenly.
- Inject the mixture into the mold, accounting for approximately two-thirds of the volume, while avoiding bubbles.
- Add isopropanol to the mold and press the adhesive for about 40 min.
- Use the same program to configure the 5% concentrated adhesive.
- Pour out isopropanol and add the adhesive immediately.
- Place the comb vertically until the glue completely polymerizes.
- Perform the electrophoresis operation.
- Add the sample to be tested in the expected experimental control order, in reverse order, into the sampling holes of the SDS-PAGE adhesive.
- Simultaneously add the Protein Mark to determine the location of the target protein and internal reference protein.
- Place the sample in the electrophoresis tank after the sample addition is completed.
- Initially, run at 80 V for 30 min, followed by running the gel to separate the protein at 100 V for 60 min.
NOTE: Attention should be paid to the position of bromophenol blue to avoid excessive electrophoresis.
- Perform the protein transfer.
- Cut the PVDF film at the top right corner to serve as a mark.
- Place the PVDF film in a methanol solution for 5-10 s to activate.
- Soak the PVDF film in an electrophoresis buffer for approximately 15 min.
- Carefully remove the gel after electrophoresis and trim excess gel, ensuring the gel remains wet throughout the process.
- Assemble the splint in the following order: negative plate → sponge → three layers of filter paper → gel → PVDF film → three layers of filter paper → sponge → positive plate.
NOTE: Ensure there are no bubbles between the gel and PVDF film. - Place the assembled clamp into the wet rotating groove (black to black) with ice cubes positioned around it.
- Turn on the power and initiate the film transfer at 400 mA for 100 min.
- Perform PVDF film sealing, and incubation of primary and secondary antibodies.
- Gently remove the PVDF film after the transfer and rinse with TBST for 10 min, repeating the process 3-5 times.
- Seal the PVDF film in 5% skim milk for 2 h.
- Remove the PVDF film and rinse with TBST for 10 min, repeating the process 4 times.
- Place the PVDF film and diluted primary antibody together in a refrigerator at 4 °C with gentle shaking and incubate overnight.
- Remove the PVDF film the next day and rinse with TBST for 10 min, repeating the process 4 times.
- Incubate the PVDF film in HRP-labeled secondary antibody diluent for approximately 2 h.
- Rinse the PVDF film with TBST for 10 min, repeating the process 4 times.
- Expose the film and analyze it.
- Apply the prepared ECL solution onto the film evenly.
- Expose the film in a dark room with an exposure meter for approximately 2-3 min and then store it.
- Conduct the analysis using image J software.
- Make preparations for SDS-PAGE.
Representative Results
The process of experimental mouse modeling is illustrated in Figure 1. After 12 h of injection completion, an open-field video recorder was used to monitor the movement distance and immobility duration of different experimental groups of mice for 5 cycles (Figure 2A). During the 5 cycles, mice in the PI V group maintained a low level of movement distance within 3 min, while the immobility ratio within 3 min increased with each subsequent cycle (Figure 2B,C). Additionally, statistical analysis was conducted on the total movement distance of mice from different experimental groups during the 5 cycles. The PI V group showed the smallest total movement distance compared to the other experimental groups, and the difference was statistically significant (p < 0.001) (Figure 2D,E). With the exception of the control group and PI I group, the mice in the other experimental groups exhibited negative growth in D-Value weight. Among them, the PI V group showed the greatest change in weight, and the difference in weight change compared to the other experimental groups was statistically significant (Figure 2F). After evaluating the survival rate of an additional 10 mice in each experimental group, the results showed that the mortality rate of mice in group PI V reached 80% on the 5th day. However, there was no statistically significant difference in mortality rate between the other four experimental groups and the control group mice (Figure 2G).
