Quantitative Real-Time PCR using the Thermo Scientific Solaris qPCR Assay
Summary
The Solaris qPCR Gene Expression Assays are novel pre-designed qPCR primer/probe combinations designed to simplify the qPCR process without sacrificing the specificity and robustness of the assay.
Abstract
The Solaris qPCR Gene Expression Assay is a novel type of primer/probe set, designed to simplify the qPCR process while maintaining the sensitivity and accuracy of the assay. These primer/probe sets are pre-designed to >98% of the human and mouse genomes and feature significant improvements from previously available technologies. These improvements were made possible by virtue of a novel design algorithm, developed by Thermo Scientific bioinformatics experts.
Several convenient features have been incorporated into the Solaris qPCR Assay to streamline the process of performing quantitative real-time PCR. First, the protocol is similar to commonly employed alternatives, so the methods used during qPCR are likely to be familiar. Second, the master mix is blue, which makes setting the qPCR reactions easier to track. Third, the thermal cycling conditions are the same for all assays (genes), making it possible to run many samples at a time and reducing the potential for error. Finally, the probe and primer sequence information are provided, simplifying the publication process.
Here, we demonstrate how to obtain the appropriate Solaris reagents using the GENEius product search feature found on the ordering web site (www.thermo.com/solaris) and how to use the Solaris reagents for performing qPCR using the standard curve method.
Protocol
1. The Thermo Scientific Solaris qPCR Assay Design Algorithm
- The algorithm used in the design of Solaris qPCR probe/primer pairs adjusts the Tm of each component thereby enabling universal cycling conditions. Incorporation of the MGB, or minor groove binding, moiety in the probe increases the Tm and allows for shorter probes to be designed for greater assay flexibility and performance. As their name implies, MGBs are a class of antibiotics that can fit into the minor groove of the DNA helix. MGBs are attached to the 3 or 5 end of a DNA probe. In solution, the FAM reporter fluorescence of the probe is quenched by Eclipse Dark Quencher. When the probe binds to a target sequence, the resulting change in the probe s 3-D conformation allows a strong fluorescent signal. Binding of the DNA probe to target sequence is stabilized by the MGB, which allows the use of highly specific, shorter probes while maintaining the appropriate melting temperature for PCR. Thus, signal detection occurs during the annealing phase of target amplification. During the extension step, the MGB probe disassociates and the fluorescence is again quenched. Because the probe disassociates, it does not affect PCR efficiency.
- Another feature of the Solaris algorithm is the use of Superbases. Superbases are modified bases that have enhanced binding abilities to improve stability of A-T bonds and eliminate G-G self-association in guanine rich sequences. Moreover, they do not quench adjacent fluorophores attached at the 5 terminus. These bases are selectively placed, according to the algorithm, to expand the availability of target sequences. This makes it possible to use sequences, such as G-C rich regions, that would otherwise be avoided in designing primers and probes.
- Whenever possible, the Solaris algorithm incorporates exon junction spanning regions in the target site. This increases specificity by avoiding the amplification of contaminant genomic DNA.
- Importantly, the algorithm also identifies a consensus sequence for all known splice variants, such that all are covered by a single probe/primer set. So, only one assay is needed for a particular gene of interest.
- Finally, the Solaris qPCR Assay design algorithm utilizes very stringent parameters for critical design considerations including GC content, length, Tm, and stretches of homogenous nucleotides. The resulting sequences are BLAST analyzed for specificity using 3 different databases: genomic, transcript, and pseudogene.
2. Obtaining Solaris Reagents
- To obtain the appropriate Solaris reagents, visit www.thermo.com/solaris
- Once at the website click on “GENEius Product Search”. To go directly to the GENEius product search, go to www.thermo.com/SolarisSearch
- Click in the search inquiry box and choose the appropriate target organism.
- The GENEius product search can process many different gene identifiers. Here, enter one of the suggested search terms to find the gene of interest, including Ref Seq Accession, the Gene ID, the Gene Symbol, a Gene Description, the Sanger ID or Accession number, or a catalog number. Then, click on “Search”.
- Identify the gene of interest and click on “Select” on the left hand side. This will open a window that shows the different Thermo Scientific products available for the gene of interest. Under “Products” select the “qPCR Detection” Tab.
