Assessing Functional Recovery of Eupneic Diaphragm Activity Following Unilateral Cervical Spinal Cord Hemisection in Rats
Summary
Respiratory complications are the leading cause of death in individuals with cervical spinal cord injury (cSCI). Animal models of cSCI are essential for mechanistic evaluations and pre-clinical studies. Here, we introduce a reproducible method to assess functional recovery of diaphragm muscle (DIAm) activity following unilateral C2 spinal hemisection (C2SH) in rats.
Abstract
Following cSCI, activation of the DIAm can be impacted depending on the extent of the injury. The present manuscript describes a unilateral C2 hemisection (C2SH) model of cSCI that disrupts eupneic ipsilateral diaphragm (iDIAm) electromyographic (EMG) activity during breathing in rats. To evaluate recovery of DIAm motor control, the extent of deficit due to C2SH must first be clearly established. By verifying a complete initial loss of iDIAm EMG during breathing, subsequent recovery can be classified as either absent or present, and the extent of recovery can be estimated using the EMG amplitude. Additionally, by measuring the continued absence of iDIAm EMG activity during breathing after the acute spinal shock period following C2SH, the success of the initial C2SH may be validated. Measuring contralateral diaphragm (cDIAm) EMG activity can provide information about the compensatory effects of C2SH, which also reflects neuroplasticity. Moreover, DIAm EMG recordings from awake animals can provide vital physiological information about the motor control of the DIAm after C2SH. This article describes a method for a rigorous, reproducible, and reliable C2SH model of cSCI in rats, which is an excellent platform for studying respiratory neuroplasticity, compensatory cDIAm activity, and therapeutic strategies and pharmaceuticals.
Introduction
There are more than 300,000 individuals with spinal cord injury (SCI) in the United States, approximately half of whom have cervical injuries1. These injuries result in significant loss of well-being and place a financial strain on individuals, their families, and the healthcare system. Fortunately, the majority of SCIs are incomplete—providing the potential for strengthening of spared pathways1. This neuroplasticity may allow recovery of at least some function, including DIAm activity, which is important for ventilatory and non-ventilatory behaviors. Thus, promoting neuroplasticity is a promising avenue of research to help individuals with SCI2.
Rodent models of SCI have the potential to contribute substantially to the discovery of treatments to improve human health. One of the classic models of SCI used to study neuroplasticity is a unilateral transection (hemisection) of the spinal cord at C2 (C2SH), which leaves the contralateral side intact3,4,5,6,7,8,9,10,11,12,13. The effect of C2SH on phrenic output and the importance of spared contralateral pathways was first revealed over a hundred years ago by Porter12, whose seminal article laid the foundation for modern-day studies of respiratory neuroplasticity. The C2SH model interrupts descending inputs from the rostral ventral respiratory group (rVRG) in the medulla, which contains premotor neurons responsible for transmitting the output of respiratory rhythm generation14. These rVRG premotor neurons also transmit excitatory neural drive to phrenic motor neurons (Figure 1). Several investigators have taken different approaches to the C2SH model10,11,15,16, which may partly explain some of the variability in recovery across studies. Briefly, approaches vary in terms of sparing the dorsal funiculi, performing a complete hemisection, or performing a lateral partial transection that does not completely interrupt descending inputs from the ipsilateral rVRG. Generally, C2SH models are particularly useful for studying respiratory neuroplasticity due to the rates of spontaneous recovery of eupneic iDIAm electromyographic (EMG) activity over time, which can be improved by several factors, including neurotrophic signaling17,18,19,20,21. However, an initial loss of function—defined as the silencing of eupneic iDIAm EMG activity—must be first established before recovery can be clearly classified. This validation of inactivity at the time of C2SH is not done in several studies3,4,6,7,11,22,23.
Histological assessments of the excised spinal cord only provide evidence of damage to the appropriate location of ipsilateral excitatory bulbospinal pathways innervating phrenic motor neurons in the spinal cord, but histology does not substitute for physiological evidence (e.g., DIAm EMG). Furthermore, histological assessments are performed in ex vivo at terminal time points (often several weeks to months post-injury) and thus do not provide "real-time" information. Some investigators have noted that the magnitude of the lesion relates to the amount of functional deficit or lack thereof5,24,25,26. It is important to note that the validity of such claims is likely highly dependent on how "function" is classified (i.e., what the functional tasks are and how they are quantified), and the variability across studies highlights the difficulty of producing functionally identical lesions across animals. Indeed, investigators have emphasized that the relationship between the extent of injury and limb muscle locomotor function (quantified by the Basso, Beattie, and Bresnahan (BBB) score24) is not linear27,28. In previous studies, we have found no relationship between the extent of the C2SH and the extent of recovery of eupneic iDIAm EMG activity post-injury10,29,30,31, although other investigators have reported a relationship between ventilatory function and the extent of white matter sparing5. Thus, in the case of the C2SH model, an approach for functional validation of iDIAm inactivity at the time of the surgery and preferably early in the time course of chronic spinal cord injury experiments is both beneficial and necessary.
The present article underscores the use of DIAm EMG for real-time confirmation of the initial loss of DIAm EMG during breathing after the C2SH as well as subsequent confirmatory assessments at 3 days (Day 3) after the injury18,21,31,32,33. In earlier work with the C2SH model, repeated laparotomies were performed to record DIAm EMG10,13,30,34. However, more recent work has used chronic EMG electrodes, which allow the recording of EMG in anesthetized and awake rats. Additionally, chronic electrodes reduce the risk of pneumothorax and don't require repeated laparotomies, which can cause inhibition of the DIAm35,36. Although versions of the C2SH model have been used by many investigators, confirmation of the silencing of iDIAm activity was not made at the time of surgery3,4,6,7,11,22,23. Without such a confirmation of inactivity, it is difficult to know what portion of subsequent recovery to attribute to the neuroplasticity of ipsilateral versus contralateral pathways, which may have differential impacts. This is an important consideration because the inspiratory neural drive from the rVRG to phrenic motoneurons is primarily ipsilateral, with a loss of about 50% of excitatory glutamatergic inputs to phrenic motor neurons after C2SH33. However, there are remaining inspiratory excitatory inputs from the contralateral rVRG that decussate below the site of the lesion to innervate ipsilateral phrenic motor neurons and can be strengthened via neuroplasticity to promote functional recovery. By removing the predominant ipsilateral excitatory input to phrenic motor neurons, eupneic iDIAm EMG activity is lost (at least under anesthesia), while the activity of the cDIAm continues and is even enhanced. The loss of iDIAm EMG activity during breathing is thus a measure of a successful C2SH (Figure 2).
Some level of iDIAm EMG activity is present as early as 1-4 days following C2SH in awake animals23,37. Additionally, in decerebrate animals, iDIAm activity is present within minutes to hours after upper cervical hemisection and is suppressed by anesthesia38. Additionally, the success of the C2SH is validated by confirming the absence of iDIAm EMG activity during breathing (eupnea) in anesthetized rats on Day 3 post-injury. Confocal imaging studies confirmed the loss of glutamatergic synaptic inputs on phrenic motor neurons during this initial stage of injury37. At Day 3 post-injury, if there is any residual eupneic iDIAm EMG activity, this is interpreted as evidence of incomplete removal of ipsilateral descending inspiratory drive from the rVRG. The present article is divided into three sections: (1) chronic DIAm EMG recordings, (2) C2SH, and (3) EMG data acquisition in awake and anesthetized animals. This protocol describes a rigorous, reproducible, and reliable C2SH model of cSCI in rats, which is an excellent platform for studying respiratory neuroplasticity, compensatory cDIAm activity, and therapeutic strategies and pharmaceuticals.
