Summary
In this report, we describe a protocol for isolating highly purified populations of leukocytes that infiltrate tumors. This protocol is adapted from the Miltenyi Biotech protocol to enhance yield and purity for isolating cells from complex tumor tissue.
Abstract
Tumors create a unique immunosuppressive microenvironment (tumor microenvironment, TME) whereby leukocytes are recruited into the tumor by various chemokines and growth factors 1,2. However, once in the TME, these cells lose the ability to promote anti-tumor immunity and begin to support tumor growth and down-regulate anti-tumor immune responses 3-4. Studies on tumor-associated leukocytes have mainly focused on cells isolated from tumor-draining lymph nodes or spleen due to the inherent difficulties in obtaining sufficient cell numbers and purity from the primary tumor. While identifying the mechanisms of cell activation and trafficking through the lymphatic system of tumor bearing mice is important and may give insight to the kinetics of immune responses to cancer, in our experience, many leukocytes, including dendritic cells (DCs), in tumor-draining lymph nodes have a different phenotype than those that infiltrate tumors 5,6 . Furthermore, we have previously demonstrated that adoptively-transferred T cells isolated from the tumor-draining lymph nodes are not tolerized and are capable of responding to secondary stimulation in vitro unlike T cells isolated from the TME, which are tolerized and incapable of proliferation or cytokine production 7,8. Interestingly, we have shown that changing the tumor microenvironment, such as providing CD4+ T helper cells via adoptive transfer, promotes CD8+ T cells to maintain pro-inflammatory effector functions 5. The results from each of the previously mentioned studies demonstrate the importance of measuring cellular responses from TME-infiltrating immune cells as opposed to cells that remain in the periphery. To study the function of immune cells which infiltrate tumors using the Miltenyi Biotech isolation system9, we have modified and optimized this antibody-based isolation procedure to obtain highly enriched populations of antigen presenting cells and tumor antigen-specific cytotoxic T lymphocytes. The protocol includes a detailed dissection of murine prostate tissue from a spontaneous prostate tumor model (TRansgenic Adenocarcinoma of the Mouse Prostate -TRAMP) 10 and a subcutaneous melanoma (B16) tumor model followed by subsequent purification of various leukocyte populations.
Protocol
1. Isolation of Myeloid Cells from TRAMP Prostate Tumors
- All procedures were performed under the guidance of an animal review board. TRAMP mice spontaneously develop autochthonous prostate tumors as a result of a transgene (SV40) controlled by the probasin promoter 10. Any male mouse can be used for this procedure. While TRAMP tumors can be harvested at any age, it should be noted that TRAMP prostatic tumors generally remain small until the mice reach ages greater than 20 weeks. Euthanize mice by CO2 asphyxiation. Remove the urogenital tract (UGT), by cutting open the peritoneal cavity and pulling back the adipose tissue. Adipose tissue is foamy, glistening looking tissue.
- Locate the bladder; it lies directly between the two large, white seminal vesicles. Hold onto the bladder with forceps. Keeping the scissors closed, smooth away adipose tissue and locate the urethra. Cut down on the urethra as close to the pelvic girdle as possible and sever the vas deferens. While cutting down, simultaneously pull up on the bladder. This will release the entire UGT that can now be micro-dissected to obtain each of the lobes of the prostate.
- Place the UGT in room temperature PBS. Use a dissecting microscope to visualize the UGT and clear away any excess adipose tissue. Using forceps, hold onto the urethra and collect the ventral, lateral and anterior lobes of the prostate. Place the tissue in PBS while you collect the rest of the lobes.
- Remove the seminal vesicles and discard.
- Turn the remaining tissue 180°. Collect the ampullary gland and dorsal prostate lobes.
- Using forceps, tease apart the prostate tissue and place it into the digestion (dissociation) buffer.
- Place tumor tissue in 1 ml dissociation buffer (100 U/ml Collagenase type IV and 100 μg/ml DNase in RPMI + 10% FBS). Each tumor may require unique dissociation conditions; for TRAMP prostatic tumors, use 1 ml and for B16 tumors, use 5 ml (see below). Note: the grade of Collagenase (I-IV) will depend on the cell population to be isolated; myeloid cell isolation is best with type IV, whereas lymphocyte yield is higher using type I.
- Place tube in 37 °C incubator for 30 min.
