Summary
Methods to record auditory P50 sensory gating, a physiological marker of cerebral inhibition which reflects early stages of attention, are described.
Abstract
Attentional deficits are common in a variety of neuropsychiatric disorders including attention deficit-hyperactivity disorder, autism, bipolar mood disorder, and schizophrenia. There has been increasing interest in the neurodevelopmental components of these attentional deficits; neurodevelopmental meaning that while the deficits become clinically prominent in childhood or adulthood, the deficits are the results of problems in brain development that begin in infancy or even prenatally. Despite this interest, there are few methods for assessing attention very early in infancy. This report focuses on one method, infant auditory P50 sensory gating.
Attention has several components. One of the earliest components of attention, termed sensory gating, allows the brain to tune out repetitive, noninformative sensory information. Auditory P50 sensory gating refers to one task designed to measure sensory gating using changes in EEG. When identical auditory stimuli are presented 500 ms apart, the evoked response (change in the EEG associated with the processing of the click) to the second stimulus is generally reduced relative to the response to the first stimulus (i.e. the response is "gated"). When response to the second stimulus is not reduced, this is considered a poor sensory gating, is reflective of impaired cerebral inhibition, and is correlated with attentional deficits.
Because the auditory P50 sensory gating task is passive, it is of potential utility in the study of young infants and may provide a window into the developmental time course of attentional deficits in a variety of neuropsychiatric disorders. The goal of this presentation is to describe the methodology for assessing infant auditory P50 sensory gating, a methodology adapted from those used in studies of adult populations.
Introduction
Many neuropsychiatric illnesses- including attention deficit-hyperactivity disorder (ADHD), bipolar mood disorder, and schizophrenia- are considered neurodevelopmental disorders, meaning that the onset of symptoms is at least partially due to very early aberrations in brain development. The early brain developmental aberrations may be silent or only associated with nonspecific symptoms for years or even decades prior to onset of the full disorder. This model suggests that a full understanding of the disorder may require methods to measure very subtle changes in brain function very early in brain development.
P50 sensory gating reflects an early step in the attentional process1,2. P50 sensory gating refers to a specific task, an auditory evoked potential task, which correlates with behavioral and neurocognitive tests of attention and reflects cerebral inhibitory mechanisms3,4. In this task, subjects are presented with two identical broad spectrum auditory stimuli ("clicks") while an EEG response is recorded. An auditory stimulus elicits a consistent EEG-measured brain response; the response includes a wave which, in adults, occurs approximately 50 msec after the stimulus. Since the wave is in the positive direction, this wave is described as the P50 wave. Most individuals demonstrate a significantly reduced response to the second stimulus of a pair relative to the first, reflective of the brain's ability to automatically inhibit response to irrelevant repetitive information (to "gate" sensory information). Many psychiatric illnesses with attentional symptoms-including attention deficit-hyperactivity disorder (ADHD), bipolar mood disorder, and schizophrenia-are associated with impairment in sensory gating5-7.
Auditory P50 sensory gating results can be confounded by a subject's adrenergic tone8. During REM sleep, adrenergic tone is diminished making REM sleep an optimal time for task administration9,10. Because P50 sensory gating is a passive task and because infants spend much of their day in active sleep (the infant version of REM sleep), P50 sensory gating can be measured even in very young infants.
Protocol
1. Institutional Review and Oversight
- All work with human subjects requires Institutional Review Board approval and oversight. Methods reported here, when used in research, have been reviewed and approved by the Colorado Multiple Institutional Review Board (COMIRB).
2. Preparation and Recording
- Schedule the baby for 90 min at the infant's regular nap time. Have clean electrodes, conductive EEG paste, and a dark quite room available. Feed, burp, and diaper the infant before beginning.
- Wipe the head to minimize impedance. CZ has the largest amplitude P5011. Place an electrodes at CZ, on the forehead, above and below one eye, on the chin, and on the right mastoid.
- Present 50 msec broad-spectrum stimuli (clicks) as pairs with a 500 msec interstimulus interval. Pairs of clicks should be presented 10 sec apart.