Protocol
This protocol was approved by the Mayo Clinic Institutional Animal Care and Use Committee (Protocol Number: A00003105-17-R23). The animals in the present study were a mix of male and female Sprague-Dawley rats approximately 3 months old and weighing between 200 g to 350 g. The details of the reagents and the equipment used in the study are listed in the Table of Materials.
1. Electrode implantation
- Preparing the electrodes
- Ensure that the mentioned equipment is available: (1) Multistranded, stainless steel wire, (2) scalpel with #11 blade, (3) scissors, 4) dissection microscope, (5) ruler, (6) forceps, (7) 25 g needles, (8) 2 pliers (at least one with a crimping zone).
- Details of the process for making electrodes are shown in Figure 3. Repeat this process at least twice for paired differential recording electrodes.
- First, measure and cut 18 cm of the stainless steel wire.
- Tie a basic overhand knot at 4 cm from one end of the wire, as shown in Figure 3; this will serve as an anchor when the short side of the wire is implanted and sutured into the DIAm.
- Using a scalpel and a surgical dissection scope, carefully strip 2 mm of insulation immediately adjacent to the knot on the shorter side of the wire. Exercise great care not to cut strands of the multistranded wire.
NOTE: This uninsulated portion serves as the conducting surface of the electrode. Generally, signal quality begins to degrade if three or more strands are inadvertently cut, and if so, the electrode is replaced. - Next, strip ~5 mm from the end of the short side of the wire, which is then inserted into the lumen of a 25 G needle to make a suture thread-like tool that will be used to implant the electrode into the DIAm.
NOTE: Higher gauge needles (e.g., up to 28 G) may also be used depending on personal preference. Due to the thinner diameter of higher-gauge needles, the risk of pneumothorax may potentially be reduced. However, it may also be more difficult to insert the multistranded wires into the smaller lumen of the needle. - To accomplish this, remove a 25 G needle from its packaging, and using both pliers, carefully bend the needle at ~1 cm from the sharp beveled end from side to side until it breaks.
NOTE: This process must not be rushed; otherwise, the shearing forces can cause the lumen of the needle to shut, requiring the process to be repeated. - Insert the stripped end of the wire into the lumen of the broken-off 1 cm needle, ensuring that the needle rests immediately adjacent to the remaining insulated portion of the wire.
- Ensuring that there is no uninsulated portion between the needle and the remaining shorter side of the wire, crimp the needle to attach it to the wire.
- Next, use the two pliers to carefully bend the attached needle to give it a curvature similar to that of a standard surgical suturing needle.
- Finally, strip away ~5 mm from the end of the long side of the wire (Figure 3). After the electrodes are sutured into the DIAm, the long side of the wire will be tunneled for external access to allow for DIAm EMG recordings.
- Repeat the process to make a second electrode for differential recordings.
- Diaphragm EMG electrode placement
- Prior to surgery, sterilize or autoclave all surgical equipment, including (1) small animal hair clippers, (2) scalpel with #11 blade, (3) surgical scissors, (4) forceps, and (5) micro-needle holder.
- Acquire a clean cage and provide food, water, bedding, and environmental enrichment. Set aside on a heating pad. This cage will be used to house the animal after surgery.
- Sterilize the surgical field with appropriate disinfectant and put on personal protective equipment (scrubs, caps, mask, and surgical gloves). Place the previously prepared electrodes in a pool of 70% isopropyl alcohol.
- Place a heating pad in the surgical area to maintain the rat's body temperature during anesthesia and surgery. Cover the heating pad with a sterilized surgical towel.
- Weigh the rat and calculate the appropriate anesthetic dosage based on institutional animal care and use guidelines.
- Anesthetize the rat with a hind limb intramuscular injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) or other appropriate anesthetic (following institutionally approved protocols). Inject extended-release buprenorphine (1 mg/kg) and carprofen (5 mg/kg) preoperatively. Proceed once the animal no longer responds to toe pinch.
- Use clippers to shave the rat from the xiphoid process to approximately halfway to the hind limbs. Also, shave the back of the rat where electrodes will be externalized beyond the reach of the animal (usually around the shoulder blades). Clean off excess hair with a piece of gauze.
- Prepare the skin for incision by cleaning it with sterile gauze dipped in chlorohexidine gluconate (4% weight by volume).
- Place the animal in a supine position on the sterilized heating pad. Starting from the xiphoid process, use the scalpel and a clean #11 blade to make a 4-5 cm rostrocaudal incision. This should expose the underlying abdominal muscle layers.
- Make another incision (3-4 cm) along the midline in the muscle layers to expose the abdominal contents. Take care not to cut through into the abdominal organs.
- Ensure that the abdominal (i.e., inferior) surface of the DIAm is visible, although the animal's right side will be obscured by the liver.
- Using a scalpel handle or other blunt instrument, push down the liver and other organs to expose the abdominal side of the DIAm.
- Prepare the electrode for use by removing it from the pool of isopropyl alcohol and washing it with sterile saline. Then, using a micro-needle holder, orient one of the previously prepared electrodes perpendicular to muscle fibers in the mid-costal region of the DIAm. Muscle fibers spread out radially across the DIAm.
NOTE: The DIAm is thin (~2 mm) and must not be punctured. Rather, the goal is to embed the uninsulated portion of the electrode wire (Figure 3) within the DIAm. - Use the surgical microscope to carefully insert the electrode suture wire (needle end) into the DIAm. Allow the curvature of the needle to guide the electrode into and then out of the abdominal surface of the DIAm, ensuring that the entire uninsulated portion of the wire will be embedded fully in the muscle.
- Carefully pull the needle through until the knot in the wire anchors itself in the DIAm. Ensure that the uninsulated portion (2 mm) of the electrode wire is completely within the DIAm, with a knot at the point where the needle first entered the DIAm.
- Next, cut off the needle from the electrode wire and dispose of it in a sharps container.
- Using two forceps, tie a knot at the point where the suture (3-0) needle exited the DIAm. To do this, tie a loop in the short side of the wire, slide the loop down to the abdominal surface of the DIAm, and carefully tighten the loop into a knot. The electrode (uninsulated portion) should now be anchored at both its entrance and exit point.
- Cut off the excess part of the shorter side of the wire.
- Follow steps 1.2.14-1.2.18 to suture in another electrode on the same side of the DIAm, ~3 mm away from the first.
- Perform electrode pair implantation in the opposite hemidiaphragm in the same manner.
- Next, ensure that the longer side of the electrode wires are all outside of the abdominal cavity and begin to suture (3-0) the muscle layers to close the abdomen. For each pair of electrodes, make a loop and anchor it.
NOTE: Ensure that 2-3 cm of wire is left within the abdominal cavity to allow the rat to stretch without causing tension on the DIAm. - Clean off excess blood with saline and then suture (3-0) the muscle layer in a continuous pattern to close the peritoneum.
- Tunnel a 16 G catheter from the medial dorsal skin of the rat through to the open abdominal skin and pull a pair of electrode wires through to the back of the rat.
- Repeat the process with a second catheter on the other side and pull the second pair of electrode wires through.
- Suture the abdominal skin with a 3-0 suture in an interrupted suture pattern.
- Next, for each pair, tie the electrode wires into a knot and suture them in place.