- Pipette up and down with a 1 ml pipette to get an easily flowing single cell suspension.
- Filter suspension through 70 micron filter and wash 3x with MACs separation buffer supplemented with 10% FBS for myeloid cell isolation.
- Centrifuge cell suspension at 400 xg for 10 min. Then rinse the pellet with 10 ml MACs buffer and centrifuge again with the same settings.
- Resuspend the cell pellet in 100 μl MACs buffer. Next, add 1 μg anti-CD16/32 antibody (Ab) per 108 cells (200 μl 2.4.G2 hybridoma culture supernatant) and incubate at room temperature for 10 min. Note: the amount of FcR-γ blocking antibody (Ab) to be added may vary depending upon the frequency/number of FcR-γ+ cells in the tissue and the volume of the cell suspension; therefore, this step may require optimization.
- Add 100 μl of “Pan-DC” or 10 μl of anti-CD11b, anti-CD11c or anti-PDCA-1 MicroBeads per 108 total cells, depending on the desired cell population. Note: the amount of MicroBeads required may vary depending upon the frequency/number of cells of the desired population in the tissue; therefore, this step may require optimization.
- Mix well by gently flicking tube (Do Not Vortex) and incubate for 15 min at 4-8 °C, shielded from light. Do not incubate on ice.
- Wash cells by adding 10 ml of MACs buffer. Centrifuge at 400 xg for 10 min.
- Pour off supernatant completely and resuspend cells in 500 μl of MACs buffer. Tumor cell suspensions flow better through the LC columns. (Other choices: MS, LS, LD).
- Apply a fresh column to the magnet separator. Prime the column by rinsing with an appropriate amount of MACs buffer for the column selected: For LC and MS columns use 500 μl. For LS or LD columns use 1 ml. After priming the column, apply cell suspension onto the top of the column. Use one column for every 1 x 108 cells.
- Collect unlabeled cells (pass-through) and wash the column a minimum of three (up to five) times with 500 μl volume for a total of 1.5-2.5 ml of fluid wash. Perform wash step 1 by adding MACs buffer to the column; it is important to keep the column flowing. As soon as the column drips the last drop, add more buffer. Do not let column stand without flow or allow it to run dry (this cannot be understated, as drying of the column can ruin purity and lead to significant loss of cell viability.). For wash step 2, rinse the column with dissociation buffer mix (containing Collagenase + DNase as described in step 1.7); this serves as a “harsher” wash step to flush out sticky debris from dead or dying tumor cells. Perform wash step 3 with MACs buffer; if the flow through does not look completely clear after wash step 3, continue to wash for 2 additional wash steps with MACs buffer (for a total of 5 washes). For large subcutaneous tumors (e.g., B16 melanoma), during the last wash step, apply gentle pressure with a gloved fingertip to the top of the column; this releases extra debris for a cleaner, purified cell population. This step is not required for solid tissue tumors such as prostate; instead, use extra wash steps, washing a total of at least 5-6 times.
- Remove column from the magnetic separator and place it on a suitable collection tube. Pipette 2 ml of buffer onto the column. Collect the magnetically labeled cells by firmly applying the plunger supplied with the column.
- Count cell number and confirm purity for the selected cell population by flow cytometric analysis. For the identification of DC populations, suggested markers include CD45, CD11c, PDCA-1, B220 and CD11b. Suggested macrophage markers include CD45, CD11b, F4-80, and Ly6C.
2. Isolation of Adoptively Transferred T cells from Subcutaneous B16 Melanoma Tumors
This protocol works best with tumors that are 250 mm2 or less (estimated by measuring bisecting diameters of the tumor).
- B16 tumor cells were injected subcutaneously into C57BL/6 mice and tumor measurements were recorded every 2 days 8. Mice with tumors measuring 250 mm2 are euthanized by CO2 asphyxiation. Using autoclaved surgical instruments rinsed with 70% ETOH, cut tumor into small (< 3mm) pieces and incubate in 5 ml dissociation solution (RPMI medium supplemented with 5% FBS, Collagenase type I (200 U/ml) and DNase I (100 μg/ml)) for 30 min at 37 °C, Pipetting (using a 1,000 μl pipet tip) and vortexing every 10 minutes during the incubation. If myeloid cells will be subsequently isolated, substitute 5% FBS and Collagenase type I with 10% FBS and Collagenase type IV (200 U/ml), respectively.
