A Riboflavin and UV Light Treatment of Platelet Products to Eliminate Bacteria
Abstract
Source: Keil, S. D., et al. Treatment of Platelet Products with Riboflavin and UV Light: Effectiveness Against High Titer Bacterial Contamination. J. Vis. Exp. (2015).
This video demonstrates the riboflavin and UV light-based pathogen reduction process against high-titer bacterial contamination in human platelet products. This process could help to lower the risk of severe adverse transfusion events associated with bacterial contamination.
Protocol
1. Platelet Product Preparation
- Collect a standard apheresis human platelet product at an accredited blood bank. The platelet product must meet the following collection specifications: 90–360 ml volume and 0.8 –3.8 x 106 platelets/µl concentration.
- Ensure that the platelet product has rested for a minimum of 2 hr prior to processing for pathogen reduction. Store the products at 22 ± 2 °C in a platelet incubator without agitation for these 2 hr. After two hours, ensure the platelet product is agitated at 22 ± 2 °C in a platelet incubator. Use the product for up to 22 hours post-collection.
- Using a 3 ml syringe, remove a 2 ml sample to measure the pH22C of the platelet product. Use a blood gas analyzer to ensure the product has a pH >6.6. Use the same sample to measure the platelet concentration of the platelet product using a hematological analyzer. Ensure the product meets the 0.80–3.8 x 106 platelets/µl collection concentration requirement.
- Visually evaluate the swirl of the platelet product. If the product has a "0" swirl, the product will be discarded.
- Hold the platelet bag up under a direct white-light source and squeeze the platelet bag briefly. Visually observe the swirling pattern of the platelet sample. Score the product using the following scale.
NOTE: 2 – Positive Swirl: Swirling inhomogeneity visible throughout the entire bag with strong contrast. 1 – Intermediate Swirl: Some inhomogeneity visible, but only in a few places and with poor contrast. 0 – Negative Swirl: Turbid homogeneity remaining before and after squeezing the platelet bag
2. Riboflavin and UV Light Process
- Transfer the platelet product to a riboflavin and UV light illumination and storage bag using a sterile tubing docking device. After transfer, remove the empty platelet unit collection bag using a tubing sealer and discard the empty bag as biohazardous waste.
- Remove a 35 ml pouch of 500 μM riboflavin stock solution from its light protective outer wrap. Sterile dock the riboflavin kit onto the inlet line of the treatment and storage bag. Allow the contents to drain into the platelet unit.
- Hang the riboflavin pouch/treatment and storage bag set from the riboflavin pouch and gently express residual air out of the treatment and storage bag and into the riboflavin bag. Ensure a small amount of platelet product is also expressed into the riboflavin pouch to rinse any residual riboflavin into the platelet product. Allow the riboflavin and platelet solution to drain back into the treatment and storage bag, and then clamp the inlet line. Remove the empty riboflavin bag using a tubing sealer and discard the empty bag as biohazardous waste.
- Sterile dock a 6" tubing line with a female syringe connector onto the inlet line of the illumination and storage bag. Attach a 10 ml syringe barrel (remove the plunger) onto the female syringe connector.
- Add 5 ml of stock bacteria into the syringe barrel via a pipette. Open the clamp on the inlet line and allow the bacteria to drain into the platelet product. Rinse the syringe barrel by allowing some platelet product to flow back into the 10 ml syringe barrel. Remove the 10 ml syringe barrel.
- Aseptically attach a 30 ml syringe and flush the inlet port at least two times to ensure the stock bacteria is mixed into the platelet product. After the product is well mixed, use a clean 5-10 ml syringe and remove a 2 ml pre-treatment sample. Using a new syringe, ensure any air introduced from the inoculation step or sampling step is removed.
- Titer the pre-treatment sample per section 3.
- Remove the inlet line from the treatment and storage bag and weigh the product.
- Place the product into the illuminator and follow the onscreen commands to start a standard platelet treatment.
- Enter in Operator ID.
- Secure and mount the bag to the Illuminator platen.
- Enter the Sample ID into the Illuminator using either the barcode reader or the manual entry keypad.
- Scan product type.
- Close the quartz clamp.
- The illumination device will automatically deliver 6.24 J/ml of energy. The typical treatment time takes 5-10 min.
- Following treatment, sterile dock a 6" tubing line with a female syringe connector onto the sample bulb line of treatment and storage bag. Ensure the product is well mixed, and then attach a 5 ml syringe and remove a 2 ml post-treatment sample.
- Titer the post-treatment sample per section 3.
3. Bacterial Titer
- Estimate the titer of the pre-treatment and post-treatment samples and prepare the appropriate number of 5 ml dilution tubes needed to perform an end-point serial dilution of each sample. Aseptically fill the required tubes with 1.8 ml of peptone water.