Using a high-power microscope, significant cellular swelling, necrosis, and inflammatory cell infiltration were observed in the PI IV and PI V groups of mice (Figure 3A,B). Using the rating criteria as provided in Supplementary Table 2, the pancreatic pathology of different experimental groups of mice was evaluated, and observed significant differences in the pancreatic pathology score compared to the control group mice (p < 0.001) (Figure 3C; Supplementary Figure 1). In addition, compared to the control group,the levels of serum amylase and lipase in the measured mice were significantly higher in the PI II to PI V experimental groups, and the differences were statistically significant. Interestingly, there was no statistically significant difference in the PI I group mice (Figure 3D,E). The ELISA method was employed to assess the levels of inflammatory markers14, including TNF-α and IL-6, in the serum of mice. The findings revealed that TNF-α and IL-6 levels in the PI V group mice were significantly higher than those in the other experimental groups, and the differences were statistically significant (Figure 3F,G). Compared with the control group, the PCT levels increased in all four experimental groups, but only the difference in the PI V group was statistically significant (p < 0.05) (Figure 3H).
The apoptotic status of pancreatic tissues in different experimental groups of mice
By performing TUNEL staining on the pancreatic tissues of each group of mice, the cellular necrosis status in the pancreatic tissues of different experimental groups was observed (Figure 4A). The grayscale values (OD) per unit area of pancreatic tissue sections and the positive rate of cellular necrosis were semi-quantitatively analyzed using Image J software. The results showed that compared to the other experimental groups, the level of cell necrosis in the pancreatic tissues of the PI V group of mice was significantly increased, and the difference was statistically significant (p < 0.001) (Figure 4B,C). Protein immunoblotting experiments were performed to assess the expression levels of cysteinyl aspartate specific proteinase-3 (Caspase-3), a cellular necrosis marker, in the pancreatic tissues of mice from different experimental groups (Figure 4D). Quantification of caspase-3 expression showed that the expression level of caspase-3 protein in the pancreatic tissues of the PI V group was significantly increased, and the difference was statistically significant (p < 0.001). The protein expression levels were quantified and normalized to the internal control GapDH (Figure 4E). Additionally, fresh pancreatic acinar cell suspensions were labeled with Annexin V-FITC/PI and analyzed by flow cytometry. It was found that compared to the other experimental groups of mice, the PI V group had a significantly higher positive rate of cell death, which was statistically significant (p < 0.001) (Figure 4F,G).
The content of HMGB-1 in peripheral serum and the expression level of HMGB-1 in pancreatic tissue
In order to investigate the relationship between HMGB-1 protein and pancreatic injury, immunohistochemical staining and quantification were performed on the pancreatic tissues of mice in each experimental group. There was a statistically significant difference between the experimental and control groups (p < 0.001) (Figure 5A,B). ELISA was used to measure the levels of HMGB-1 in the serum of mice from different experimental groups. The results showed that compared to the control group, the serum levels of HMGB-1 were significantly higher in all experimental groups, with the highest level observed in the PI V group, and the difference was statistically significant (p < 0.001) (Figure 5C). Furthermore, Western blot analysis detected elevated expression of HMGB-1 protein in the pancreatic tissues of mice in all experimental groups, with statistically significant differences compared to the control group (p < 0.001) (Figure 5D,E).
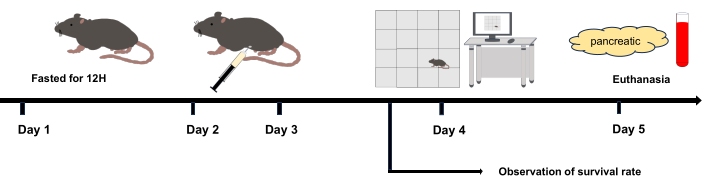
Figure 1: Experimental flowchart. Day 2 and Day 3: Intraperitoneal injection. Day 4: The open field experiment was initiated after 12 h of the last intraperitoneal injection. Day 5: Euthanized 36 h after the intraperitoneal injection. Please click here to view a larger version of this figure.