- Under the “Solaris Gene Expression Assay” heading, you will see one optimally designed Solaris probe/primer assay. This one assay will cover all known splice variants of your gene target. Choose the desired quantity. They are available in 100, 200, 400, and
1000 X 25 μl reactions (if the assay is identified as made-to-order, the 100 pack size will not be available). Click on “Add to Cart”. - Next, select the appropriate master mix for the thermal cycler that will be used. To determine which master mix will work best with the machine available, click on “Find out which Master Mix works best with your PCR Cycler!”
- Find the qPCR cycler that will be used on the menu to determine which mix to use.
- Return to the GENEius product search window, and select the appropriate master mix. The master mix comes in 100, 200, 400, and 1000 reaction sizes. Click “Add to Cart”.
- Suggested reference genes and verso cDNA synthesis kits can also be ordered for a complete qRT-PCR analysis.
- When the Solaris qPCR reagents arrive, store them at -20°C until ready for use. The following reagents should be received: A 20X solution containing two primers provided at 800 nM each and one gene specific probe provided at 200 nm, sequence information for both the probe and the primers, and Solaris qPCR Gene Expression Master Mix at a 2X concentration. Solaris qPCR Gene Expression Assays are stable for a minimum of 12 months. Avoid repeated freeze thawing.
3. qPCR setup
- Begin the qPCR protocol with cDNA. Total RNA should be extracted using a spin column method. The Thermo Scientific Verso cDNA synthesis kit is recommended for reverse transcription to generate cDNA.
- Prepare for qPCR by thawing the primers, probes, and master mix on ice. Also have on hand PCR grade water and opaque white qPCR plates, which can improve the sensitivity of real-time PCR.
- The master mix contains all components for real time PCR including: 1) Thermo-Start DNA polymerase, a hot-start version of Thermoprime DNA polymerase. This type of polymerase is used to prevent non-specific amplification during the reaction-set up and requires activation step at 95°C 15 min. 2) dNTPs in the Solaris Master mix, dTTP replaces dUTP to maximize amplification efficiency 3)Proprietary reaction buffer, optimized to work with Solaris primer/probe assays. This reaction buffer contains blue dye to increase contrast between reagent and plastic. 4)ROX, if your qPCR instrument requires ROX.
- Once the solutions have thawed, mix by flicking the tube (do not vortex). Spin them down to prevent loss of reagent.
- Next, prepare a 15 mL sterile Falcon tube to set up the reaction mix for the gene of interest, plus one for the reference gene, scaling up depending on the number of samples. Prepare enough such that three replicates can be prepared for each sample, including a no template control (NTC) and standard curve samples, if the standard curve method will be used for analysis.
- For each sample run on a 96 well plate, mix the following: 5 μL of the 2X Solaris qPCR Master Mix, .5 μl of the Solaris Primer/Probe set (20X), and PCR grade water such that the final reaction volume, once the cDNA is added will be 25 μL. For each sample run on a 384 well plate mix the following: 12.5 μL of the 2X Solaris qPCR Master Mix, 1.25 μl of the Solaris Primer/Probe set (20X), and PCR grade water such that the final reaction volume, once the cDNA is added will be 10 μL.
- Using a multi channel pipettor, transfer the master mix to the appropriate wells of the plate. The blue dye included in the master mix will aid in tracking pipetting progress on the white plates. Then, add the 1-5 μL cDNA template (for a 96 well plate) or 1-2 μL of cDNA template (for a 384 well plate). Pipette up and down to mix. Place the plate in a centrifuge and spin down to remove any bubbles, as these will interfere with the fluorescence readings.
- Program the thermal cycler: The enzyme must be activated with a 95°C 15 min (1 cycle). Then 40 cycles of 95°C, denaturation for 15 seconds, and annealing and extension at 60°C for 60 seconds.
- Place the plate in the qPCR instrument. Start the program.
