Procedures for Rat in situ Skeletal Muscle Contractile Properties
Summary
This video demonstrates the surgical preparation and procedures needed to study the contractile responses of the rat medial gastrocnemius muscle preparation in situ. This preparation allows measurement of skeletal muscle contractile properties under physiological conditions. The animal is anesthetized and the muscle is separated from surrounding tissue at its distal end. The Achilles tendon is attached to a force transducer, allowing measurement of the muscle’s contractile response at 37 degrees C with an intact circulation.
Abstract
There are many circumstances where it is desirable to obtain the contractile response of skeletal muscle under physiological circumstances: normal circulation, intact whole muscle, at body temperature. This includes the study of contractile responses like posttetanic potentiation, staircase and fatigue. Furthermore, the consequences of disease, disuse, injury, training and drug treatment can be of interest. This video demonstrates appropriate procedures to set up and use this valuable muscle preparation.
To set up this preparation, the animal must be anesthetized, and the medial gastrocnemius muscle is surgically isolated, with the origin intact. Care must be taken to maintain the blood and nerve supplies. A long section of the sciatic nerve is cleared of connective tissue, and severed proximally. All branches of the distal stump that do not innervate the medial gastrocnemius muscle are severed. The distal nerve stump is inserted into a cuff lined with stainless steel stimulating wires. The calcaneus is severed, leaving a small piece of bone still attached to the Achilles tendon. Sonometric crystals and/or electrodes for electromyography can be inserted. Immobilization by metal probes in the femur and tibia prevents movement of the muscle origin. The Achilles tendon is attached to the force transducer and the loosened skin is pulled up at the sides to form a container that is filled with warmed paraffin oil. The oil distributes heat evenly and minimizes evaporative heat loss. A heat lamp is directed on the muscle, and the muscle and rat are allowed to warm up to 37°C. While it is warming, maximal voltage and optimal length can be determined. These are important initial conditions for any experiment on intact whole muscle. The experiment may include determination of standard contractile properties, like the force-frequency relationship, force-length relationship, and force-velocity relationship.
With care in surgical isolation, immobilization of the origin of the muscle and alignment of the muscle-tendon unit with the force transducer, and proper data analysis, high quality measurements can be obtained with this muscle preparation.
Protocol
1. Introduction
- The MacIntosh lab has been using the medial gastrocnemius muscle preparation for several years, and before that, the whole gastrocnemius muscle preparation, as developed with Dr. Phil Gardiner.1
2. Anesthesia
- Adult Sprague-Dawley rats (200-300 g) are typically used in our lab for the study of contractile properties in situ. The rat can be constrained in a plexiglass device, commercially available, or by covering with a towel, and holding.
- We use ketamine/xylazine, (100 mg·ml-1, each) mixed in 85:15, and administer 0.1 ml per 100 g of rat weight intramuscularly 2,3. Sodium pentobarbital (50-60 mg·kg-1, intraperiteneal) or isoflurane (2-3.5%, inhaled) may also be used4.
- While the anesthetic is taking effect, a precise weight can be obtained and the left hind-limb shaved. This is also a good time to be sure all electronics are turned on and ready. This includes computers, strain gauge amplifier, oil heater and others.
- Check for return of reflex response periodically and supplement the anesthesia as needed (0.05 to 0.1 ml per 100 g).
3. Initiating Surgery
- We use a plexiglass platform on which to immobilize the rat for surgery. Simple masking tape does the job. Be sure the animal is stretched from left hindlimb to right forelimb. Add a drop of lubricant to the eyes. We use paraffin oil. Otherwise, the eyes will dry out with ketamine anesthesia.
- A small surgical light is useful. This one emits enough heat to help keep the animal warm. Alternatively, a water heating blanket can be used. Check to be sure corneal or toe-pinch reflexes are absent before proceeding. Sterile technique is not necessary, because this procedure is acute. The animal will not recover from the anesthesia. We euthanize the rat with anesthetic overdose (0.2 ml, intracardiac) when all procedures are completed.
- The first incision is through the skin from the heel to the vertebral column. We use scissors, because the depth of the cut can be controlled and the scissors are used to separate the skin from the underlying tissues. Rats have excellent haemostasis, so as long as you avoid large blood vessels, bleeding will be limited. Keep exposed surfaces covered with isotonic saline-soaked gauze, whenever possible.