- After incubation, pass cell suspension through a 70 μm cell strainer and wash twice with 10 ml MACs buffer (prepared per manufacturer’s instructions, Miltenyi Biotech). Optional – for very large tumors (>300 mm2), inflammatory cells can be pre-enriched using density gradient centrifugation (Percoll or Ficoll).
- Aspirate the wash supernatant and add 1 μg anti-CD16/32 antibody/108 cells (200 μl of clone 2.4.G2 culture supernatant) and incubate at room temperature for 10 min.
- Without washing, add 1 μg anti-Thy1.1 PE antibody per 108 cells, mix well by gently flicking the tube and incubate for 30 minutes on ice, shielded from light.
- Wash the cells to remove unbound primary antibody by adding 10 ml MACs buffer and centrifuge at 400 xg for 5 minutes.
- Aspirate the supernatant and add 20 μl anti-PE microbeads (Miltenyi Biotech) per 107 total cells, according to manufacturer instructions.
- Mix well by gently flicking tube (do not vortex) and incubate at 4 °C (do not use ice) for 15 minutes, shielded from light. Caution: vortexing can diminish the integrity of the beads.
- Wash cells by adding 10 ml of MACs buffer and centrifuge at 400 xg for 5 minutes.
- Aspirate supernatant, and resuspend cells in 500 μl MACs buffer. For tumors of a larger size (>300 mm2), use 1 ml buffer.
- Place one LC (Large Cell) column in the magnetic field separator. Tumor cell suspensions flow best through these columns. Rinse the column with 500 μl MACs buffer to prime the column. Most tumors will also flow through LS and LD columns, but require further digestion and mechanical disruption to achieve the appropriate level of single cell suspension. Wash the column with 1 ml MACs buffer to prime the column.
- Apply cell suspension onto the column and allow to completely flow through (without letting the column run dry). Next, wash with 500 μl MACs buffer, and repeat washing 3x. Only add new buffer when the column reservoir is empty, but do not let the column stand without flow. This will cause clogging and diminished purity.
- This is a critical step for large subcutaneous tumors: After the last wash step, with a gloved finger tip, apply gentle pressure to the top of the column while still on the magnetic separator. This releases extra debris for a more purely enriched population. Next, wash the column 1 more time with 500 μl MACs buffer.
- Remove column from the separator and place it in a clean 15 ml conical tube, add 2 ml MACs buffer onto the column. Flush out the magnetically-labeled cells by firmly pushing the plunger into the column.
- Centrifuge the eluted fraction at 400 xg for 5 min. Purity at this step is typically between 75-80%. To achieve >90% purity, repeat the magnetic separation procedure as described in step 1.10-1.12 using a new MS column. The MS column is smaller and more compact so the suspension will run more slowly through the column, but will yield higher numbers and purity of the cell of interest.
- Count cell number for further experiments and check purity by flow cytometric analysis. For isolation of adoptively transferred T cells such as those described in this protocol, allelic markers for identification include CD45 and Thy1.1 (transferred cells) and Thy1.2 (host T cells).
3. Representative Results
The yield of a particular cell population (i.e. macrophages, DC, T cell, etc.) will vary depending on the size of the tumor and treatments that were administered during tumor growth. A prostate from an untreated 14-16 week old TRAMP mouse should yield between 8×105-1×106 CD11c+/PDCA-1+ (DC) cells at 90-95% purity or 1×106-1.5×106 F4/80+/CD11b+ (macrophages) at 80-90% purity from 300 mg of tissue following the isolation protocol above as shown in Figure 1. The number of each of these cells slightly increases upon adoptive transfer of tumor-antigen specific T cells. Poor purity is usually a result of insufficient washing, allowing the column to dry (which can result in tumor debris retention in the column), or insufficient Fc receptor blocking.
Similarly, the total cells isolated from B16 tumors will also vary depending on tumor size at the time of tissue harvest and adoptive transfer of T cells (transfer of 5×106) with or without DC vaccine (transfer of 1×105). Very few antigen-specific T cells infiltrate the tumor unless an antigen-pulsed DC vaccine is also given one day after T cell transfer. If a DC vaccine is administered to a mouse bearing a small, palpable (< 50 mm2) tumor, a yield of approximately 3×105 Thy1.1+ T cells, with a purity of 80-85%, would be considered a “good” harvest as shown in Figure 2A. However, if larger tumors are harvested, total yield and purity will be reduced.
