A High-Throughput Method For Zebrafish Sperm Cryopreservation and In Vitro Fertilization
Summary
This is a high-throughput sperm cryopreservation protocol for zebrafish. Sperm cryopreserved using this protocol has an average of 25% fertility in subsequent vitro fertilization and is stable over many years.
Abstract
Protocol
Part A: Sperm Freezing Solutions
1. Ginsberg Fish Ringers (Make fresh every 3 days ;Ginsburg, 1963)
NOTE: ORDER OF ADDITION IS IMPORTANT TO PREVENT PRECIPITATION
In 450 ml sterile ddH2O dissolve:
- NaCl 3.25 g
- KCl 0.125 g
- CaCl2•2 H2O 0.175 g
Then add: - NaHCO3 0.10 g
Bring final volume to 500 ml with sterile ddH2O and store at 4°C.
2. Freezing Medium (Make fresh daily)
- WITHOUT Methanol
- Ginsburg Fish Ringers 10 ml (room temp.)
- Powdered Skim Milk 1.5 gm
- WITH Methanol
NOTE: ORDER OF ADDITION IS IMPORTANT TO PREVENT PRECIPITATION
- Ginsburg Fish Ringers 9 ml (room temp.)
- Methanol 1 ml
- Powdered Skim Milk 1.5 g
After assembling freezing media, mix well for 20 min. on orbital shaker or rocker. Before using, aliquot into 1 ml eppendorf tubes, avoiding surface bubbles.
Part B: Materials needed for sperm freezing
Materials needed for sperm freezing
- 10 μl disposable pipettes (Fisherbrand cat# 22-358697)
- 250ml beakers for fish water containing MESAB/Tricaine
- watch glasses (Pyrex cat# 9985-75)
- P20 pipetman (or equivalent) and tips
- Sponge fish holder (to hold male while squeezing)
- Dissecting microscope with above stage lighting
- Flat forceps (e.g. Millipore type)
- 2.0ml cryogenic vials (Corning cat# 430488)
- 10X10 cryoboxes (Nalgene cat#03-337-7AA)
- Styrofoam container filled with ~ 15 cm of finely crushed dry ice1
- 15 ml conical tubes (Falcon # 352099)
- Dewar flask containing liquid nitrogen
- Long tongs (e.g. CMS Fisher Health Care cat# 10-316B)
- Cryogloves
- Recovery tank for males
- One eppendorf tube filled with freezing media without methanol
- One eppendorf tube filled with freezing media with methanol
- Cryofreezer (e.g. Taylor-Warton K-series)
1 A simple method for making finely crushed dry ice is to wrap it in a towel and pulverize it with a hammer. A more efficient method is to use a dry ice grinder, e.g. Clawson model RE-2.
Part C: Sperm Freezing Method
- MARK CAPILLARY TUBES: Prior to beginning, mark the 10μl capillary tubes with a lab pen at the level of 3.33 μl (i.e. 16.7 mm from bottom; see Fig. 1.1).
- ANESTHETIZE MALE: Place male(s) in beaker containing MESAB/tricaine diluted in fish water (recipe in “The Zebrafish Book”)
- DRY FISH: Once anesthetized, lift fish out of anesthetic using a plastic spoon and gently blot fish dry on a paper towel, paying special attention to drying the ventral side. Water activates sperm so it is important to thoroughly dry around the cloaca. Position fish on sponge holder, ventral side up (Fig. 1.2). If region around anal fin is still moist, gently dry with a kimwipe. Take care to not squeeze sperm prematurely!
- Place marked capillary tube in rubber mouth pipette adapter that is included with capillary tubes. The adaptor is a rubber hose that has a mouthpiece on one end and a capillary holder on the other. Alternatively, the capillary tube can be connected to a 50μl Hamilton syringe via a short piece of tygon tubing of the appropriate diameter.
- Place male under the scope and expose the urogenital opening by carefully spreading the pelvic fins apart using the end of the capillary tube.
- COLLECT SPERM: Expel sperm by gently stroking the sides of the fish with smooth forceps (e.g. Millipore), or by gently squeezing the sides of the fish between your index finger and thumb. Collect sperm in capillary tube as it is expelled using gentle suction (Fig. 1.2). Avoid feces that may be expelled with sperm.
- NORMALIZE SPERM VOLUME TO 3.3 μl: If the volume of sperm reaches, or exceeds pen mark on capillary tube (i.e. 16.7 mm or greater), then proceed to step 8. If sperm volume does not reach target volume, then normalize volume to pen mark using Freeze Medium WITHOUT Methanol (Fig. 1.3). Minimum amount of sperm that is acceptable varies with quality of sperm. Good sperm is white and opaque. Poor sperm looks watery. In general, we accept as minimums: 1 μl good sperm (or 1/3 target) and 2 μl poor sperm (or 2/3 target). Volume is now normalized to 3.3 μl.