Representative qPCR results using Solaris
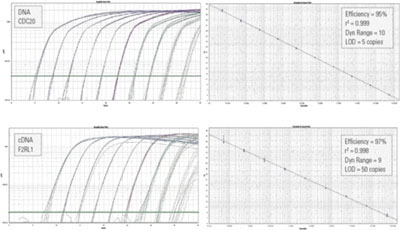
Figure 1. Solaris assays give reliable detection even at very low input concentrations, as judged by the PCR efficiency and r2 values. Ten 10-fold dilutions of cDNA synthesized from synthetic RNA amplicon sequence or DNA amplicon sequence was amplified on an ABI 7900HT instrument using Solaris qPCR Gene Expression Assay for F2RL1 or CDC20, respectively. The log-scale amplification curves and standard curves are shown along with the performance of each assay including efficiency, r2 value, dynamic range out of 10 log10 dilutions, and the lower limit of detection.
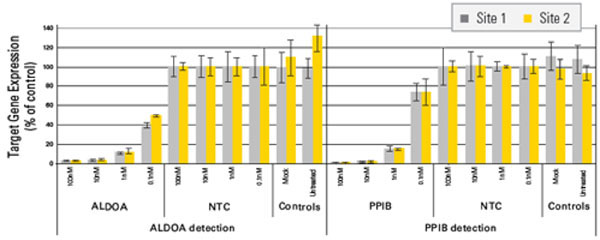
Figure 2. Solaris Assays give consistent results even between different researchers and laboratories. siRNAs targeting ALDOA and PPIB, as well as a Non-targeting Control (NTC), were transfected into HeLa cells at 100, 10, 1 and 0.1 nM final concentrations. Cells were harvested and total RNA isolated 48 hrs post-treatment. cDNA was synthesized using Thermo Scientific Verso cDNA Synthesis Kit. An aliquot of the cDNA was amplified in two geographically separated laboratories using Solaris qPCR Gene Expression Assays for detection of ALDOA, PPIB, and GAPDH on a Roche LightCycler 480 (384-well) platform. Knockdown was calculated using the ΔΔCq method (normalized to GAPDH reference gene and NTC-treated cells). The same levels of knockdown were demonstrated for both gene targets between both researchers at both sites.
Discussion
Thermo Scientific Solaris qPCR Assays provide a simple, improved method for performing qPCR analysis. Using a high stringency algorithm, incorporating MGB technology and Superbases, these primer and probe sets maximize the fidelity and robustness of qPCR experiments. One primer and probe set recognizes all known splice variants of a given gene. The target site, whenever possible, includes exon spanning regions, to ensure that genomic DNA is not amplified. These features make it possible to run numerous samples simultaneously, greatly reducing the amount of time needed to perform complex experiments. Since the master mix is blue, it is possible to use white opaque plates, which improve the sensitivity of the qPCR, without losing track of pipetting.
Taken all together, the design features of the Solaris qPCR Assay make this novel qPCR assay a simple, yet high-performance assay that is both specific and robust.
Disclosures
The authors have nothing to disclose.
Materials
| Name of the reagent / material | Company | Catalogue Number | Comments |
| Solaris aPCR Gene Expression ROX Master Mix (Intro Pack Size) | Thermo | AB-4351/INT | 100 x 25 μL rxns |
| Solaris qPCR Gene Expression ROX Master Mix | Thermo | AB-4351/A | 200 x 25 μL rxns |
| Solaris qPCR Gene Expression ROX Master Mix | Thermo | AB-4351/B | 400 x 25 μL rxns |
| Solaris qPCR Gene Expression ROX Master Mix | Thermo | AB-4351/C | 1000 x 25 μL rxns |
| Solaris qPCR Gene Expression Assays | Thermo | AX-XXXXXX-XX-XXXX* | 100 rxns (1 x 125 μL) 200 rxns (2 x 125 μL) 400 rxns (4 x 125 μL) or 1000 rxns (10 x 125 μL) |
| Verso cDNA Synthesis Kit | Thermo | AB-1453/A AB-1453/B |
40 x 20 μL rxns 100 x 20 μL rxns |
| ABgene 96-Well Plate (Skirted, Low Profile, White) ** | Thermo | AB-0800/W | 25 plates |
| ABgene Diamond Ultra 384-Well PCR Plate, White ** | Thermo | AB-2150/W | 25 plates |
| Absolute qPCR Adhesive Seal | Thermo | AB-1170 | 50 sheets |
*Catalog number is product specific. Please refer to www.thermo.com/solaris to select the appropriate assay
**For cycler compatibility and color choices, see our lates catalog or visit www.thermo.com/pcrplastics