- Record until there are 15 min of continuous active sleep (the infant equivalent of REM sleep). Allow the infant to complete their natural sleep period. This may take >90 min to achieve. Record baby's status (e.g. eyes open or closed, movement, etc.) during the entire sleep period.
3. Data Analysis
- Identify 15 min of continuous, active sleep. This period will have high frequency, low voltage brain waves, low muscle tone, large eye movements, and will be noted for having eyes closed.
- Filter data to remove 60 Hz interference and low frequency (<1 Hz drift)12.
- Identify epochs by the presentation of the first sound in each pair. Average at least 60 epochs. The greater the number of epochs used, the greater signal to noise ratio possible.
- Filter the averaged tracing using a bandpass filter between 10-50 Hz. This allows the component to be identified more easily. Using the filtered averaged tracing, measure P50 amplitude in response to the first and second stimulus independently. P50 waves are identified as the first wave between 40 and 100 msec after stimulus presentation; amplitude is measured from the preceding trough to the peak.
- Divide the amplitude of the P50 wave in response to the second sound by the amplitude of the P50 wave in response to the first sound to obtain a P50 sensory gating ratio.
Representative Results
P50 sensory gating ratios should generally range from 0 to slightly greater than 1.0. Values above 2 generally only occur in the case of P50 amplitudes below 1.0 μV, may represent poor signal to noise ratio and should either be discarded or truncated to a value of 2.0 11. Mean values from infants drawn from the general population should average about 0.4 13. Figure 1 provides two sample tracings: one from an infant with typical sensory gating; one from an infant with poor sensory gating.
P50 sensory gating can be computed by subtraction: amplitude of response to the first sound minus amplitude of response to the second sound. However, there is wide variability in attenuation of signal from its source through the scalp to the measuring electrode. Things which can effect attenuation include thickness of the skull, debris (e.g. dirt) on the scalp, and moisture content on the surface of the scalp. This variation in attenuation of signal across individuals and across the same individual over time decreases reliability of results from a subtraction method. Thus, P50 sensory gating is generally reported as a ratio: amplitude of response to the second sound divided by amplitude of response to the second sound.
P50 sensory gating ratios can be utilized as a continuous measure. However, the creation of a ratio introduces additional variability. An alternative is to dichotomize results into “normal” versus “impaired” P50 sensory gating. In adults, ratios < 0.40 are considered normal and >0.50 are considered impaired14. Ratios between 0.40-0.50 are considered indeterminate and can either be added to either group or excluded from further analysis. Appropriate cutoff points for infants have not been firmly established; however, preliminary work suggests similar cutoff points as used in adults are appropriate15.
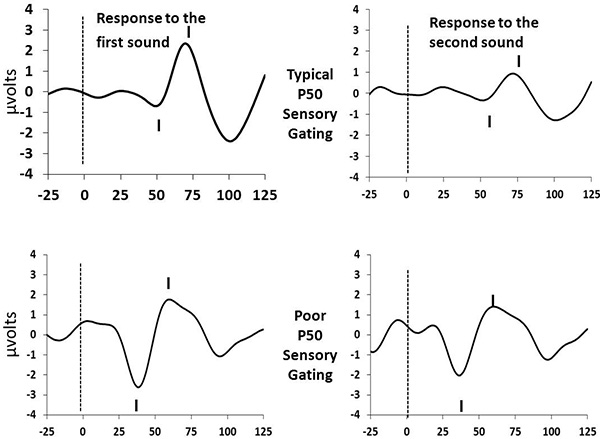
Figure 1. Sample Tracings. Click here to view larger image.
Discussion
P50 sensory gating is thought to reflect a complex interaction of inhibitory neurocircuits located primarily in the hippocampus, dorsolateral prefrontal cortex, and thalamus16. Thus, alterations in P50 sensory gating in infants may suggest altered development in these regions of the brain.