- Provide the animal with a subcutaneous saline injection (~1 ml per 50 g of animal mass). Put the animal in a clean cage over a heating pad to recover.
NOTE: Laparotomy can cause inhibition of DIAm activity35,36. Allow at least 72 h before quantitative analysis of any EMG measurements.
2. Cervical spinal hemisection
- Prepare sterile surgical area as before.
- Prior to surgery, autoclave or sterilize all surgical equipment. Equipment required: (1) small animal hair clippers, (2) scalpel with #11 blade, (3) surgical scissors, (4) forceps, (5) rongeurs, (6) retractors, (7) angled dissecting knife.
- Approximately 72 h after the DIAm EMG electrode implantation, weigh the rat and calculate the appropriate anesthetic dosage based on institutional animal care and use guidelines.
- Prior to anesthetizing the rat, place the rat in a Bowman-style cage, and record bilateral DIAm EMG as outlined in step 3.2.
- Anesthetize the rat with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) or other appropriate anesthetic as per the local Institutional Animal Care and Use Committee protocol. Preoperatively inject extended-release buprenorphine (1 mg/kg) subcutaneously and carprofen (5 mg/kg) intraperitoneally.
- Proceed once the animal no longer responds to toe pinch. Check the depth of anesthesia with a toe pinch approximately every 15 min.
- Prior to beginning surgery, place the rat in a prone position and record EMG in the anesthetized rat as outlined in step 3.3.
NOTE: Ensure that all recordings are performed with the rat in the same position. - Shave the hair from the neck at about the ear level and down to the scapulae and remove it with wet gauze.
- Clean the skin with chlorohexidine gluconate (4% weight by volume) and cover the animal with sterile surgical covers (except for the upper dorsum).
- Using a scalpel, make a 4 cm rostro-caudal incision. Retract the skin and cut the acromiotrapezius muscle. Then dissect the rhomboid muscle to expose the spinalis muscles.
NOTE: The acromiotrapezius is the middle trapezius muscle, and its muscle fibers run approximately parallel to the spine. The rhomboid muscle is exposed in the plane below the acromiotrapezius and its muscle fibers run diagonally. - Retract the spinalis muscles from C1 to C3.
NOTE: The spinalis muscles run immediately adjacent to the spine. - Carefully perform a laminectomy at C2 using a rongeur while looking through a surgical microscope to ensure that no major arteries or nerves are damaged.
- Cut and retract the dura mater at C2.
- Connect exposed electrode wires from the rat's back to the amplifier while performing the spinal hemisection. Refer to step 3 for more details.
- While monitoring DIAm EMG, insert the angled dissecting knife just below the point where the dorsal root enters the spinal cord and section all the way to the midpoint of the ventral surface in the manner shown in Figure 2A.
NOTE: If using an electronic heat pad, it may be necessary to turn it off while monitoring EMG to avoid noise. - Within seconds after this sectioning, ensure that the eupneic DIAm EMG activity ipsilateral to the injury ceases and a compensatory increase in the cDIAm EMG activity is evident (Figure 2C).
- If eupneic iDIAm EMG activity persists, perform another cut with the dissecting knife, as highlighted in Figure 2C. However, do not cut across the midline; cutting to the contralateral side will most likely lead to the death of the animal.
- Suture the muscles and then the skin with sterile sutures (3-0).
- To maintain hydration, subcutaneously inject 1 mL of saline per 50 g of animal mass and place the animal in a clean cage with a heating pad for recovery. Monitor the animal as it recovers from the surgery.
- Approximately 72 h later (Day 3 Post-C2SH), record the EMG activity in awake rats as per the protocol step 3.2. Be careful not to damage the stitches on the dorsum of the rat.
- Next, anesthetize the rat once again (following institutionally approved protocols), using a lighter dose of anesthetic than would be required for a surgical plane (between ⅓ to ½ of a normal dose). Additional doses may be given as long as the rats display an intact corneal reflex and respond to toe pinch.
- Monitor DIAm EMG activity following protocol step 3.
NOTE: Place the rat in the same position as the one used for the initial assessment of iDIAm EMG activity. - If eupneic iDIAm EMG activity (i.e., activity in phase with inspiratory cDIAm EMG activity) is absent on Day 3, conclude that the initial C2SH was successful and include the same animal for further analyses.
3. Data acquisition and analysis
- General settings and data extraction
- Set band-pass filters of the preamplifier to 100 Hz (high-pass) and 1000 Hz (low-pass).
NOTE: These filter settings eliminate movement artifacts, and markedly reduce 60 Hz noise and ECG contamination (which has peak power at ~75 Hz) while retaining the vast majority of the frequency content of the intramuscular DIAm EMG signal. Based on the Nyquist theory, digitize with a sampling frequency of 2000 Hz to avoid aliasing and allow adequate resolution to note motor unit activity in the compound EMG. If performing specialized frequency content analyses of the sub-100 Hz frequency range, it is important to set the preamplifier filter settings to ensure that the frequencies of interest are not filtered. - Monitor two channels (left and right hemidiaphragm EMG) in visualization software on a computer and save the data in an appropriate format for further analyses.
- Collect at least 1-2 min of eupneic recordings to have enough data for analyses.
- Ensure that the data is collected at three or more time points:
- Prior to C2SH, establish a baseline eupneic recording in awake and anesthetized conditions.
- During the C2SH surgery to establish silencing of eupneic iDIAm EMG activity in anesthetized conditions.
- At Day 3 post-C2SH to confirm that the absence of eupneic iDIAm EMG activity persists and establish an initial point of the extent of the deficit in awake animals that is not as influenced by spinal shock2,39 or administration of respiratory depressants like buprenorphine.
- Analyze the DIAm EMG recordings using any software that allows calculations to be performed on digitized signals (e.g., Matlab, Python, R). The basic process is as follows:
- Load the raw EMG files. Depending on the filtering settings, it may be necessary to account for a DC offset in the signal.
NOTE: An easy fix is to subtract the mean of the raw EMG signal from itself. - Calculate the root-mean-square of the EMG signal with a moving window of 50 ms. This can be done by moving a 50 ms calculation window forward by one sample point at a time until the end of the signal is reached.
NOTE: Analyses of individual DIAm EMG events provide the most statistical power and allow clustering of eupneic and non-eupneic activity. Thus, detection of the onset and offset of individual breaths in the DIAm EMG is paramount, as highlighted in a previous report40.
- Load the raw EMG files. Depending on the filtering settings, it may be necessary to account for a DC offset in the signal.
- Perform the filtering of eupneic activity by visualizing the histograms of the instantaneous respiratory rate and burst duration, as shown in a previous report41.
- Set band-pass filters of the preamplifier to 100 Hz (high-pass) and 1000 Hz (low-pass).
- Awake recordings.
- Perform awake recordings by placing rats in a Bowman-style rodent cage. A cylindrical Bowman-style cage can accommodate adult rats up to 750 g.
- Unscrew metal rods on one side of the Bowman-style cage and gently coax the rat inside. Screw metal rods back on, trapping the rat in the cylindrical cage.
NOTE: Acclimate the rats to the cage for at least 30 min before proceeding with recording. If possible, it is best to acclimate rats to the Bowman-style cage for multiple days before any measurements. It is good practice to provide treats while the rats are in the cage during this acclimatization phase. - To minimize external visual stimuli, use a paper towel or similar object to make a tent around the front of the cage where the rat's head is. In addition, minimize loud noises and ensure the physical comfort of the rat at all points.