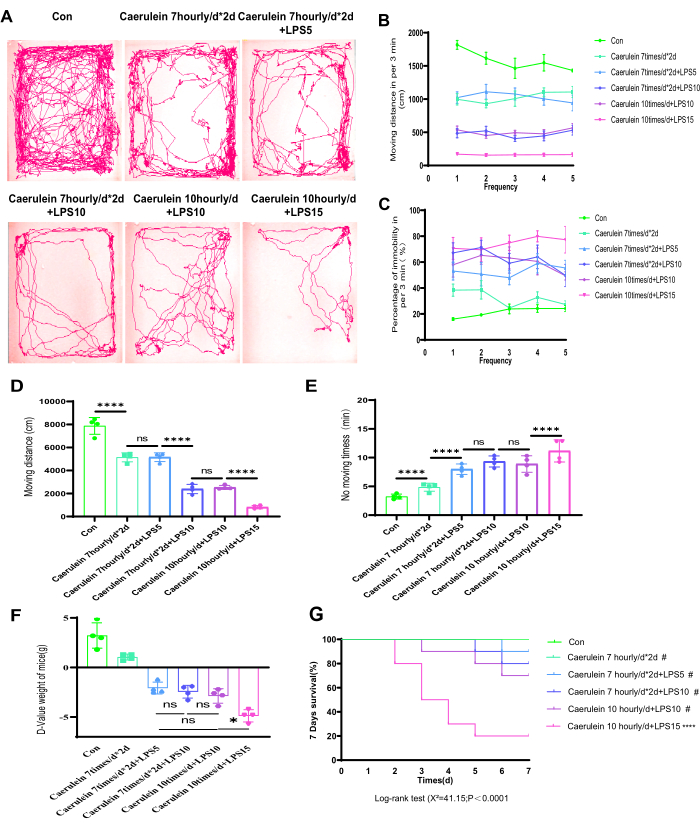
Figure 2: Macroscopic changes in the PI mice model. (A) The real-time movement trajectory plot of mice is depicted. (B) The total distance of movement within each monitoring period for different experimental groups of mice. (C) The percentage of immobility time within each monitoring period for different experimental groups of mice. (D,E) The total movement distance and immobility time percentage during 15 min were analyzed for mice in different experimental groups. (F) The D-value representing the weight of mice before and after the modeling process is shown. (G) The survival of 10 mice in each group was observed for the 7 days following the intraperitoneal injection. Data are expressed as means ± SEM, n = 4. "ns" denotes not significant, *P < 0.05, ****P < 0.001. Please click here to view a larger version of this figure.
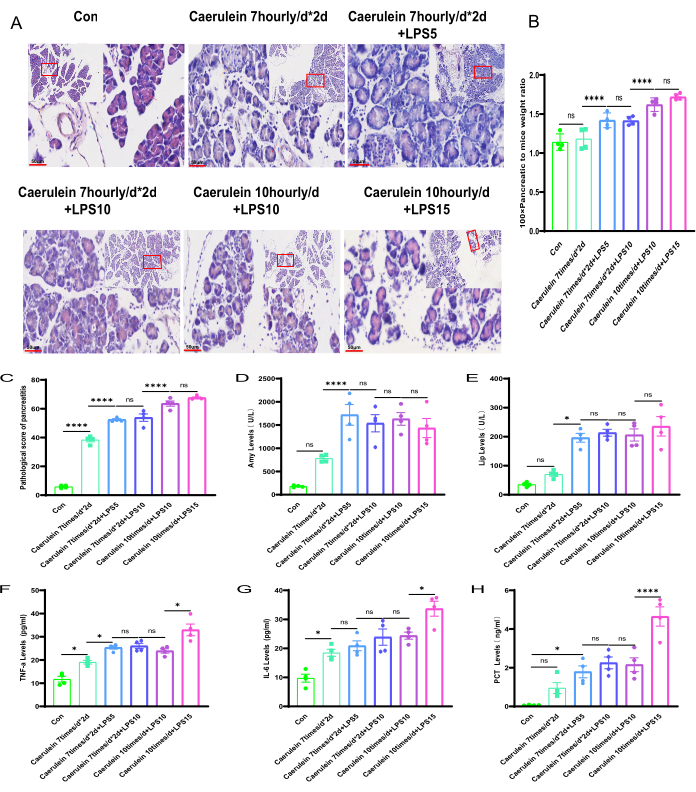
Figure 3: Pathological changes in mice pancreatitis among different experimental groups. (A) The H&E-stained histological sections of the mice pancreatic tissue in different experimental groups (paraffin-embedded pancreatic tissue sections stained with hematoxylin and eosin, magnifications of 100-fold and scale bars 200 µm, and 400-fold and scale bars 50 µm, respectively. Yellow long arrows indicate islets; red long arrows indicate acinar cells; brown long arrows indicate blood vessels; blue long arrows indicate ducts). (B) Calculation of the ratio of pancreatic weight to body weight in mice. (C) Pathological scoring of mice pancreas. (D,E) Detection of serum amylase and lipase levels in mice. (F–G) ELISA measurement of serum TNF-α and IL-6 levels in mice. (H) Assessment of PCT level.Data are expressed as means ± SEM, n = 4. "ns" denotes not significant, *P < 0.05, ****P < 0.001. Please click here to view a larger version of this figure.