- After the skin is separated from the underlying connective tissue, the superficial muscle layer is cut. Be sure not to go too deep; you do not want to damage the sciatic nerve or blood vessels, or the muscle of interest. Begin over the gastrocnemius muscle and cut proximally, along the same line as the skin incision. Peek underneath to locate and avoid the sciatic nerve. Once you see the nerve, you can follow its path with your incision, but stay well above the nerve. There is a clear seam between muscles. Locate this, and cut along that seam towards the knee. Watch for and avoid blood vessels.
4. Prepare pilot hole in femur for bone pin
- Cut through the thin layer of muscle over the caudal aspect of the femur, exposing the bare bone. Using a hand-held rotary tool, drill, or pin vice, (0.9 mm carbon steel bur), make a small pilot hole through the cortex, and just into the medulla. Do not drill too deeply, or excessive bleeding will result. This hole will be used later to place a bone pin to immobilize the femur on the myograph base.
5. Isolate innervation of the medial gastrocnemius muscle
- The next step requires a dissecting microscope. Locate the place where the popliteal nerve disappears behind the medial gastrocnemius muscle. Gently spread out the various branches, and cut all that do not innervate the medial gastrocnemius muscle. Innervation can be determined by microstimulation. At this stage, also make sure the superficial branches of the sciatic nerve are cut.
- Now clear the connective tissue away from the sciatic nerve, so it can be slipped into the nerve cuff (later). Be sure to treat the nerve gently. Any stretch of the nerve can lead to inexcitability, and end your experiment prematurely.
6. Isolate the Achilles tendon and gastrocnemius muscle
- Blunt dissection, with occasional help from scissors, can be used to separate the gastrocnemius muscle from other tissues. The tendon of the plantaris can be pulled out from under the Achilles tendon, tied and cut. The tie is merely used to help hold the tendon and can be cut away right after the tendon is cut and the plantaris is separated from the gastrocnemius muscle for a substantial length. Place a #1 silk ligature around the Achilles tendon, and tie in a square knot. Do not pull too tightly, or the tendon will be damaged. We use bone rongeurs to cut the calcaneus, leaving a small piece of bone still attached to the Achilles tendon. Keep the cutting surfaces horizontal to be sure the bone is cut, not just the tendon. This ensures the Achilles tendon can be affixed to the myograph later.
- Along the underside of the gastrocnemius, the soleus can be seen. Again, blunt dissection can be used to separate the soleus muscle from the gastrocnemius muscle. We want to isolate the medial gastrocnemius, so it is the only muscle still attached to the Achilles tendon. Cut the soleus tendon, close to the distal end. Then you can separate the medial and lateral gastrocnemius muscles, and cut the lateral tendon, leaving just the medial gastrocnemius muscle attached to the Achilles tendon. Pull the lateral gastrocnemius muscle away from the medial, along about 50% of its length. Beyond this length, the fibres intersect and damage will result from further pulling. Check to be sure the muscle is free of connective tissue.
7. Cut the tibia
- Place a ligature around the shank, just above the midpoint. This needs to be tight, without cutting into the tissue. A second loop before tying helps prevent slipping. Again use a square knot to secure.
- Using a small saw, cut the lower leg away. This cut should be about midway along the tibia.
- Insert a sharp probe into the medulla of the tibia. This probe will be used to immobilize the tibia on the myograph base.
- Attach 2 elastics to the skin, using Michel clips. These and additional elastics will be used to hold the skin up around the muscle to form a container that will be filled with warmed paraffin oil.
[OPTIONAL]
8. Insert sonometric crystals (optional step)5
- Place the animal on a heating pad at low setting. A rectal probe, if not already inserted can be inserted at this time. Use a small drop of paraffin oil to lubricate.
- Using microstimulation, identify the ends of a fascicle within the muscle. Poke a 21 gauge needle into the site of both the origin and insertion of the muscle.
- Slide a sonometric crystal into the hole made by the needle, and seal with vet-bond surgical glue. Use a very small drop of glue; making sure that it is placed right over the crystal. Applying the glue to a piece of paper cut with an acute angle helps in applying the glue accurately. We are placing one additional crystal at the insertion of the fascicle that was identified by microstimulation.
[CONTINUE]
9. Mount in apparatus (see Figure 1)
- Position the rat on the myograph base, with the tibial probe oriented toward the lever. Place the probe in the holder, to immobilize the leg.
- Pull the skin up at the sides, to form a container and secure with elastics.
- Set drill bit (1/16th inch) into the femur, using the pilot hole that was created during surgical preparation. Ensure that you support the femur on the anterior portion as you place the drill bit (in a pin vice) as breakage of the femur may result, terminating the experiment prematurely. Affix the pin vice to a cross-bar to immobilize the femur.