NOTE: Obvious signs of discomfort include fidgeting, rapid breathing, and struggling against the cage. If any of these signs are observed, it may be necessary to return the rat to the home cage and attempt recordings when the rat is calmer. - For smaller rats (<300 g), insert filler materials (e.g., paper towel, cotton balls) into extra spaces in the cage to discourage the rat from turning 180 degrees.
- After the rat is acclimated, carefully connect the wires exiting the rat dorsum to a preamplifier, which feeds the analog signals into an analog-to-digital converter, and follow the procedures outlined in step 3.1.
- Anesthetized recordings
- Place rats in the same position each time, preferably prone to more closely mimic the awake rat posture.
- Connect the wires exiting the rat dorsum to a preamplifier, which feeds the analog signals into an analog-to-digital converter, and follow the procedures outlined in step 3.1.
- Animal recovery
- Place rats inside an empty, clean rat cage with no bedding for recovery.
- Place the cage on a heating pad.
- Monitor the rat at regular intervals (<15 min) for the first 3 h or until the rat begins to move. Monitor at less regular intervals (30 min) until the animal is awake again.
- After the rat is ambulatory, place them in a clean cage with bedding, access to food and water, and environmental enrichment.
- Continue to monitor the rat at least once daily throughout the time course of the experiment. Pay special attention to changes in weight/ability to feed as well as the integrity of the incision sites and sutures.
Representative Results
The approach presented in this article minimizes inter-operator variability by setting clear criteria for evaluating DIAm EMG in a rat model of C2SH. First, the cessation of eupneic iDIAm EMG activity immediately after C2SH must be observed, as shown in Figure 2. If not, a secondary transection can be performed until eupneic iDIAm activity disappears. Second, on Day 3 post-C2SH, the continued absence of eupneic iDIAm EMG must be verified while animals are anesthetized. Figure 4A shows an example of a successful C2SH as determined by the continued absence of eupneic iDIAm EMG activity under anesthesia on Day 3 post-injury. Figure 4B shows DIAm EMG activity in the same animal under unanesthetized awake conditions, highlighting the reduction—but not absence—of iDIAm activity compared to the pre-injury baseline. Notably, the cDIAm EMG activity is increased in both awake and anesthetized conditions. The anesthetized recordings on Day 3 provide validation that the initial C2SH was successful, but quantitative analyses should be performed in awake animals. In awake animals, DIAm EMG activity at Day 3 post-C2SH represents the starting point for recovery. Figure 4B shows that iDIAm EMG activity is reduced, but not absent, on Day 3 in awake rats.
In some cases, C2SH is not accompanied by total cessation of eupneic iDIAm EMG activity (Figure 5A). An inadequate C2SH occurs in fewer than 5% of all cases using the approach outlined in the present manuscript; however, it is essential that an evaluation of DIAm EMG activity at Day 3 post-C2SH be performed on each rat to validate the success of the C2SH. Figure 5A shows an example of DIAm EMG activity from a rat on Day 3 post-injury, in which the C2SH was inadequate in eliminating eupneic iDIAm EMG activity by Day 3. It is important to note that validation of the efficacy of the C2SH was performed while the animal was anesthetized. Continued eupneic EMG activity suggests that at least a portion of the descending ipsilateral bulbospinal pathway was spared, which can complicate the interpretation of the results. Figure 5B shows that iDIAm EMG activity is reduced, but not absent, at Day 3 in awake rats. However, the sparing of ipsilateral descending inputs may lessen the initial deficit.
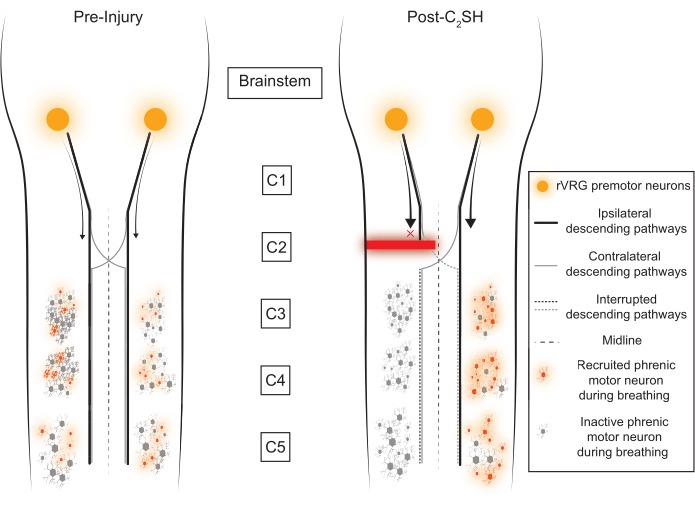
Figure 1: Conceptual framework. The rostral ventral respiratory group (rVRG) sends descending inputs to phrenic motor neurons in the cervical spinal cord (C3-C5). The majority of these descending axons innervate the ipsilateral phrenic motor neuron pool, but a small fraction cross the midline to activate the contralateral motor neuron pool. Immediately after a C2SH, descending inputs from the rVRG to the ipsilateral phrenic motor neuron pool are disrupted. Please click here to view a larger version of this figure.
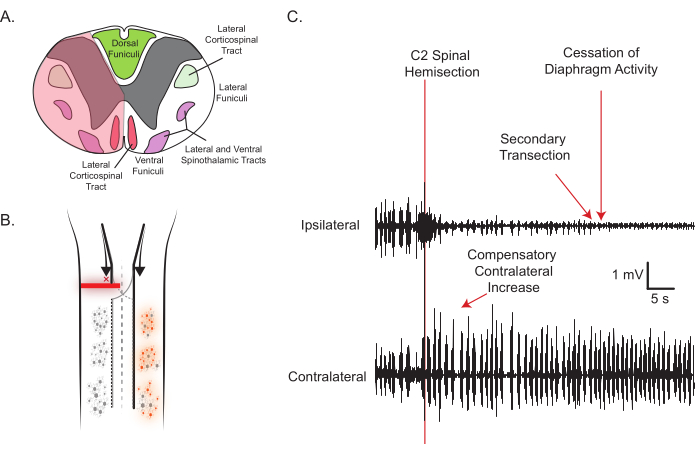
Figure 2: Representative C2SH and DIAm EMG activity. (A) The transverse view of the rat spinal cord and tran site (shaded red) with important structures labeled is shown. The spinal lesion is performed by inserting a dissecting knife right below the point where the dorsal root enters and cutting through to approximately the midline (shaded translucent red area). Note the severing of major motor tracts in the lateral and ventral funiculi, but the sparing of the dorsal funiculi. (B) The longitudinal view, depicting the silencing of ipsilateral motor neuron activity, complements the raw EMG traces used for real-time confirmation of a satisfactory lesion (C). As the spinal cord is lesioned, eupneic iDIAm EMG activity ceases, suggesting ipsilateral inputs to phrenic motor neurons have been severed; meanwhile, the cDIAm EMG immediately increases its activity as shown. Please click here to view a larger version of this figure.