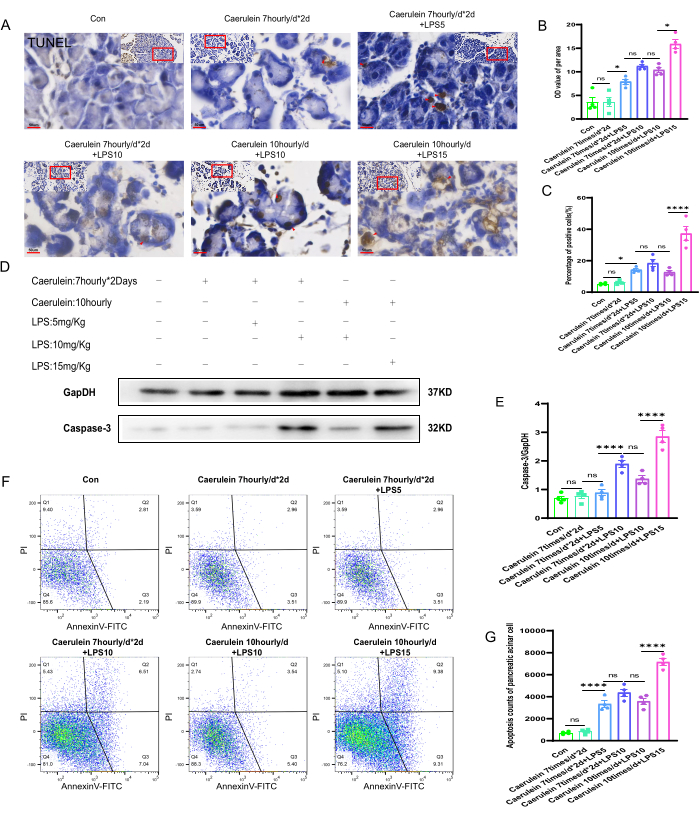
Figure 4: Apoptosis of pancreatic tissue in mice. (A) Representative images of TUNEL staining were obtained from pancreatic paraffin sections of mice and enlarged area images. (400x, scale bar 50 µm, brownish yellow is positive cells). (B,C) Quantitative analysis of the grayscale value per unit area of TUNEL-stained sections of mice pancreatic tissue and the percentage of positively stained dead cells. (D) The expression level of Caspase-3 in pancreatic tissue was detected by Western blot. (E) Quantification of Caspase-3 expression in pancreatic tissue. (F) Flow cytometry was employed to evaluate the extent of cell death in mice pancreatic acinar cells. (G) Enumeration of late-stage dead cells in mouse pancreatic acinar cells.Data are expressed as means ± SEM, n = 4. "ns" denotes not significant, *P < 0.05, ****P < 0.001. Please click here to view a larger version of this figure.