- Place the distal stump of the sciatic nerve through the nerve stimulating cuff (cathode towards muscle) and tuck back close to the origin of the muscle. Connect the stimulator to these wires.
- Attach the Achilles tendon to the lever, which has strain gauge sensors on it. Tie snuggly, but leave some room for later adjustment. Be sure the alignment of the muscle is perpendicular with the lever.
- Fill the container formed by the skin with warmed paraffin oil.
- Turn on the heat lamp and position shields made of tinfoil over the head of the animal, and the force transducer. Additional foil shields may be necessary to ensure the core temperature of the animal does not exceed 38 °C. The length of the muscle can be adjusted until slack of the string is removed from the connection between the medial gastrocnemius and the force transducer.
10. Set maximal voltage and reference length
- While the muscle is warming up, set up the stimulator and test the stimulation voltage. It is important to be sure the stimulation is set at very brief pulse duration. We use 50 μs. Maximal voltage should be less than 1 V. Starting at 0.5 V, increase voltage until twitch amplitude does not increase. Maximal voltage is the lowest voltage that activates all motor units. We typically stimulate at double the maximal voltage, or 3 V whichever is higher.
- Typically, experiments will begin with the muscle at the length that gives the largest twitch contraction. A twitch is obtained with a single stimulating pulse. The muscle length is increased by about 1 mm for another twitch. This is repeated as long as twitch amplitude is increasing. Once twitch amplitude decreases, the length will be returned to the one that gave the largest amplitude twitch.
- Following this initial setting of length, we test the system with what we refer to as a “conditioning tetanic contraction”. The stimulator is set to deliver pulses at 200 Hz for 500 ms. Delivery of this stimulation will result in a completely fused tetanic contraction. The force generated by the muscle will tighten all connections, including the knots on the muscle. After a suitable rest for dissipation of potentiation4, the reference length of the muscle is reset (see part 9.2). Usually, the conditioning tetanic contraction will have permitted some fascicle length shortening, so the muscle will have to be stretched a little to get back to the length that gives the largest amplitude twitch contraction.
11. Initiate the experiment
- A typical experiment will include length adjustment and/or stimulation with a variety of patterns of stimulation. Data collection may involve just force, or force, with length, fascicle length and electromyogram.
[OPTIONAL]
12. Force-frequency relationship2
- Set the train duration long enough to reach a plateau of force at any frequency of interest. The maximum isometric force is attained in the medial gastrocnemius muscle at 200 Hz. Train duration must be at least 200 ms; longer is better for lower frequencies, but will result in some fatigue if several contractions are used to determine the full range of the force-frequency relationship. We typically use contractions at 0, 20, 40, 60, 80, 100 and 200 Hz. This range of frequencies will allow the full range of the force-frequency relationship to be described. A suitable rest between contractions must be allowed to avoid fatigue (1-10 min).
13. Force-length relationship6
- With the estimated optimal length established previously, a force-length relationship may be determined by systematically adjusting the length of the muscle from -4 mm to +4 mm using a servo-motor apparatus. This should be done using maximal stimulation (200 Hz) but we have found that very brief contractions will yield the same true optimal length7. Submaximal contractions, like twitches, can be used, but this will yield a different length-dependence of force8.
- An important aspect of the determination of the force-length relationship is the need to estimate the passive force at the fascicle length at which the measured force occurs. Active force is calculated as the difference between total force and the appropriate passive force. Since the parallel elastic structures bear the passive force and these are in series with the series elastic structures, the passive force contribution to total force must decrease as the series elastic structures are stretched during a contraction3. Once the passive force is known for all relevant muscle lengths, the appropriate passive force can be estimated with continuous measurement of fascicle length using sonomicrometry, or by estimating the compliance of the measurement system and the in-series structures of the muscle-tendon unit. If this is not done, the optimal length of the preparation and peak forces, are underestimated.
14. Force-velocity relationship9
- If you would like to determine the force-velocity relationship, you will need to attach the force transducers to a system, which allows control of either load or rate of length change. The easiest and most cost-effective method is to use air pressure to restrict shortening to a controlled load. In our system, the muscle is attached to the lever on one side of the fulcrum and the air pressure impedes length change on the other side. A length sensor needs to be employed so that you are able to detect the change in muscle length during isotonic contractions. This arrangement permits afterloaded contractions with pneumatic resistance. Dual tanks can permit isometric contraction with release to an isotonic load. A total of 15-20 contractions should be obtained, with loads ranging from nearly unloaded to maximum isometric force. When fitting the data to an equation, loads above 90% of isometric should not be used9.