- ADD CRYOPROTECTANT TO SPERM : Suck up Freezing Medium WITH Methanol to the orange mark (approx. total volume is now 20 μl; Fig. 1.4). Expel sperm and cryoprotectant mixture onto clean area of a watch glass, paying special attention to not introduce bubbles. Gently mix by pipetting up and down ~ 4 times (avoid introducing bubbles).
- PLACE 10μl SPERM INTO EACH OF 2 CRYOVIALS: Pipette 10 μl of the sperm:cryoprotectant solution into the bottom of two separate cryovials that have been labeled with relevant information (Fig. 1.5). Moving quickly, cap vials and drop them into the bottom of room temperature 15 ml Falcon tubes- one cryovial/tube. Cap Falcon tube and insert the pair of cryovial-containing Falcon tubes into crushed dry ice.
- FREEZE SPERM FOR 20 MIN. ON DRY ICE: Dry ice should be in fine powder form, not pellets. The tubes should be inserted into the dry ice deep enough that only their caps show (Fig. 1.6). In order to keep track of tubes in dry ice, give each pair of Falcon tubes a number from 1-20 (written on the caps) and record the time they go into the dry ice.
- PLACE CRYOVIALS INTO LIQUID NITROGEN: After the sperm has been frozen on dry ice for 20 min., transfer them to a liquid nitrogen- containing dewar flask. Sperm is stored here until time to place into liquid nitrogen freezer. When placing in the freezer boxes, place freezer box in a bath of liquid nitrogen so that samples do not heat up. Use long metal tongs to recover vials from dewar flask and handle with cryogloves. Alternatively, cryovials can be transferred directly from dry ice into the freezer box if the freezer box is kept immersed in liquid nitrogen in a Styrofoam container.
- Store cryovials long term in a cryogenic liquid nitrogen freezer. To maintain viability of sperm, it is important that vials are stored immersed in liquid nitrogen. In our experience, vials stored in the vapor phase lose viability over time.
Speed is important! The time between adding the cryoprotectant and placing sperm vials in dry ice (i.e. steps 6-10) should be no more than 30 sec.
Part D: In Vitro Fertilization Method Using Cryopreserved Sperm
We find that this works best with two people. One person squeezes eggs from females while the other person thaws the sperm.
- Set a water bath to 33°C.
- Remove cryovial from liquid nitrogen freezer and transfer to liquid nitrogen-containing dewar flask until ready to thaw.
- Squeeze eggs from females into 35mm plastic petri dish. If possible, try to obtain 3 clutches of eggs and combine into one dish. If you cannot get three good clutches within 1 min of your first clutch, then proceed to step 4 (one good clutch is sufficient). Definition of good clutch: >150 uniformly nice looking eggs (i.e. yellowish, with no white debris indicative of degradation). Keep dish covered while sperm is thawed.
- Remove vial from liquid nitrogen and remove cap. Quickly immerse vial ~1/2 way into 33°C water bath for 8-10 sec.
- Quickly add 70 μl room temperature Hanks to vial and mix by pipetting up and down. Immediately add to eggs and mix gently with pipette tip.
- Without delay, activate sperm and eggs by adding 750 μl fish water. Swirl to mix. Incubate 5 min at room temperature.
- After 5 min, fill dish with fish water and incubate dish at 28°C. After 2-3 hrs, count and transfer fertile embryos to 100mm dishes (50/dish). Count infertile eggs so that fertilization frequency can be determined.
- Take good care of the larvae! For first 5 days, change water daily and remove dead embryos. Put only larvae that have swim bladders into nursery on day 5.
Part E: Representative Results
We used this crypreservation methodology to make a library of 8600 frozen sperm samples for TILLING between the years 2001 and 2003. We have thawed 106 of these samples and determined their fertility. Fertility is the ratio of viable embryos produced in an in vitro fertilization divided by the total number of eggs that were fertilized with the frozen sperm. Fresh (non cryporeserved) sperm typically has >95% fertility; cryopreserved sperm has much lower fertility: an average of 29% (n=106 vials). The vial-to-vial variability in fertility is large, ranging from less than 1% to as high as 62%. This variability is represented by the large error bars representing standard deviation in Fig. 2. However in 106 sperm samples thawed to date we have only once experienced 0% fertility in both sperm samples and failed to recover the corresponding TILLING mutation.
By thawing sperm vials from our library over the course of several years (2001-2008) we have found that the average fertility of sperm samples frozen by this method remains stable over time (Fig. 2). The average fertility of frozen sperm samples thawed in 2001 was 23.5% (based on 11 sperm samples) and in 2008 was 25% (based on 21 sperm samples).