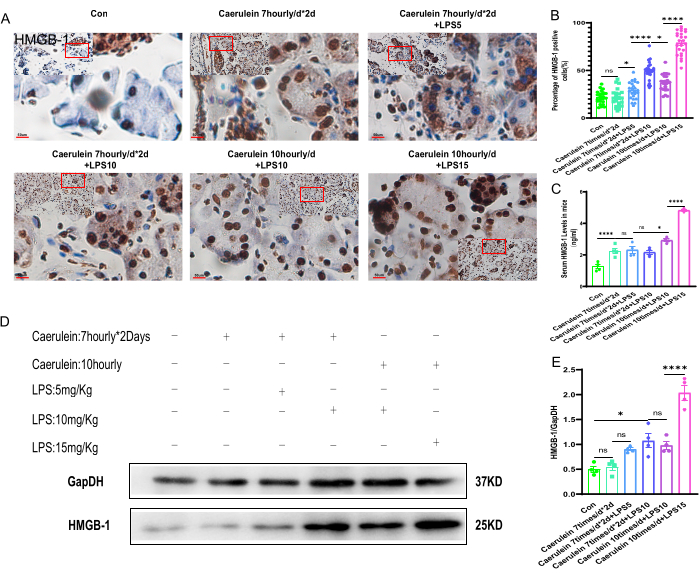
Figure 5: The HMGB-1 expression in mice pancreatic tissue. (A) Representative images and enlarged images (x400, scale bar 50 µm) of immunohistochemical staining of HMGB-1 on pancreatic sections. The brownish-yellow color indicates positive cells (n = 4). (B) The percentage of HMGB-1 positive cells in pancreatic tissue sections (n = 24). (C) The level of HMGB-1 in mouse serum (n = 4). (D,E) A Western blot was performed to detect the expression level of HMGB-1 in pancreatic tissue and to obtain the quantification results (n = 4).Data are expressed as means ± SEM, "ns" denotes not significant, *P < 0.05, ****P < 0.001. Please click here to view a larger version of this figure.
Supplementary Figure 1: Multiple organ injury in the PI V group mice model. (A) Representative images of histological changes in the pancreas, lung, liver, and kidney tissues collected from the CON and PI V group mice were analyzed using H&E staining (200x magnification, scale bar 50 µm). Pathological alterations associated with acinar cell necrosis are indicated by black arrows. Yellow arrows indicate pathological changes characterized by interstitial hemorrhage and edema in the alveoli. Hepatocyte edema and necrosis are denoted by green arrows. Glomerular hemorrhage-related pathological changes are marked by red arrows. (B) Calculation of histological scores was performed on pancreas, lung, liver, and kidney tissues obtained from the CON group and PI V group mice. Please click here to download this File.
Supplementary Table 1: Intraperitoneal injection protocol. Please click here to download this File.
Supplementary Table 2: Pathological scoring criteria for severity of pancreatitis. Please click here to download this File.
Discussion
Currently, there is a lack of effective means to improve the high mortality rate in patients with severe acute pancreatitis. It is crucial to investigate the efficacy of drugs in enhancing immune stability mechanisms. An urgent need exists for an ideal animal model for severe acute pancreatitis. Mice with a C57BL/6J genetic background are widely used in biomedical research, including studies on SAP pathophysiology. Over 70 years of genetic differentiation in B6J mice have resulted in the spontaneous deletion of several exons15, leading to reduced sensitivity to Caerulein-induced pancreatic injury16. Additionally, existing animal models of acute pancreatitis have limitations such as surgical trauma or applicability only to larger animals, which hinder scientific investigations using these models. Therefore, it is highly valuable to establish a stable, efficient, and convenient animal model using this specific gene strain.
Caerulein is an analog of cholecystokinin that induces pancreatic tissue damage by causing relative obstruction of pancreatic digestive fluid and enzyme secretion through high-frequency injections within a short period when mice have fasted and stored sufficient amounts of digestive fluid17. LPS is the main component found in bacterial cell walls and can cause MODS and SIRS in mice18. A previous study demonstrated that combining Caerulein injections at different frequencies with varying doses of LPS administered via intraperitoneal injection induced similar pathological changes observed in human SAP19,20. This study used a non-invasive approach to induce varying degrees of pancreatic injury by combining different frequencies of Caerulein injections with different doses of LPS administered intraperitoneally into mice. Ensuring uniform absorption by the peritoneum was crucial during protocol development.To fulfill this requirement, it is crucial to strictly adhere to the steps of intraperitoneal injection in order to ensure precise control over each drug administration. Additionally, gentle rubbing and application of appropriate pressure on the injection site with a sterile cotton swab after each medication injection are essential for uniform distribution of the medication throughout the peritoneal cavity. Furthermore, accurate and precise placement of the pinhead into the peritoneal cavity, with the needle pointing towards the central area of the upper abdomen, is of utmost importance. Prior training should be conducted to enable operators to accurately perceive any absence of resistance when inserting the needle into the abdominal cavity. The utilization of a micro-quantitative infusion pump instead of manual operation could potentially enhance drug injection quality; unfortunately, this was not implemented in this study. Nevertheless, by employing different injection schemes, we induced varying degrees of pancreatic injury and effectively observed differences in pancreatic injury caused by different frequencies and doses under identical technical conditions induced by Caerulein combined with LPS injections. This provides valuable insights for the future use of Caerulein in non-invasive animal models for severe acute pancreatitis induction.