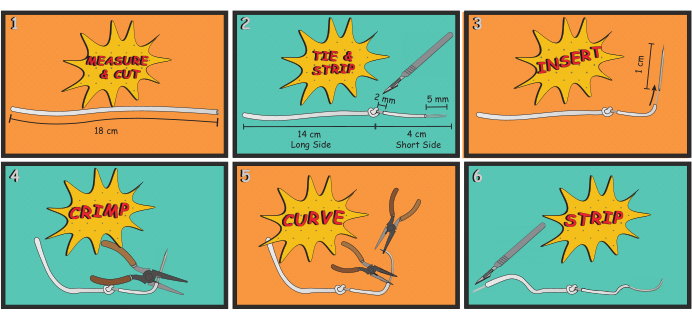
Figure 3: Electrode manufacturing. The process for manufacturing DIAm EMG electrodes is shown. The basic process involved measuring and cutting an appropriate length of wire (Step 1), tying an anchoring knot and stripping way insulation to expose the electrode (Step 2), attaching a 25 gauge needle to the wire (Steps 3 and 4), bending the needle to give it a curvature similar to a surgical suture needle (Step 5), and stripping away insulation to expose a portion of the wire that will be connected to the amplifier for recording EMG (Step 6). Please click here to view a larger version of this figure.
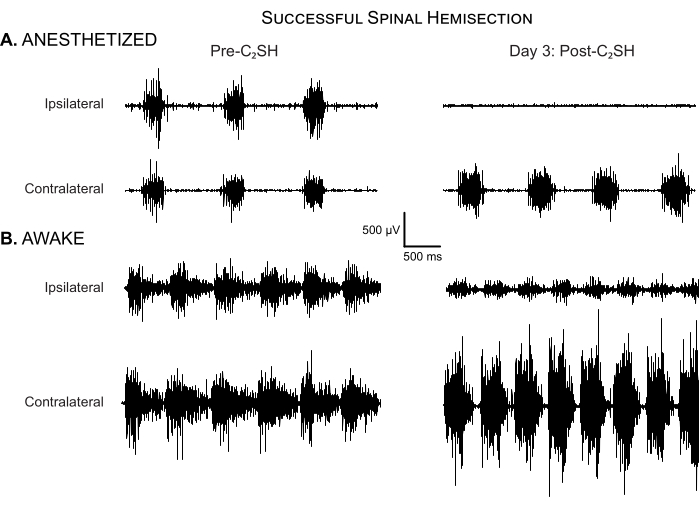
Figure 4: Successful spinal hemisection. (A) DIAm EMG activity is shown in an anesthetized rat before C2SH and at Day 3 post-injury. There is no evidence of eupneic iDIAm EMG activity in this rat at day 3 post-injury, indicating that the initial C2SH was successful. Note the compensatory increase in cDIAm EMG activity and increase in respiratory rate compared to the pre-injury condition. (B) DIAm EMG activity is shown in the same rat under unanesthetized awake conditions on Day 3 post-C2SH. There is clear evidence of reduced-albeit not completely silenced-iDIAm EMG activity. Note the compensatory increase in cDIAm EMG activity and increase in respiratory rate compared to the pre-injury condition. Please click here to view a larger version of this figure.
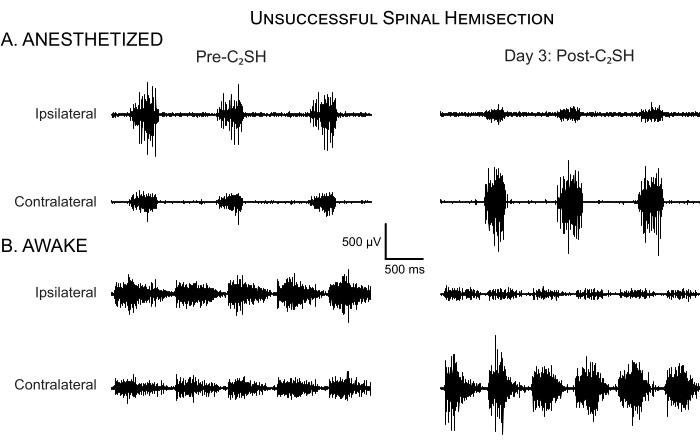
Figure 5: Unsuccessful spinal hemisection. (A) DIAm EMG activity is shown in an anesthetized rat before C2SH and at Day 3 post-injury. In this rat, eupneic iDIAm EMG activity persisted at Day 3 post-injury, indicating that the initial C2SH was inadequate. Accordingly, this rat was excluded from further analyses of functional recovery. Note the compensatory increase in the cDIAm EMG activity despite an unsuccessful C2SH, but minimal increase in respiratory rate. (B) DIAm EMG activity is shown in the same run under unanesthetized awake conditions on Day 3 post-C2SH. There is evidence of reduced iDIAm EMG, increased cDIAm EMG activity, and a slight increase in respiratory rate. Please click here to view a larger version of this figure.
Discussion
C2 spinal hemisection
The procedure described in this article emphasizes assessments of DIAm EMG activity that serve as a validation of a C2 spinal lesion that transects the lateral and ventral funiculi while sparing the dorsal funiculi (Figure 2A). The proposed surgical approach has two major benefits. First, it spares the dorsal funiculi, which preserves ambulatory function in rats, while still severing ipsilateral inputs to phrenic motor neurons. Second, by monitoring DIAm EMG, we can validate the efficacy of the C2 lesion in eliminating eupneic iDIAm EMG activity initially during surgery while the animals are anesthetized. At Day 3 post-injury, while the animals are anesthetized, we then verify that there is indeed continued silencing of eupneic iDIAm EMG activity. It was previously shown that rVRG excitatory inputs on phrenic motor neurons and NMDA receptor expression in phrenic motor neurons are reduced at 3 to 7 days post-injury and that both glutamatergic synaptic input and NMDA receptor expression increase over time after 7 days post-injury33,42. These data, combined with histological confirmation of the C2 lesion10,13,29,30,33, suggest that continued inactivity under anesthesia on Day 3 post-injury provides information about the efficacy of the initial C2SH. Across studies conducted in multiple years, relatively stable rates of spontaneous recovery under anesthesia between 30%-40% were seen at 14 days post-C2SH18,19,20,29,31,43, suggesting that this method of verifying the success of the C2SH is reproducible and reliable.
The C2SH procedure involves several critical steps. Importantly, the C2SH model proposed here spares the dorsal funiculi and thus does not lead to limb motor deficits. In full C2 spinal hemisection models involving the dorsal funiculi, limb motor deficits are considerably greater3,4,5,6,7,37. Thus, an added benefit of the C2SH model (Figure 2A) is that in addition to the silencing of eupneic iDIAm EMG activity in anesthetized C2 lesioned rats, the rats are functionally similar to sham laminectomy rats in terms of ambulation and other functions. Accordingly, they are generally able to feed and groom themselves, which reduces the caretaking burden while still allowing studies of DIAm neuromotor control and respiratory neuroplasticity. To ensure that the C2 model is implemented appropriately, great care must be taken to avoid excessive lesioning. Inserting the dissecting knife right below where the dorsal root enters the spinal cord and cutting the ventral portion sparingly are both helpful rules of thumb. The goal is to ensure that eupneic iDIAm EMG activity ceases; this can be done with multiple small cuts if needed. Indeed, in the week following the C2SH, it will become clear if there was excessive damage to the spinal cord if the animals have difficulty ambulating and reaching for food pellets.