Macrophages (F4/80+/CD11b+) are usually a smaller percentage of the total cells in B16 tumors. Figure 2B shows that utilizing CD11b, from a tumor that is 250 mm2, yields approximately 1×106 macrophages at 90-95% purity. Additionally, Figure 2B shows that the DC population in B16 tumors are heterogeneous. Unlike prostate tumors, two subpopulations: CD11c+/PDCA-1+ (plasmacytoid DC) and CD11c+/PDCA-1– (conventional DC) can be obtained from B16 melanoma tumors. An estimated 2×106 pDC and 4×106 cDC are expected from 250 mm2 B16 tumors at 80-90% purity. Reduced purity is usually a result of not clearing the tumors cells from the column. Step 2.12 is a critical step to remove the small clump of melanoma cells that persists at the base of the column. Following this step improves the effectiveness of the wash steps and results in better purity.
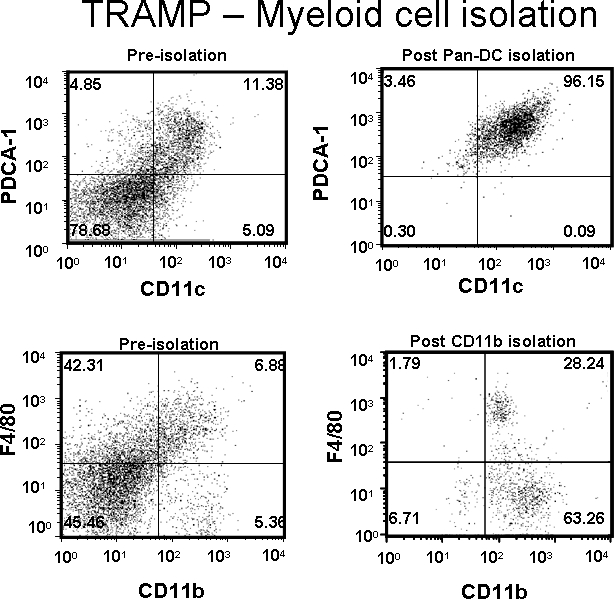
Figure 1. DCs (CD11c+/PDCA-1+) and macrophages (F4/80+/CD11b+) were isolated from a TRAMP prostate tumor. Dot plot values represent percentage of cells of interest pre- or post-purification.
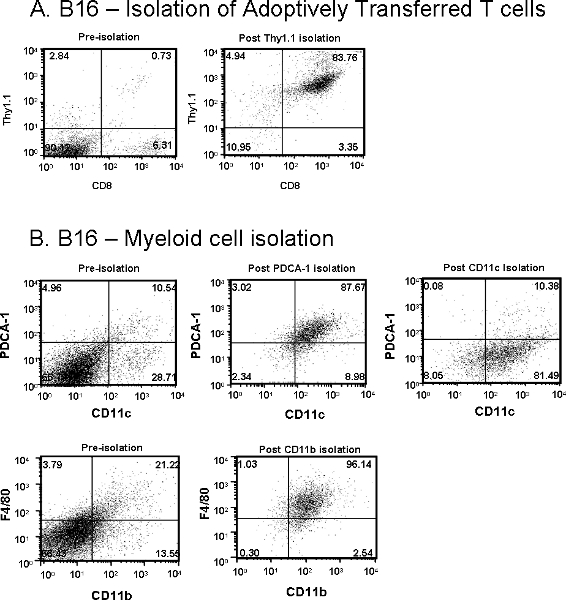
Figure 2. (A) Adoptively transferred Thy1.1+/CD8+ T cells and (B) Myeloid cells including CD11c+/PDCA-1+ plasmacytoid DCs, CD11c+/PDCA-1– conventional DCs and F4/80+/CD11b+ macrophages were isolated from subcutaneous B16 melanoma tumor from Thy1.2+ mice. Dot plot values represent percentage of cells of interest pre- or post-purification.