The combination of Caerulein and LPS resulted in significantly elevated serum amylase and lipase levels, increased pancreatic weight, and histological changes, including extensive infiltration of inflammatory cells, pancreatic acinar cell edema, necrosis, and hemorrhage, as demonstrated by hematoxylin and eosin (HE) staining. Moreover, with an increasing dose of LPS, the pathological changes in the pancreas became more pronounced. However, it is noteworthy that there were no discernible differences in pancreatic pathology scores between the PI IV and PI V groups, while the extent of pancreatic tissue cell necrosis was more pronounced in the PI III group compared to the PI IV group. This observation suggests that the dosing frequency of Caerulein may serve as a primary factor contributing to pancreatic injury, while LPS exacerbates its progression, leading to systemic inflammatory damage18,21. HMGB-1 is a protein that functions depending on its location22. Extracellular HMGB-1 acts as a protein involved in the warning signals of inflammation and is closely associated with the severity of acute pancreatitis23,24,25. In this study, the serum HMGB-1 levels in mice from all experimental groups were significantly higher compared to the control group, with the PI V group showing the most significant increase. Immunohistochemical and protein electrophoresis experiments also confirmed the high expression of HMGB-1 in pancreatic tissue. This important protein may serve as a therapeutic target for inhibiting the inflammatory storm in severe acute pancreatitis.
In summary, it is crucial to develop a non-invasive, simple, and easy-to-perform model of SAP in mice. In this experimental protocol, the induction of pancreatic injury using Caerulein combined with LPS is reliable and effective. By administering Caerulein intraperitoneally for ten consecutive days at a dose of 50 µg/kg, followed by a single intraperitoneal injection of 15 mg/kg of LPS, a stable, reliable, cost-effective, and efficient animal model of SAP can be established.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by Research Projects in Health and Medical Science in Huainan City (No. HNWJ2023005); Municipal Guiding Science and Technology Plan Program in Huainan City (No.2023151); Anhui Provincial College Students' Innovation and Entrepreneurship Training Program (No. S202310361254); the ninth batch of the "50·Stars of Science and Technology" innovation teams in Huainan City and Anhui Provincial Key Clinical Specialty Construction Project. We would like to express our gratitude to the Laboratory Department of the First Affiliated Hospital of Anhui University of Science and Technology for providing the relevant testing data.
Materials
| 20× Citric Acid Antigen Repair Solution (pH 6.0) | Wuhan servicebio Technology Co.,Ltd, China | G1202-250 ml | |
| Amylase | Mindray,China | ||
| Annexin V-FITC/PI | Wuhan servicebio Technology Co.,Ltd, China | G1511 | diluted at 1:20 |
| Anti-HMGB1 Rabbit pAB | Wuhan servicebio Technology Co.,Ltd, China | GB11103 | diluted at 1:1800 |
| BCA protein quantitative detection kit | Wuhan servicebio Technology Co.,Ltd, China | G2026-200T | |
| BD FACSCanto II Flow Cytometer | BD Life Sciences, San Jose, CA, 95131, USA | BD FACSCanto II | |
| BSA | Wuhan servicebio Technology Co.,Ltd, China | GC305010-100g | |
| C57BL/6J | Cavion Experimental Animal Co., Changzhou, China | license number SCXY (Su) 2011–0003 | |
| Ceruletide | MCE, New Jersey, USA | 17650-98-5 | 50 µg/kg |