15. Representative Results:
Sample contractions are presented in Figure 2. These contractions were obtained to illustrate the force-frequency relationship. Calculating the peak active force of these contractions, and plotting the active force against frequency, yields Figure 3; the force-frequency relationship. The data presented in Figure 2 can be fit to the equation: AF=c/(1+eˆ((a-F)/b)+d); AF is active force, F is frequency and a, b, c and d are constants.
For the rat medial gastrocnemius muscle, the half-maximal frequency of stimulation is typically 50-60 Hz in non-fatigued muscle2. Results will fit closely to the line described by the equation above.
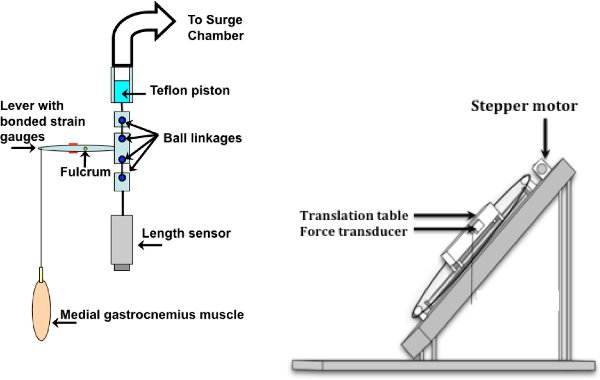
Figure 1. Apparatus and muscle set-up: Pneumatic set-up is shown on the left, isometric on the right. The lever system shown on the left is attached to the translation table when dynamic contractions are desired (adapted from13). The stepper motor is controlled by a computer (adapted from14).
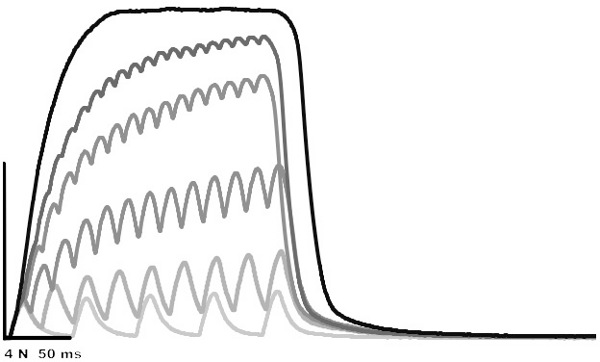
Figure 2. Superimposed isometric contractions: 20, 40, 60, 80, 100 and 200 Hz. The amplitude of the 200 Hz contraction is 8.13 N.
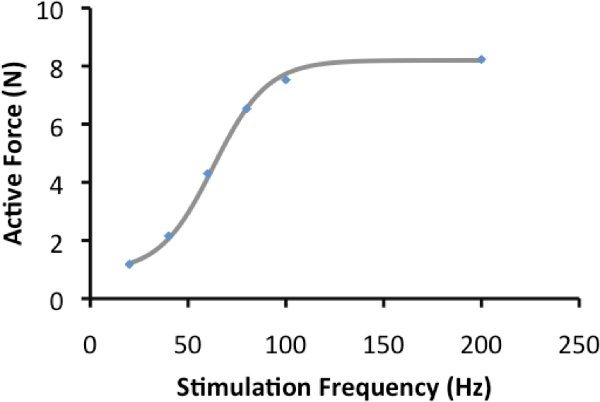
Figure 3. Force-frequency relationship: Active force of contractions in figure 2 are plotted and the line represents the line of best fit.
Discussion
Good quality contractile results can be obtained with care in surgical preparation, secure mounting in the apparatus and good quality electronics. When a student is learning this surgery, some common slips include: stretching the sciatic nerve, disrupting the blood flow, and excessive bleeding. The nerve must be handled with care to prevent damage. You will know you have damaged the nerve if the maximal tetanic force at optimal length is substantially less than that shown in Figure 3, or if the stimulation voltage needed to maximally activate all motor units is greater than 5 volts. It is relatively easy to avoid the blood vessels serving this muscle during the surgery. Disruption to these vessels can occur when the drill bit is being placed in the caudal surface of the femur. If the pilot hole is not on the flat surface of the femur, the drill may slip. When this happens, there is a possibility that the popliteal vessels get disrupted. If blood pools around the muscle after set-up, it is a sign that you have disrupted these vessels. Excessive bleeding can also occur if a large vein is cut and not tied. Large veins to watch out for are the ones around the ankle.
