Microfluidic Fabrication of Polymeric and Biohybrid Fibers with Predesigned Size and Shape
Summary
Two adjacent fluids passing through a grooved microfluidic channel can be directed to form a sheath around a prepolymer core; thereby determining both shape and cross-section. Photoinitiated polymerization, such as thiol click chemistry, is well suited for rapidly solidifying the core fluid into a microfiber with predetermined size and shape.
Abstract
A “sheath” fluid passing through a microfluidic channel at low Reynolds number can be directed around another “core” stream and used to dictate the shape as well as the diameter of a core stream. Grooves in the top and bottom of a microfluidic channel were designed to direct the sheath fluid and shape the core fluid. By matching the viscosity and hydrophilicity of the sheath and core fluids, the interfacial effects are minimized and complex fluid shapes can be formed. Controlling the relative flow rates of the sheath and core fluids determines the cross-sectional area of the core fluid. Fibers have been produced with sizes ranging from 300 nm to ~1 mm, and fiber cross-sections can be round, flat, square, or complex as in the case with double anchor fibers. Polymerization of the core fluid downstream from the shaping region solidifies the fibers. Photoinitiated click chemistries are well suited for rapid polymerization of the core fluid by irradiation with ultraviolet light. Fibers with a wide variety of shapes have been produced from a list of polymers including liquid crystals, poly(methylmethacrylate), thiol-ene and thiol-yne resins, polyethylene glycol, and hydrogel derivatives. Minimal shear during the shaping process and mild polymerization conditions also makes the fabrication process well suited for encapsulation of cells and other biological components.
Introduction
Tissue scaffolds1, composite materials2, optical communications3, and conductive hybrid materials4 are areas of research utilizing specialized polymer fibers. Conventional methods for fiber fabrication include melt extrusion, spinning, drawing, casting and electrospinning. Most of the polymer fibers produced by these methods exhibit round cross-sections engendered by surface tension between the polymer and air during fabrication. However, fibers with nonround cross-sections may enhance the mechanical properties of composite materials5,6, increase surface area-to-volume ratios, control wetting or wicking7, and be utilized as waveguides8 or polarizers9.
Production of specialized polymer fibers by microfluidic systems employing one stream (sheath flow) to surround and shape another stream (core flow) are appealing because of the mild conditions and capacity for continuous production of highly reproducible fibers. Initial experiments produced round fibers with sizes dependent on the relative flow rates of the prepolymer and sheath fluids10-12. The discovery that grooves in the top and bottom of the microfluidic channel could deflect the sheath to produce a predetermined shape for the core stream13,14 led to technology for generating more complex fiber shapes10-12,15-17.
NRL investigators have demonstrated the following critical technical features13-21:
- A variety of shaping features can be used to direct the sheath fluid to shape the core stream: grooves or ridges can be configured as stripes, chevrons, or herringbones.
- A toolbox of these features can be mapped to the desired flow outcome.
- Microchannels can be created using lithography, molding, milling, or printing techniques. The substrate materials must not dissolve or erode in the prepolymer or sheath solutions, and for photoinitiated polymerizations, the external layers must be transparent to ultraviolet light.
- The shape created by a single set of shaping features can be altered by changing the flow rates through the channel. COMSOL Multiphysics simulations of fluid flow in the microchannels are capable of predicting the resulting fluid and fiber shapes.
- Matching the viscosity and phase (hydrophilicity) of the sheath and core fluids is critical to avoid buckling type instability, arising from variation in shear strain across the fluid interface. If there is a large viscosity or phase mismatch viscous buckling can occur, possibly deforming the final fiber shape or even clogging the microchannel.
- Fibers can be formed by casting or polymerization, but polymerization provides more control over shape.
- Polymerization (solidification of the core fluid) must occur prior to exiting the microchannel. However, slower polymerization within the channel may cause an increase in viscosity, affecting the fiber shape or even clogging the channel. The time and location of polymerization events must be carefully controlled.
- Due to their rapid reaction kinetics, photo-induced free radical polymerizations, especially thiol-based click chemistries, are particularly well suited for fiber production.
- The relative flow rates can be changed during fabrication to create nonuniform fiber diameters.
- Multiple groups of shaping features can be integrated into a single channel for the following reasons:
- To separate the shaping and sizing functions
- To create multilayer or hollow fibers
- To produce multiple fibers from a single microfluidic channel
- Liquid crystal mesogens incorporated in the polymer at very low concentrations exhibit birefringence under polarized light, suggesting that polymer molecules can be aligned along the axis of the fibers.
- Cells can be incorporated in biocompatible hydrogel prepolymers and survive the fabrication process with high viability22.
When fabricating polymer fibers using hydrodynamic focusing by a sheath stream to shape a prepolymer stream, selection of polymer materials is a practical first step. The appropriate polymers, corresponding initiator chemistries, and sheath fluids should be identified within the following guidelines:
- Polymer and sheath fluids are miscible and are of similar viscosity. For example, an aqueous monomer solution could utilize water as a viable sheath fluid, but could not employ hexane as the sheath fluid.
- The polymerization mechanism must have fast enough rate kinetics to solidify the core fluid after shaping and immediately before the fiber exits the channel.
After the materials have been selected, a microchannel to generate the desired fiber shape and size must be designed. To determine the required shaping features (stripes, herringbones, chevrons), computational fluid dynamics software can be utilized to predict the fluid flow patterns. The shaping features transport the sheath fluid around the core fluid. In general, stripes move the sheath fluid across the top and bottom of the channel from one side to the other, whereas herringbones and chevrons move the fluid away from the sides toward the top and/or bottom of the channel and then back toward the center of the channel directly under the point of the structure. The number of repetitive grooves in the top and bottom of the channel impacts the degree to which the sheath fluid is directed. The ratio of flow rates of the core and sheath fluid also mediate the effect. Simulations using COMSOL Multiphysics software have proven reliable in evaluating the interactions of the shaping features and flow-rate ratios to predict the cross-sectional shape. These simulations also provide useful insight into diffusion of solutes between the core and sheath with the size of the channel, viscosity, and flow rates proposed.