A number of other investigators are now using this sperm freezing protocol in their labs. Some have reported good success with fertility rates and sample-to-sample variability similar to what we have observed. Others have reportedly failed to make this protocol work in their hands. We believe that likely sources of problems are:
- Failure to use the exact cryovials or conical tubes we have suggested in the protocol, leading to a different rate of cooling when in dry ice;
- Failure to use powdered dry ice, leading to a sub-optimal cooling rate;
- Using male fish with poor fertility: you should get at least 1 μl of sperm from a single male. You can also test male fertility by doing an in vitro fertilization with fresh sperm, however this can be misleading because it takes very little fresh sperm to get near 100% fertility.
- Using sub-standard quality eggs in the in vitro fertilization. Using anything but perfect clutches always leads to near 0% fertility, even from sperm samples that actually have good fertility (based on the fertility of the duplicate vial).
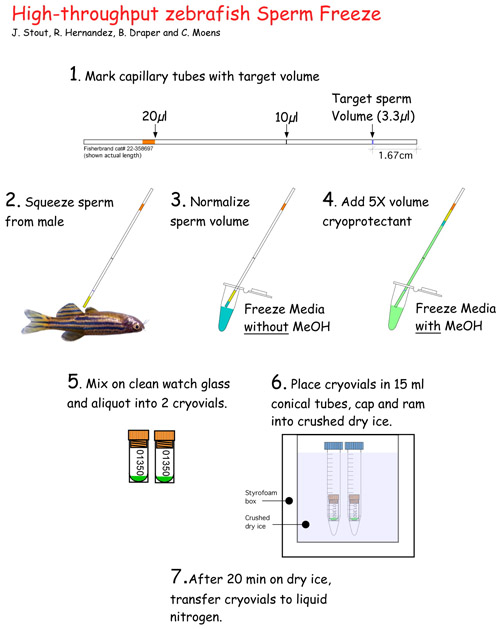
Figure 1. Schematic of sperm freezing methodology.
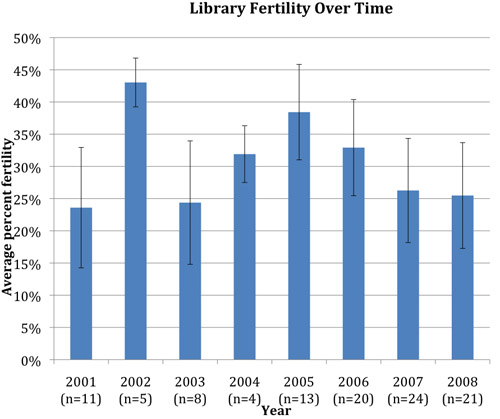
Figure 2. Cryopreserved sperm fertility remains stable over time. Percent fertility is the number of viable embryos produced in an in vitro fertilization divided by the total number of eggs that were fertilized with the frozen sperm. “n” refers to the number of cryopreserved sperm vials whose fertility was tested in the corresponding year. Error bars represent standard deviation. Since each sperm vial is from a different male, vial-to-vial variability is observed.
Discussion
A number of other investigators are now using this sperm freezing protocol in their labs. Some have reported similar fertility and the same sample-to-sample variability that we have observed. Others have reportedly failed to make this protocol work in their hands. We believe that likely sources of problems are:
- Failure to use the exact cryovials or conical tubes we have suggested in the protocol, leading to a different rate of cooling when in dry ice;
- Failure to use powdered dry ice, leading to a sub-optimal cooling rate;
- Using male fish with poor fertility: you should get at least 1 μl of sperm from a single male. You can also test male fertility by doing an in vitro fertilization with fresh sperm, however this can be misleading because it takes very little fresh sperm to get near 100% fertility. Larger, more robustly colored males typically give more higher quality sperm.
- Using sub-standard quality eggs in the in vitro fertilization. Using anything but perfect clutches always leads to near 0% fertility, even from sperm samples that actually have good fertility (based on the fertility of the duplicate vial).
Acknowledgements
This work was supported by NIH R01 HG002995 “TILLING the Zebrafish Genome: a Reverse Genetics Approach” to CBM.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| 10 μl disposable pipettes | Fisher | 22-358697 | ||
| Watch glasses | Pyrex | 9985-75 | ||
| 2 ml cryogenic vials | Corning | 430488 | ||
| 10 x 10 cryoboxes | Nalgene | 03-337-7AA | ||
| 15 ml conical tubes | Falcon | 352099 | ||
| Tongs | Fisher Health Care | 10-316B | ||
| Cryofreezer | Taylor-Warton | K-series | For example |
References
- Ginsburg, A. S. Sperm-egg association and its relationship to activation of the egg in salmonid fishes. J. Embryol. Exp. Morphol. 11, 13-33 (1963).
- Harvey, B., Kelley, R. N., Ashwood-Smith, M. J. Cryopreservation of zebra fish spermatozoa using methanol. Can. J. Zoology. 60, 1867-1870 (1982).