In these brain regions, GABAergic interneuron inhibition of glutaminergic pyramidal cells likely accounts for the gating process, with the inhibition provided by GABAergic cells. The GABAergic interneurons are themselves modulated by adrenergic and cholinergic input. The adrenergic input is intermittent and inhibits the interneurons which decreases their ability to inhibit pyramidal cells and thus blocks sensory gating. One of the difficulties in recording awake infants is that even very mild stressors, such as applying electrodes, can increase adrenergic tone an interfere with the ability to measure functional gating abilities.
In contrast to the impact of intermittent adrenergic input, cholinergic stimulation of interneurons is tonic and results in enhanced interneuron firing, thus improving sensory gating17. Factors which decrease cholinergic input, such as genetic phenotypes related to decreased cholinergic receptor expression, lead to chronic problems in sensory gating18. Similarly, decreased neuronal release of choline, as occurs during non-REM sleep, also decreases interneuron activity and sensory gating. Nicotine is an interesting compound as acute use stimulates nicotinic cholinergic receptors and improves sensory gating19,20, while repeated or chronic nicotine use has no effect when used alone but blocks the effects of other agonists21, presumably because chronic stimulation leads to receptor desensitization22. Infants who are exposed to prenatally exposed to nicotine through maternal cigarette use have impaired P50 sensory gating12.
This awareness of the fundamental biology of sensory gating was the rationale for a randomized trial of supplementation during pregnancy with the dietary component choline, an agonist at the cholinergic receptor on interneurons. Supplementation occurred in from early in the second trimester through approximately 3 months post birth. Infants who had received choline supplementation had accelerated development of sensory gating15.
Infant P50 sensory gating ratio assessment is feasible23, reliable24, and stable between infancy and four years of age25. Impairments in infant P50 sensory gating are associated with prenatal risk factors for later psychiatric illness12,26 and with increased risk for developing psychiatric symptoms at 4 years of age27. However, infants with impaired P50 sensory gating have never been followed beyond 4 years of age; thus it remains unclear if infant P50 sensory gating is predictive of later psychopathology or if efforts to alter infant P50 sensory gating will be useful as primary prevention strategies.
The adaptation of auditory P50 sensory gating paradigm for infant study allows exploration of questions related to when during development cerebral inhibition develops and when that development goes awry for individuals vulnerable to later attention-associated neuropsychiatric illnesses. Similar efforts with other physiological tasks, such as mismatch negativity, eye tracking, and prepulse inhibition may offer other windows into early development. It is hoped that the adaptation of tasks for use in early infancy may increase understanding of the developmental time course of these disorders as well as suggest novel primary prevention strategies17.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded, at least in part, by the National Institutes of Health (NIH grant numbers MH086383, MH056539 and MH015442) and by the Institute for Children’s Mental Disorders.
Materials
| E-Prime Version 2.0 Professional | Psychology Software Tools | EPR-225142 | Stimulus presentation software |
| Alcohol Prep Pads with Pumice | Compumedics | 95000016 | Cleans skin in preparation for electrode placement |
| Ten20 Paste | Compumedics | 92100032 | EEG conductive paste that has adhesive qualities to hold electrodes in place |
| Grass Ag/AgCl Surface Electrodes (10mm) | Grass Technologies | FS E5SHC-48 | 10 mm electrodes are used to record EEG signals from the surface of the scalp and serve as reference and ground electrodes |
| Grass Ag/AgCl Surface Electrodes (6 mm) | Grass Technologies | F E6SHC-48 | 6mm electrodes are used to record eye movements (EOG) and muscle tension (EMG) from the surface of the skin |
| Paper Surgical Tape | Micropore | 1530-0 | low adhesive tape used to keep electrodes in place |
| NuAmps Amplifier | Neuroscan | N/A | 40-channel monopolar digital amplifier for recording EEG |
| Harmon/Kardon computer Speakers | Harman/Kardon | HK206 | Used to present auditory "clicks" |
References
- Braff, D. L., Light, G. A. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 174 (1), 75-85 (2004).
- Boutros, N. N., Belger, A., Campbell, D., DGÇÖSouza, C., Krystal, J. Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiat. Res. 88 (2), 119-130 (1999).
- Cullum, C. M., Harris, J. G., et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizo. Res. 10 (2), 131-141 (1993).