Electrode placement and EMG recordings
Chronic DIAm EMG electrodes have several clear benefits over other approaches. Electrodes can (and should) be implanted several days before the C2SH procedure, allowing sufficient time for recovery and not requiring a laparotomy and a C2SH during the same surgical session. This is important because it is well-accepted that laparotomy causes inhibition of the DIAm35,36. By implanting chronic DIAm electrodes, there is also no need for repeated laparotomies, which were performed in earlier work10,13; there is also a reduced risk of pneumothorax as electrodes are not being inserted into the DIAm during each session. However, several potentially serious adverse events can occur as a result of electrode placement. Although the risk of pneumothorax is reduced by avoiding repeated electrode insertions, it is not completely nullified, and indeed, pneumothorax can occur, causing either immediate death or prolonged problems. In order to reduce this risk, it is best practice to ensure that the needle that is being threaded through the DIAm does not perforate the superior surface of the DIAm. Additionally, avoid using sharp forceps that may inflict damage to the DIAm while manipulating the electrode wires. Occasionally, the rats may chew the stitches at their abdomen, potentially disemboweling themselves if left to their own devices. Rats should be observed at regular intervals after electrode placement to detect these types of behaviors early. In some cases, it may be possible to anesthetize the rats to repair damage to stitches. However, it is best if such behavior is mitigated by providing adequate pain relief during and after the surgery and by ensuring that the sterile field is not broken during surgery. In extreme cases, it may be necessary to euthanize rats that repeatedly remove their stitches.
The recording and analysis of the EMG signals is not the primary focus of the present study and is highly dependent on the particular equipment and software available in each lab. Although equipment information is provided in the Table of Materials, a wide variety of hardware options exist for amplifying and recording EMG activity, and the specifics will depend on a mixture of features, availability, and affordability for each lab. However, there are some general principles of recording EMG that are important to mention. One potential issue is the movement or destruction of implanted electrodes, which can limit quantitative assessments of DIAm EMG. If the electrodes have dislodged from the DIAm, or if the rats have managed to consume or otherwise damage the externalized wires, it may not be possible to record EMG activity, or the noise level may change. This can be avoided by confirming that the electrodes are firmly secured in the DIAm, and a signal with a high signal-to-noise ratio (SNR) can be recorded from them during the DIAm electrode placement surgery. In addition, externalizing the electrode wires high on the dorsum and cutting off excess wire such that the rats are unable to access the electrode wires are both conducive to the success of chronic electrodes. As an alternative to externalized wires, head caps37 or telemetry44,45 may be reasonable options. Both approaches can obtain recordings in awake animals more easily but may be slightly more difficult to implement than simply externalizing the multistranded wires. All nearby electronic sources can potentially be sources of noise. It is paramount that all equipment is grounded appropriately, shielded cables are used, and the rats are not touched while active recordings are taking place. In addition to turning off electronic heat pads during recording, lights, nearby equipment, electrically operated surgical tables, and other such devices may need to be temporarily turned off to achieve low-noise recordings. To minimize environmental influences, electronic equipment that does not need to be on for the DIAm EMG recordings should be turned off. When possible, recordings should be made in an electrically shielded room. Over time, tissue scarring and fibrosis around the DIAm EMG electrodes can decrease the conductivity of the electrode, reducing SNR. Additionally, even slight changes in posture can have large impacts on the DIAm EMG signal; thus, to minimize these potentially complicating issues, DIAm EMG should be recorded with the rats in the same posture across recording sessions.
Another important consideration is the filtering and sampling settings. The type of electrode (i.e., intramuscular vs. esophageal/surface) is extremely relevant when it comes to determining the frequency content of—and consequently, appropriate high- and low-pass filters for—a signal. Considerable effort has been expended to determine the optimal filters for surface/esophageal DIAm EMG46. Similar studies in the rat DIAm have not been performed, but previously, we showed that effectively, the entirety of the frequency content of the DIAm EMG recorded using chronic intramuscular electrodes in rats was below 1000 Hz, with the centroid frequency around 300 Hz47. In a direct comparison of the mean and median frequencies of the power spectrum of biceps brachialis EMG in humans obtained via both surface and intramuscular electrodes, Christensen et al.48 found that both the mean and median frequencies were approximately 3-fold higher for intramuscular electrodes compared to surface electrodes. In the DIAm, investigators have reported centroid frequencies of around 100 Hz when using bipolar esophageal electrodes49,50,51,52,53,54, which would suggest that the 300 Hz, which was previously determined for intramuscular electrodes, approximately matches the same trend shown by Christensen et al.48. Despite this, multiple published studies utilizing intramuscular DIAm EMG in rodent models have placed their high-pass filters at 300 Hz4,37,55,56 and some have even gone as high as 500 Hz57. It is uncontroversial that such a filtering approach would substantially reduce the amplitude of the DIAm EMG signal by at least one-half. We propose a far less destructive approach: (1) high-pass filtering at 100 Hz because the majority of the ECG power spectrum is below 100 Hz while comparatively little of the DIAm EMG power spectrum is below 100 Hz51,52, and (2) subsequently removing the leftover ECG by waveform matching58. Thus, the appropriate filters for DIAm EMG for most studies may be set between 100 Hz and 1000 Hz, with a sampling frequency of at least 2000 Hz to capture all the relevant features within the data. Detailed analyses of the DIAm EMG can then be performed using the techniques published in previous reports40,41,47,58.
Awake and anesthetized animals
Notably, some studies have highlighted that C2SH models of cSCI do not lead to complete inactivity of eupneic iDIAm EMG23,37. In one sense, this is not surprising, as it has been noted previously that "breakthrough" activity occurs during behaviors necessitating higher drive (e.g., deep breaths and the response to airway occlusion)4,7,29,42. These data suggest that it is probably not appropriate to think of the C2SH model as a true model of continuous inactivity. Indeed, it seems that drive is sufficiently high in awake animals for eupneic iDIAm EMG activity to be present as early as four days after a complete C2SH37, although it is not present consistently at one day post-injury23. In all cases, anesthesia suppresses iDIAm activity after upper cervical spinal hemisection, as highlighted in previous reports23,38 and shown in Figure 4 and Figure 5. There are no published data available on DIAm EMG activity in awake rats with the C2SH with spared dorsal funiculi proposed in the present manuscript (Figure 2A). Future work should provide a detailed characterization of the time course of DIAm EMG activity in awake animals with this C2SH model. That said, it is still prudent to perform validation of the continued absence of eupneic iDIAm inactivity on Day 3 post-injury under anesthesia to mimic the experimental conditions during which the spinal cord was initially lesioned/transected. When eupneic iDIAm EMG activity in awake animals at Day 3 post-injury may be present, it is noted that even half-doses of anesthetics will usually lead to a complete cessation of eupneic iDIAm EMG activity. With these doses, the animals are calm and sedate. In addition to verifying the silencing of eupneic iDIAm EMG activity during C2SH and at Day 3 post-injury, it is recommended that the spinal cord should be extracted after the terminal experiment to perform histological confirmation of the site and extent of the C2SH9,29,59. Histological confirmation of the injury—when combined with functional confirmation of silenced eupneic iDIAm EMG activity—provides strong evidence of a successful C2SH.
The present article presents a C2SH of cSCI in rats that leads to the immediate silencing of eupneic iDIAm EMG activity with continued silencing under anesthesia at Day 3 post-injury. Due to the sparing of the dorsal funiculus in the C2SH model proposed in the present manuscript, limb motor function is preserved, thereby avoiding off-target effects. This allows longitudinal studies of the effects of a high-cervical lesion on DIAm neuromotor control to be performed in rats that are otherwise relatively healthy, thus adding minimal caretaking burden for investigators. The validation of inactivity under anesthesia at Day 3 post-injury ensures that a clear baseline is established for assessing the subsequent extent of recovery of eupneic iDIAm EMG. This approach provides a rigorous, reliable, and reproducible method to perform a C2SH in rats. This model has the potential to greatly improve the understanding of the time course of respiratory neuroplasticity and its intersection with potential therapeutic strategies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the NIH funding source (NIH R01HL146114).