| Chemiluminescence imager | Cytiva CO.,LTD.;USA | ||
| Citric acid antigen repair Solution (Dry powder pH 6.0) | Wuhan servicebio Technology Co.,Ltd, China | G1201-5 L | |
| Collagenase IV | Wuhan servicebio Technology Co.,Ltd, China | GC305014 | 0.5 mg/mL |
| DAB (SA-HRP) Tunel Cell Apoptosis Detection Kit | Wuhan servicebio Technology Co.,Ltd, China | G1507-100 T | |
| Dimension EXL with LM Integrated Chemistry System | Siemens Healthcare Diagnostics Inc.Brookfield,USA | YZB/USA 8311-2014 | |
| ECL developer | Wuhan servicebio Technology Co.,Ltd, China | ||
| Eosin dye (alcohol soluble) | Wuhan servicebio Technology Co.,Ltd, China | G1001-100 ml | |
| EthoVision XT | Noldus, Netherlands | ||
| FITC-labeled goat anti-rabbit IgG | Wuhan servicebio Technology Co.,Ltd, China | GB22303 | diluted at 1:50 |
| Fully automatic blood cell analyzer | Zybio Inc. China | Zybio-Z3 CRP | |
| GapDH | Wuhan servicebio Technology Co.,Ltd, China | GB11103 | diluted at 1:1500 |
| Hematoxylin blue return solution | Wuhan servicebio Technology Co.,Ltd, China | G1040-500 ml | |
| Hematoxylin differentiation solution | Wuhan servicebio Technology Co.,Ltd, China | G1039-500 ml | |
| Hematoxylin dye | Wuhan servicebio Technology Co.,Ltd, China | G1004-100 ml | |
| HMGB-1 ELISA kits | njjcbio Co., Ltd, China | ||
| HOMOGENIZER | Wuhan servicebio Technology Co.,Ltd, China | KZ-III-F;IC111150 100222 | |
| HRP-labeled goat anti-rabbit IgG | Wuhan servicebio Technology Co.,Ltd, China | GB23303 | diluted at 1:1500 |
| IL-6 ELISA kits | Wuhan servicebio Technology Co.,Ltd, China | GEM0001 | |
| Lipase | Mindray,China | ||
| Lipopolysaccharide | Wuhan servicebio Technology Co.,Ltd, China | GC205009 | 15 mg/kg |
| Low temperature high speed centrifuge | Changsha Pingfan Apparatus&Instrument Co.,Ltd.,China | TGL-20M | |
| Membrane breaking liquid | Wuhan servicebio Technology Co.,Ltd, China | G1204 | |
| microtome | Jinhua Craftek Instrument Co., Ltd.;China | CR-601ST | |
| Nylon mesh | Wuhan servicebio Technology Co.,Ltd, China | 200-mesh | |
| One-step TUNEL cell apoptosis detection kit (DAB staining method) | Wuhan servicebio Technology Co.,Ltd, China | G1507-100T | |
| Paraffin tissue embedding machine | PRECISION MEDICAL INSTRUMENTS CO.,LTD;Changzhou,China | PBM-A | |
| Pathological tissue drying apparatus | PRECISION MEDICAL INSTRUMENTS CO.,LTD;Changzhou,China | PHY-III | |
| Phosphate-buffered saline | Wuhan servicebio Technology Co.,Ltd, China | G4202-100ML | |
| PMSF | Wuhan servicebio Technology Co.,Ltd, China | G2008-1 ml | |
| Positive fluorescence microscope | Olympus Corporation,Tokyo, Japan | BX53 | |
| Pro Calcitonin | Mindray,China | ||
| PVDF membrane | Millipore, USA | 0.22 µm | |
| RIPA | Wuhan servicebio Technology Co.,Ltd, China | G2002-100 ml | |
| SDS-PAGE | Beyotime Biotechnology,China | P0012A | |
| TNF-αELISA kits | Wuhan servicebio Technology Co.,Ltd, China | GEM0004 | |
| Ultrasonic water bath | DONGGUAN KQAO ULTRASONIC EQUIPMENT CO.,LTD.;China | KQ-200KDE | |
| Western Blot | Bio-Rad Laboratories, Inc.,USA | ||
| Western blot imaging System | Global Life Sciences IP Holdco LLC, JAPAN | Amersham ImageQuant 800 | |
| Whirlpool mixer | SCILOGEX;USA |
References
- Gliem, N., Ammer-Herrmenau, C., Ellenrieder, V., Neesse, A. Management of severe acute pancreatitis: An update. Digestion. 102 (4), 503-507 (2021).
- Duan, F., et al. GDF11 ameliorates severe acute pancreatitis through modulating macrophage M1 and M2 polarization by targeting the TGFbetaR1/SMAD-2 pathway. Int Immunopharmacol. 108, 108777 (2022).