- Kisley, M. A., Noecker, T. L., Guinther, P. M. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 41 (4), 604-612 (2004).
- Olincy, A., Ross, R. G., Harris, J. G., Freedman, R. Neurophysiological studies of the P50 auditory evoked potential in adult attention deficit disorder: comparison with schizophrenia. Schizo. Res. 36, 257-25 (1999).
- Adler, L. E., Pachtman, E., Franks, R., Freedman, R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol. Psychiat. 17, 639-654 (1982).
- Martin, L. F., Mei-Hua, H., et al. Physiology of schizophrenia, bipolar disorder and schizoaffective disorder. Am. J. Psychiatr. 164 (12), 1900-1906 (2007).
- Adler, L. E., Gerhardt, G. A., et al. Sensory physiology and catecholamines in schizophrenia and mania. Psychiatr. Res.. 31 (3), 297-309 (1990).
- Kisley, M. A., Olincy, A., Freedman, R. The effect of state on sensory gating: comparison of waking. REM and non-REM sleep. Clin. Neurophysiol. 112, 1154-1165 (2001).
- Kisley, M. A., Olincy, A., et al. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiology. 40 (1), 29-38 (2003).
- Nagamoto, H. T., Adler, L. E., Waldo, M. C., Griffith, J., Freedman, R. Gating of auditory response in schizophrenics and normal controls: Effects of recording site and stimulation interval on the P50 wave. Schizo. Res. 4 (1), 31-40 (1991).
- Hunter, S. K., Kisley, M. A., McCarthy, L., Freedman, R., Ross, R. G. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: Parental psychosis, maternal depression, and nicotine. 37 (6), 1200-1208 (2011).
- Siegel, C., Waldo, M., Mizner, G., Adler, L. E., Freedman, R. Deficits in sensory gating in schizophrenic patients and their relatives: Evidence obtained with auditory evoked responses. Arch. Gen. Psychiat. 41, 607-612 (1984).
- Ross, R. G., Hunter, S. K., et al. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiat. 170 (3), 290-298 (2013).
- Tregellas, J. R., Davalos, D. B., et al. Increased hemodynamic response in the hippocampus, thalamus, and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizo. Res. 92 (1-3), 262-272 (2007).
- Ross, R. G., Stevens, K. E., et al. Cholinergic mechanisms, early brain development, and risk for schizophrenia. J. Child Psychol. Psychiat. 51 (5), 535-549 (2010).
- Leonard, S., Gault, J., et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiat. 59 (12), 1085-1096 (2002).
- Stevens, K. E., Kem, W. R., Freedman, R. Selective alphay 7 nicotinic receptor stimulation normalizes chronic cocaine-induced loss of hippocampal sensory inhibition in C3H mice. Biol. Psychiat. 46 (10), 1443-1450 (1999).
- Adler, L. E., Hoffer, L. J., Griffith, J., Waldo, M. C., Freedman, R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol. Psychiatr. 32 (7), 607-616 (1992).
- Griffith, J. M., O’Neill, J. E., et al. Nicotinic receptor desensitization and sensory gating deficits in schizophrenia. Biol. Psychiat. 44, 98-106 (1998).
- Giniatullin, R., Nistri, A., Yakel, J. L. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 28 (7), 371-378 (2005).
- Kisley, M. A., Polk, S. D., Ross, R. G., Levisohn, P. M., Freedman, R. Early postnatal development of sensory gating. Neurophysiol. Basic Clin. 14, 693-697 (2003).
- Hunter, S. K., Corral, N., Ponicsan, H., Ross, R. G. Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport. 19 (1), 79-82 (2008).
- Gillow, S., Hunter, S., Ross, R. Stability of P50 sensory gating in preschoolers. J. Invest. Med. 58 (1), 154-155 (2010).
- Hunter, S. K., Mendoza, J. H., et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am. J. Psychiatr. 169 (6), 616-624 (2012).
- Hutchison, A. K., Hunter, S. K., et al. Diminished infant P50 sensory gting predicts increased 40-month-old attention, anxiety/depression and externalizing. J. Atten. Disord.. , (2013).