Materials
| 25 G Needle | Cardinal Health | 1188825100 | Covidien Monoject Hypdermic Standard Needles: 25 G x 1" (0.508 mm x 2.5 cm) A |
| 3-0 Vicryl Violet Braided | Ethicon | J774D | 3-0 Suture |
| Adson-Brown Forceps | Fine Science Tools | 11627-12 | Tip Shape: Straight, Tips: Shark Teeth, Tip Width: 1.4mm, Tip Dimensions: 2 x 1.4 m, Alloy / Material: Stainless Steel, Length: 12 cm |
| Bowman Style Cage | Braintree Scientific | POR-530 | Weight range: 250 up to 750 g; Maximum length: 9" (228 mm); Basic unit is constructed of .5" (123 mm) jeweled acrylic. |
| Castroviejo Needle Holder | Fine Science Tools | 12565-14 | Tip Shape: Straight, Tip Width: 1.5 mm, Clamping Length: 10 mm, Lock: Yes, Scissors: No, Alloy / Material: Stainless Steel, Length: 14 cm, Serrated: Yes, Feature: Tungsten Carbide |
| Clip Lead 1m TP Shielded | Biopac Systems, Inc | LEAD110S | Shielded lead wires for EMG |
| Data Acquisition Software | LabChart | LabChart 7.3.8 | Data recording, visualization, and analysis software for multi-channel recordings and real-time assessments |
| Data Analysis Software – Matlab 2023b | Mathworks, Inc. | Version 23.2 | General purpose programming language for post hoc analysis |
| Dissecting Knife | Fine Science Tools | 10056-12 | Cutting Edge: 4 mm, Thickness: 0.5 mm, Alloy / Material: Stainless Steel, Length: 12.5 cm, Blade Shape: Angled 30° |
| Dumont #3 Forceps | Fine Science Tools | 11293-00 | Style: #3, Tip Shape: Straight, Tips: Standard, Tip Dimensions: 0.17 x 0.1 mm, Length: 12 cm, Alloy / Material: Dumostar |
| Electromyogram Amplifier | Biopac Systems, Inc | EMG100C | EMG amplifier |
| Friedman Rongeur | Fine Science Tools | 16000-14 | Tip Shape: Curved, Cup Size: 2.5mm, Alloy / Material: Stainless Steel, Length: 13cm, Joint Action: Single |
| Friedman-Pearson Rongeurs | Fine Science Tools | 16021-14 | Alloy / Material: Stainless Steel, Length: 14cm, Joint Action: Single, Cup Size: 1mm, Tip Shape: Curved |
| Isolated Power Supply Module | Biopac Systems, Inc | IPS100C | Operates 100-series amplifier modules indepdent of the Biopac Systems, Inc.'s MP series Data Acquisition System |
| Kelly Hemostats | Fine Science Tools | 13019-14 | Tips: Serrated, Tip Width: 1.5mm, Clamping Length: 22mm, Alloy / Material: Stainless Steel, Length: 14cm, Tip Shape: Curved |
| Knife Curette | V. Mueller | VM101-4414 | Tip: Sharp, Tip Diameter: 2 mm |
| Micro Dissecting Scissors | Biomedical Research Instruments, Inc. | 11-2420 | Length: 4", Angle: Straight, Blade Length: 23 mm |
| Multistranded stainless steel wire | Cooner Wire, Inc. | AS 631 | AWG 40; Overall diameter: 0.011 mm (with insulation), 0.008 mm (without insulation). |
| PowerLab 8/35 | ADInstruments | PL3508 | Data acquisition system |
| Scalpel Blade #11 | Fine Science Tools | 10011-00 | Blade Shape: Angled, Cutting Edge: 20 mm, Thickness: 0.4 mm, Alloy / Material: Carbon Steel |
| Scalpel Handle #3 | Fine Science Tools | 10003-12 | Alloy / Material: Stainless Steel, Length: 12 cm |
| Sprague Dawley Rat | Inotiv | Order code: 002 | Sprague Dawley outbred rats (female and male) |
| Surgical Microscope | Olympus | SZ61 | Surgical microscope |
| Suture Cutting Scissors | George Tiemann & Co. | 110-1250SB | Alloy / Material: Stainless Steel, Tip Shape: Straight, Tips: Sharp/Blunt, Length: 4.5" |
| Vannas Spring Scissors | Fine Science Tools | 15000-08 | Tips: Sharp, Cutting Edge: 2.5 mm, Tip Diameter: 0.05 mm, Length: 8 cm, Alloy / Material: Stainless Steel, Serrated: No, Tip Shape: Straight |
| Weitlaner Retractor | Codman | 50-5647 | Prongs: 2 x 3 Blunt, Length: 4.5" |
References
- Center NSCIS. . Spinal cord injury model systems 2022 annual report – complete public version. , (2023).
- Punjani, N., Deska-Gauthier, D., Hachem, L. D., Abramian, M., Fehlings, M. G. Neuroplasticity and regeneration after spinal cord injury. N Am Spine Soc J. 15, 100235 (2023).
- El-Bohy, A. A., Goshgarian, H. G. The use of single phrenic axon recordings to assess diaphragm recovery after cervical spinal cord injury. Exp Neurol. 156 (1), 172-179 (1999).
- Fuller, D. D., et al. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 211 (1), 97-106 (2008).
- Fuller, D. D., et al. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 165 (2-3), 245-253 (2009).
- Golder, F. J., et al. Respiratory motor recovery after unilateral spinal cord injury: Eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 23 (6), 2494-2501 (2003).
- Golder, F. J., Reier, P. J., Davenport, P. W., Bolser, D. C. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 91 (6), 2451-2458 (2001).
- Goshgarian, H. G. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 72 (1), 211-225 (1981).
- Keomani, E., et al. A murine model of cervical spinal cord injury to study post-lesional respiratory neuroplasticity. J Vis Exp. 87, e51235 (2014).
- Miyata, H., Zhan, W. Z., Prakash, Y. S., Sieck, G. C. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 79 (5), 1640-1649 (1995).
- Moreno, D. E., Yu, X. J., Goshgarian, H. G. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 116 (3), 219-228 (1992).
- Porter, W. T. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 17 (6), 455-485 (1895).
- Zhan, W. Z., Miyata, H., Prakash, Y. S., Sieck, G. C. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 82 (4), 1145-1153 (1997).
- Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W., Feldman, J. L. Pre-botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 254 (5032), 726-729 (1991).
- Vinit, S., Gauthier, P., Stamegna, J. C., Kastner, A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 23 (7), 1137-1146 (2006).
- Warren, P. M., et al. Rapid and robust restoration of breathing long after spinal cord injury. Nat Commun. 9 (1), 4843 (2018).
- Fogarty, M. J., Dasgupta, D., Khurram, O. U., Sieck, G. C. Chemogenetic inhibition of TrkB signalling reduces phrenic motor neuron survival and size. Mol Cell Neurosci. 125, 103847 (2023).
- Gransee, H. M., Zhan, W. Z., Sieck, G. C., Mantilla, C. B. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One. 8 (5), e64755 (2013).
- Gransee, H. M., Zhan, W. Z., Sieck, G. C., Mantilla, C. B. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma. 32 (3), 185-193 (2015).
- Mantilla, C. B., Greising, S. M., Stowe, J. M., Zhan, W. Z., Sieck, G. C. TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp Neurol. 261, 190-195 (2014).