- Titer each sample using a 10-fold serial dilution scheme. Aseptically transfer 0.2 ml from the undiluted sample to the first dilution tube (10-1). Vortex this dilution tube and transfer 0.2 ml to the next dilution tube. Continue the serial dilution through the required number of dilutions.
- Aseptically transfer 100 µl of each dilution to be plated to an appropriate agar plate (Table 1).
- Using a sterile cell spreader, evenly distribute the sample onto each agar plate.
- Invert and incubate the plates at the appropriate conditions (Table 1) for 1-2 days, checking for growth periodically.
- Count plates with 30–300 colonies. Plates containing greater than 300 colonies are considered too numerous to count (TNTC). Plates containing less than 30 colonies are considered to be below the limit of quantification (LOQ).
- Calculate the titer of each test sample and record the results as CFU/ml.
- Use the following equation to calculate CFU/ml:

- If there are no colonies on the lowest dilution plate, use the following equation to calculate the Limit of Detection (LOD):
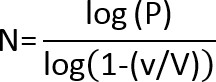
and
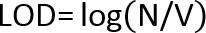 3
3
Where:
N =lowest number of particles (CFU) in a product that can be detected with confidence.
P = probability that a microorganism will be undetected; for a 95% confidence of detecting a microorganism, P = .05.
V = total product volume in ml
v =volume plated (ml) x dilution level of plate
- Use the following equation to calculate CFU/ml:
- For Negative Control, plate 0.5 ml of the peptone water that is used for the serial dilutions onto each of the two agar plates. Use the same plate type as the samples to be tested. Invert and incubate the plate at the appropriate conditions for 1-2 days, checking for growth periodically.
- If growth is observed on either agar plate, the bottle of peptone water used must be discarded; the results of the study are also invalidated.
- For Positive Control, re-titer the stock culture of bacteria that was used. Invert and incubate the plates at the appropriate conditions for 1-2 days, checking for growth periodically. The titer shall be ± 1.0 log CFU/ml of the recorded stock titer (See section 1.5) for the study to be considered valid.
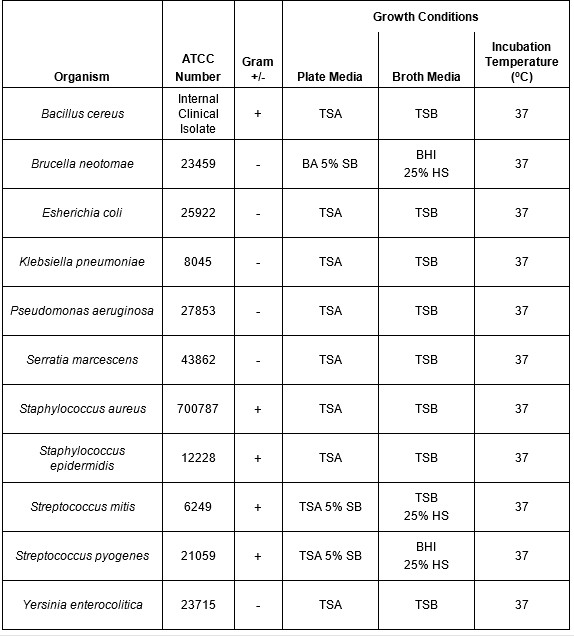
Table 1: Growth conditions for bacterial species evaluated using riboflavin and UV light. A panel of both Gram-negative and Gram-positive bacteria that have been shown to contaminate platelet products were selected for this study. The growth conditions (temperature and media type) used to propagate and titer the organisms are shown.
Disclosures
The authors have nothing to disclose.
Materials
| Mirasol Illuminator | Terumo BCT | N/A | |
| Mirasol Treatment/Storage Bag | Terumo BCT | N/A | |
| Hematology Analyzer | Beckman-Coulter | Ac•T diff | |
| Blood Gas Analyzer | Siemens | RapidLab 1265 | |
| Sterile Docking Device | Terumo BCT | TSCD | |
| Platelet Incubator | Helmer | PC2200 | |
| Orbital Incubator Shaker | Lab-Line | 4628 | |
| Dry Incubator | Fisher | Iso-temp | |
| Class II A/B3 Biosafety Cabinet | Thermo-forma | 1286 | |
| Tubing Sealer | Sebra | Smart Sealer II | |
| Table Top Centrifuge | IEC | Centra GP8R | |
| 10 ml Syringe | BD | 309604 | |
| 30 ml Syringe | BD | 309650 | |
| 5 ml Dilution Tube | VWR | 211-0058 | |
| 50 ml Conical Tube | Corning | 430290 | |
| Micropipette | Gilson | P-200 | |
| Micropipette | Rainin | R-200 | |
| 25 ml Disposable Serological Pipette | Costar | 4489 |