The in situ muscle preparation is a valuable approach to the study of muscle contractile properties. Individual motor units can be activated10, but usually all motor units are activated synchronously. This is a disadvantage relative to the normal asynchronous activation that occurs by voluntary motor unit recruitment, so represents a limitation. However, on the positive side, synchronous activation allows quantification of an average response of all motor units.
There are two approaches that have been used to avoid synchronous activation. One is to use an electrode cuff with several pairs of stimulating wires. This allows activation of a portion of the motor units with each pair, and stimulation can rotate through the pairs to achieve asynchronous activation. This method of activation can be combined with anodal block11to attempt to activate motor units in the appropriate sequence according to the size principle12. In this approach, all motor units are activated with a proximal pair of electrodes, and a block is imposed with direct current stimulation. The amplitude of stimulus for the block can be modulated to inhibit motor units for which activation is not desired. Apparently the block affects large axons at the lowest voltage, and progressively affects smaller units.
The in situ rat gastrocnemius muscle preparation is a valuable physiological approach to the study of skeletal muscle contraction and biochemical properties in health and in disease.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Research supported by the Natural Science and Engineering Research Council of Canada.
Materials
| Name of the equipment | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| Clippers | Good quality pet clippers | ||
| Surgical lamp | Dyna-Lume | Any of several will do | |
| Myograph | Custom built | ||
| Stimulator | Grass | S-88 | Any of several will do |
| Strain gauge amplifier | CWE | PM-1000 | |
| Telethermometer | YSI | YSI-400 | |
| Robotic platform | Arrick Robotics | MD-2 | |
| Sonometric amplifier | Sonometrics | Sonolab | |
| Computer and data collection | PC with NI board | Custom software (labview) | |
| Block heater | Lab-line | Multi-block | |
| Nerve cuff | Custom made | ||
| Microstimulator | Custom made |
References
- MacIntosh, B. R., Gardiner, P. F. Posttetanic potentiation and skeletal muscle fatigue: interactions with caffeine. Canadian Journal of Physiology and Pharmacology. 65, 260-268 (1987).
- Dormer, G. N., Teskey, G. C., MacIntosh, B. R. Force-frequency and force-length properties in skeletal muscle following unilateral focal ischeaemic insult in a rat model. Acta. Physiol. (Oxf.). 197, 227-239 (2009).
- MacIntosh, B. R., MacNaughton, M. B. The length dependence of muscle active force: considerations for parallel elastic properties. J. Appl. Physiol. 98, 1666-1673 (2005).
- Tubman, L. A., MacIntosh, B. R., Rassier, D. E. Absence of myosin light chain phosphorylation and twitch potentiation in atrophied skeletal muscle. Canadian Journal of Physiology and Pharmacology. 74, 723-728 (1996).
- MacNaughton, M. B., MacIntosh, B. R. Impact of length during repetitive contractions on fatigue in rat skeletal muscle. Pflugers. Arch. 455, 359-366 (2007).
- MacNaughton, M. B., MacIntosh, B. R. Reports of the Length Dependence of Fatigue are Greatly Exaggerated. J. Appl. Physiol. 101, 23-29 (2006).
- Rassier, D. E., MacIntosh, B. R. Length-dependent twitch contractile characteristics of skeletal muscle. Canadian Journal of Physiology and Pharmacology. 80, 993-1000 (2002).
- Rack, P. M. H., Westbury, D. R. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. Journal of Physiology. 204, 443-460 (1969).
- Devrome, A. N., MacIntosh, B. R. The biphasic force-velocity relationship in whole rat skeletal muscle in situ. J. Appl. Physiol. 102, 2294-2300 (2007).
- Drzymala-Celichowska, H., Krutki, P., Celichowski, J. Summation of motor unit forces in rat medial gastrocnemius muscle. J Electromyogr. Kinesiol. 20, 599-607 (2010).
- Petrofsky, J. S. Control of the recruitment and firing frequencies of motor units in electrically stimulated muscles in the cat. Medical & Biological Engineering & Computers. 16, 302-308 (1978).
- Bawa, P., Binder, M. D., Ruenzel, P., Henneman, E. Recruitment order of motoneurons in stretch reflexes is highly correlated with their axonal conduction velocity. Journal of Neurophysiology. 52, 410-420 (1984).
- Dormer, G. N. . Fundamental Contractil Properties of Skeletal Muscle Following a stroke in a Rat Model. Master’s Thesis. , (2008).
- MacNaughton, M. B. . The Length dependence of Fatigue and of Repetitive Contractions. Master’s Thesis. , (2005).