- Sieck, G. C., Gransee, H. M., Zhan, W. Z., Mantilla, C. B. Acute intrathecal BDNF enhances functional recovery after cervical spinal cord injury in rats. J Neurophysiol. 125 (6), 2158-2165 (2021).
- Fuller, D. D., Golder, F. J., Olson, E. B., Mitchell, G. S. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 100 (3), 800-806 (2006).
- Bezdudnaya, T., Hormigo, K. M., Marchenko, V., Lane, M. A. Spontaneous respiratory plasticity following unilateral high cervical spinal cord injury in behaving rats. Exp Neurol. 305, 56-65 (2018).
- Basso, D. M., Beattie, M. S., Bresnahan, J. C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12 (1), 1-21 (1995).
- Cloud, B. A., et al. Hemisection spinal cord injury in rat: The value of intraoperative somatosensory evoked potential monitoring. J Neurosci Methods. 211 (2), 179-184 (2012).
- Hurd, C., Weishaupt, N., Fouad, K. Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp Neurol. 247, 605-614 (2013).
- Fouad, K., Hurd, C., Magnuson, D. S. Functional testing in animal models of spinal cord injury: Not as straight forward as one would think. Front Integr Neurosci. 7, 85 (2013).
- Fouad, K., Popovich, P. G., Kopp, M. A., Schwab, J. M. The neuroanatomical-functional paradox in spinal cord injury. Nat Rev Neurol. 17 (1), 53-62 (2021).
- Fogarty, M. J., et al. Novel regenerative drug, SPG302 promotes functional recovery of diaphragm muscle activity after cervical spinal cord injury. J Physiol. 601 (12), 2513-2532 (2023).
- Prakash, Y. S., Miyata, H., Zhan, W. Z., Sieck, G. C. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 22 (3), 307-319 (1999).
- Sieck, G. C., Mantilla, C. B. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 169 (2), 218-225 (2009).
- Brown, A. D., et al. Mitochondrial adaptations to inactivity in diaphragm muscle fibers. J Appl Physiol. 133 (1), 191-204 (2022).
- Rana, S., Zhan, W. Z., Mantilla, C. B., Sieck, G. C. Disproportionate loss of excitatory inputs to smaller phrenic motor neurons following cervical spinal hemisection. J Physiol. 598 (20), 4693-4711 (2020).
- Mantilla, C. B., Rowley, K. L., Zhan, W. Z., Fahim, M. A., Sieck, G. C. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 146 (1), 178-189 (2007).
- Ford, G. T., Whitelaw, W. A., Rosenal, T. W., Cruse, P. J., Guenter, C. A. Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis. 127 (4), 431-436 (1983).
- Road, J. D., Burgess, K. R., Whitelaw, W. A., Ford, G. T. Diaphragm function and respiratory response after upper abdominal surgery in dogs. J Appl Physiol Respir Environ Exerc Physiol. 57 (2), 576-582 (1984).
- Rana, S., Sunshine, M. D., Greer, J. J., Fuller, D. D. Ampakines stimulate diaphragm activity after spinal cord injury. J Neurotrauma. 38 (24), 3467-3482 (2021).
- Ghali, M. G., Marchenko, V. Dynamic changes in phrenic motor output following high cervical hemisection in the decerebrate rat. Exp Neurol. 271, 379-389 (2015).
- Ditunno, J. F., Little, J. W., Tessler, A., Burns, A. S. Spinal shock revisited: A four-phase model. Spinal Cord. 42 (7), 383-395 (2004).
- Khurram, O. U., Gransee, H. M., Sieck, G. C., Mantilla, C. B. Automated evaluation of respiratory signals to provide insight into respiratory drive. Respir Physiol Neurobiol. 300, 103872 (2022).
- Khurram, O. U., Mantilla, C. B., Sieck, G. C. Neuromotor control of spontaneous quiet breathing in awake rats evaluated by assessments of diaphragm emg stationarity. J Neurophysiol. 130 (5), 1344-1357 (2023).
- Rana, S., Zhan, W. Z., Sieck, G. C., Mantilla, C. B. Cervical spinal hemisection alters phrenic motor neuron glutamatergic mRNA receptor expression. Exp Neurol. 353, 114030 (2022).
- Mantilla, C. B., Greising, S. M., Zhan, W. Z., Seven, Y. B., Sieck, G. C. Prolonged c2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 114 (3), 380-386 (2013).
- Jensen, V. N., Romer, S. H., Turner, S. M., Crone, S. A. Repeated measurement of respiratory muscle activity and ventilation in mouse models of neuromuscular disease. J Vis Exp. 122, e55599 (2017).
- Navarrete-Opazo, A., Mitchell, G. S. Recruitment and plasticity in diaphragm, intercostal, and abdominal muscles in unanesthetized rats. J Appl Physiol. 117 (2), 180-188 (2014).
- Redfern, M., Hughes, R., Chaffin, D. High-pass filtering to remove electrocardiographic interference from torso emg recordings. Clin Biomech (Bristol, Avon). 8 (1), 44-48 (1993).
- Seven, Y. B., Mantilla, C. B., Zhan, W. Z., Sieck, G. C. Non-stationarity and power spectral shifts in emg activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol. 185 (2), 400-409 (2013).
- Christensen, H., Sogaard, K., Jensen, B. R., Finsen, L., Sjogaard, G. Intramuscular and surface emg power spectrum from dynamic and static contractions. J Electromyogr Kinesiol. 5 (1), 27-36 (1995).
- Belman, M. J., Sieck, G. C. The ventilatory muscles. Fatigue, endurance and training. Chest. 82 (6), 761-766 (1982).
- Belman, M. J., Sieck, G. C., Mazar, A. Aminophylline and its influence on ventilatory endurance in humans. Am Rev Respir Dis. 131 (2), 226-229 (1985).
- Levine, S., Gillen, J., Weiser, P., Gillen, M., Kwatny, E. Description and validation of an ecg removal procedure for emgdi power spectrum analysis. J Appl Physiol. 60 (3), 1073-1081 (1986).
- Schweitzer, T. W., Fitzgerald, J. W., Bowden, J. A., Lynne-Davies, P. Spectral analysis of human inspiratory diaphragmatic electromyograms. J Appl Physiol Respir Environ Exerc Physiol. 46 (1), 152-165 (1979).
- Sharp, J. T. The respiratory muscles in emphysema. Clin Chest Med. 4 (3), 421-432 (1983).
- Sinderby, C., Spahija, J., Beck, J. Changes in respiratory effort sensation over time are linked to the frequency content of diaphragm electrical activity. Am J Respir Crit Care Med. 163 (4), 905-910 (2001).
- Dougherty, B. J., et al. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir Physiol Neurobiol. 183 (3), 186-192 (2012).
- Lane, M. A., et al. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol. 235 (1), 197-210 (2012).
- Burns, D. P., Murphy, K. H., Lucking, E. F., O’halloran, K. D. Inspiratory pressure-generating capacity is preserved during ventilatory and non-ventilatory behaviours in young dystrophic mdx mice despite profound diaphragm muscle weakness. J Physiol. 597 (3), 831-848 (2019).
- Dow, D. E., Mantilla, C. B., Zhan, W. Z., Sieck, G. C. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc. 2006, 1204-1207 (2006).
- Rana, S., Sieck, G. C., Mantilla, C. B. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J Neurophysiol. 117 (2), 545-555 (2017).

.