Discussion
This protocol can be modified, based on the size and source of the tumor (subcutaneous, spontaneous tumor, or orthotopic tumors). For larger tumors, it is recommended to increase the amount of dissociation buffer, MACs buffer, and number of washes. The heartiness of the cells isolated can depend on the TME from which they are being enriched. For example, in our experience, cells isolated from spontaneous prostate tumors require gentler dissociation than cells isolated from subcutaneous B16 melanoma tumors. During dissection of the tumor, eliminate as much adipose, skin, or other debris that can prevent effective enzymatic dissociation. It is critical to completely digest tumor masses and obtain a single cell suspension to ensure proper Ab labeling and to prevent columns from clogging. For myeloid cell isolation, the amount of fetal bovine serum recommended in the MACs isolation buffer was increased from 2% to 10% to improve cell viability. It is also essential to keep all buffers and cells cold throughout the protocol to prevent non-specific Ab binding and clogging of the column. In conclusion, to obtain highly enriched populations of antigen presenting cells and tumor antigen-specific cytotoxic T lymphocytes, we have modified and optimized an antibody-based isolation procedure utilizing the Miltenyi Biotech technology. Utilizing this protocol, both adoptively transferred and endogenous leukocyte populations may be enriched using Abs directed against cell type-specific surface markers. One advantage of the described procedures is the reduction of tumor debris carry-over following extensive and rigorous washing. This includes the use of collagenase in the wash buffer as well as identification of a point at which added pressure to the column can eliminate column clogs by tumor cells. Obtaining a sufficient number of immune cells from tissues, especially at a purity that is suitable for functional analysis, can be a difficult task. However, in our experience, the protocol herein yields the highest number of cells, at the greatest purity, with the most consistency.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Dr. Scott Durham for review of the manuscript and video. This work is supported in-part by the intramural research program of the NIH, NCI.
Materials
| Reagent | Company | Catalouge Number | Comments |
| Collagenase I | Gibco | 17100-017 | Use when selecting for T cells |
| Collagenase IV | Gibco | 17104-019 | Use for myeloid selection |
| DNase | Calbiochem | 260913 | |
| RPMI | Gibco | 21870 | |
| Dulbecco’s PBS | Lonza | 17-5158 | |
| Fetal Bovine Serum | Lonza | 14-501F | |
| MACs Buffer | 2% FBS for T cells 10% FBS for myeloid cells |
||
| Thy1.1 PE | ebioscience | 551401 | |
| Anti-PE microbeads | Miltenyi Biotech | 120-000-294 | |
| Anti-Pan DC microbeads | Miltenyi Biotech | 120-003-183 | |
| Anti-CD11b microbeads | Miltenyi Biotech | 120-000-300 | |
| LC column | Miltenyi Biotech | 130-042-202 | |
| MS column | Miltenyi Biotech | 130-042-201 |
References
- Shurin, M. R. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies. Cancer Metastasis Rev. 25, 333-356 (2006).
- Lewis, C. E., Pollard, J. W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 66, 605-612 (2006).
- Anderson, M. J., Shafer-Weaver, K., Greenberg, N. M., Hurwitz, A. A. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J. Immunol. 178, 1268-1276 (2007).
- Ganss, R., Hanahan, D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 58, 4673-4681 (1998).
- Shafer-Weaver, K. A. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. 癌症研究. 69, 6256-6264 (2009).
- Watkins, S. K., Zhu, Z., Riboldi, E., Shafer-Weaver, K. A., Stagliano, K. E. R., Sklavos, M. M., Ambs, S., Yagita, H., Hurwitz, A. A. Foxo3a programs tumor associated dendritic cells to become tolerogenic in Human and Murine Prostate Cancer. Journal of Clinical Investigation. 121, (2011).
- Shafer-Weaver, K. A., Hurwitz, A. A. Tumor-Specific CD8+ T Cells Infiltrating Prostatic Tumors are Induced to Become Suppressor Cells. , (2009).
- Singh, V., Ji, Q., Feigenbaum, L., Leighty, R. M., Hurwitz, A. A. Melanoma progression despite infiltration by in vivo-primed TRP-2-specific T cells. J. Immunother. 32, 129-139 (2009).
- Matheu, M. P., Cahalan, M. D. Isolation of CD4+ T cells from Mouse Lymph Nodes Using Miltenyi MACS Purification. J. Vis. Exp. (9), e409 (2007).
- Hurwitz, A. A., Foster, B. A., Allison, J. P., Greenberg, N. M., Kwon, E. D. The TRAMP mouse as a model for prostate cancer. Curr. Protoc. Immunol. Chapter 20, (2001).