- Zhang, X. P., et al. Preparation method of an ideal model of multiple organ injury of rat with severe acute pancreatitis. World J Gastroenterol. 13 (34), 4566-4573 (2007).
- Bluth, M. H., Patel, S. A., Dieckgraefe, B. K., Okamoto, H., Zenilman, M. E. Pancreatic regenerating protein (reg I) and reg I receptor mRNA are upregulated in rat pancreas after induction of acute pancreatitis. World J Gastroenterol. 12 (28), 4511-4516 (2006).
- Qiu, F., Lu, X. S., Huang, Y. K. Effect of low molecular weight heparin on pancreatic micro-circulation in severe acute pancreatitis in a rodent model. Chin Med J (Engl). 120 (24), 2260-2263 (2007).
- Lombardi, B., Estes, L. W., Longnecker, D. S. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 79 (3), 465-480 (1975).
- Liu, Y., et al. Deletion of XIAP reduces the severity of acute pancreatitis via regulation of cell death and nuclear factor-kappaB activity. Cell Death Dis. 8 (3), e2685 (2017).
- Niederau, C., Ferrell, L. D., Grendell, J. H. Caerulein-induced acute necrotizing pancreatitis in mice: Protective effects of proglumide, benzotript, and secretin. Gastroenterology. 88, 1192-1204 (1985).
- Zhou, X., et al. DPP4 inhibitor attenuates severe acute pancreatitis-associated intestinal inflammation via Nrf2 signaling. Oxid Med Cell Longev. 2019, 6181754 (2019).
- Yang, J., et al. Heparin protects severe acute pancreatitis by inhibiting HMGB-1 active secretion from macrophages. Polymers (Basel). 14 (12), 2470 (2022).
- Kong, L., et al. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 12 (10), 928 (2021).
- Tan, J. H., et al. ATF6 aggravates acinar cell apoptosis and injury by regulating p53/AIFM2 transcription in severe acute pancreatitis. Theranostics. 10 (18), 8298-8314 (2020).
- Schmidt, J., et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 215 (1), 44-56 (1992).
- Luo, C., et al. Abdominal paracentesis drainage attenuates severe acute pancreatitis by enhancing cell apoptosis via PI3K/AKT signaling pathway. Apoptosis. 25 (3-4), 290-303 (2020).
- Fontaine, D. A., Davis, D. B. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes. 65 (1), 25-33 (2016).
- Wan, J., et al. Pancreas-specific CHRM3 activation causes pancreatitis in mice. JCI Insight. 6 (17), e132585 (2021).
- Sah, R. P., et al. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 144 (5), 1076-1085 (2013).
- Wang, K., et al. Activation of AMPK ameliorates acute severe pancreatitis by suppressing pancreatic acinar cell necroptosis in obese mice models. Cell Death Discov. 9 (1), 363 (2023).
- Jin, C., Li, J. C. Establishment of a severe acute pancreatitis model in mice induced by combined Rain Frog Peptide and lipopolysaccharide and exploration of its mechanism. Acta Exp Bio Sinica. 36 (2), 91-96 (2003).
- Tan, J. H., et al. ATF6 aggravates acinar cell apoptosis and injury by regulating p53/AIFM2 transcription in severe acute pancreatitis. Theranostics. 10 (18), 8298-8314 (2020).
- Roy, R. V., et al. Pancreatic Ubap2 deletion regulates glucose tolerance, inflammation, and protection from Caerulein-induced pancreatitis. Cancer Lett. 578, 216455 (2023).
- Chen, R., Kang, R., Tang, D. The mechanism of HMGB1 secretion and release. Exp Mol Med. 54 (2), 91-102 (2022).
- Murao, A., Aziz, M., Wang, H., Brenner, M., Wang, P. Release mechanisms of major DAMPs. Apoptosis. 26 (3-4), 152-162 (2021).
- Liu, T., et al. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg. 104 (9), 1215-1225 (2017).
- Li, N., Wang, B. M., Cai, S., Liu, P. L. The role of serum high mobility Group Box 1 and Interleukin-6 levels in acute pancreatitis: A meta-analysis. J Cell Biochem. 119 (1), 616-624 (2018).

.