If a complex shape is desired, such as the “double anchor” described in Boyd et al.23, it is useful to separate the functions of shaping and sizing. A complex shape can be created with one set of features and then a strategically placed single-groove structure placed at the entrance of a second sheathing stream can be used to decrease the cross-sectional area of the polymerizable stream without significantly altering its shape.
Another example of complex microchannel design can generate multilayer fibers. In this design, sequential sets of shaping features and additional cladding fluids are introduced. These concentric flows can be solidified into solid core-cladding fibers or hollow tubes. An example of this device will be presented below.
Once the design of the microfluidic device has been chosen, the microchannel fabrication process can begin. Fabrication tools that can be used include soft lithography, CNC milling, hot embossing and 3D printing. Regardless of the tools used, it is important to realize that features accidently introduced into the wall of the microfluidic channel will also direct the sheath flow and may result in highly reproducible deviations in the cross-sectional shape of all fibers made using that device. Microchannel substrate materials should also be carefully selected to be physically robust, chemically inert, and resistant to UV-damage. For example, polydimethylsiloxane (PDMS) can be easily cast, provides gasket-like seals, and is UV transparent; PDMS is useful for the transparent top of the channel, but not the sides and bottom of the channel, which need more rigidity.
Ultimately, by introducing the properly selected core and sheath fluids at the flow rates predicted by the fluid dynamics simulations, the shaping features will generate the appropriate fluid profile and the downstream UV curing lamp will solidify the designed polymer fibers. Continuous extrusion of the polymerized fibers from the channel can provide reproducible fibers in lengths limited only by the volume of the fluid reservoirs.
Protocol
This protocol describes the fabrication of a hollow fiber using photoinitiated thiol-yne click chemistry. The microchannel has chevron grooves or “stripes” as shaping features in the bottom and top of the channel (Figure 1). Three fluids are introduced and are directed in concentric streams; from the inner to outer fluid streams, these are referred to as the core, cladding, and sheath fluid. Only the cladding flow is polymerized to form the hollow fiber. The materials selected are as follows:
- Core Fluid: PEG (M.W. = 400), ~100 mPa.sec (20 ºC)
- Cladding Fluid: Thiol-yne Polymer (PETMP + ODY), Initiator (DMPA)
- Sheath Fluid: PEG (M.W. = 400), ~100 mPa.sec (20 ºC)
The microchannel device was assembled from aluminum and plastic parts fabricated by CNC milling and PDMS casting. Flow through the microchannel was controlled by three syringe pumps.
1. Design and Simulation of Microchannel
When calculating both fluid velocity and convection/diffusion within the microchannel, it is critical to assign the proper viscosity to each incoming fluid.
- Create a computer model of the desired microchannel to be imported into the computational fluid dynamics software (COMSOL). The example in Figure 1 was generated with Autodesk Inventor CAD software. The following steps are in reference to the use of COMSOL Multiphysics for calculation of fluid flow within a microchannel.
- After import of the designed microchannel into COMSOL, iterative fluid flow rates can be introduced into the Navier-Stokes solver.
- Initialize program setting and choose 3D Laminar Flow+Convection/Diffusion Equations. The low Reynolds numbers generated in the microchannels allow complete laminar flow within the device.
- Design a finite-element mesh on which to do the numerical calculations. The mesh should be more refined (have small divisions) in areas where properties change rapidly. It is suggested to refine the mesh at both the shaping feature and exit to <1 μm side-length. This provides for “crisp” visualization of the core-sheath fluid interface.
- Input material properties for fluid flow, i.e. viscosity, diffusion constant, and concentration. At this time, also set the boundary conditions for the exit flow. We suggest zero viscous stress to simulate an open outlet.
- Calculate fluid flow velocity studies by iteratively cycling through a series of input flow rates. For example, core fluid = 7.5 μl/min, sheath fluid = 30 μl/min.
- Import the velocity field solutions as the initial values to solve the convection/diffusion properties of the microchannel flow. The solution to the convection/diffusion problems will illustrate the core-sheath fluid interface and aid in predicting the shape of the final fluid flow and fiber produced.
From the computational results, the required number and type of shaping features can be predicted to attain the desired fiber shape. The fluid flow rate inputs will also correlate to the required flow rates for generating the fibers. With these predictions, a microchannel device can be fabricated for the extrusion of polymer fibers.
2. Fabrication of Sheath Flow Apparatus Components
A combination of direct micromilling, hot-embossing, and/or polymer casting can be used to create the components of the sheath flow device. Depending on the resources, choose the strategy accordingly. The example presented is a direct milling process that uses a Computer Numerical Code (CNC). There are five layers to be made (from top to bottom), which are depicted in Figure 2: 1. Inlet chuck (aluminum), 2. Fastening plate (aluminum), 3. Microchannel top layer (cyclic olefin copolymer, COC or PDMS), 4. Microchannel bottom layer (COC or polyether ether ketone, PEEK), 5. Fastening plate (aluminum). (Example files for direct milling are available in *.stl format in the Supporting Information)
- Using a design compatible with the COMSOL simulations, develop a 3D model of the system via computer aided drafting (CAD). Create a separate CAD file for each layer of the device.
- When a layer is to be fabricated via direct micromilling, import the CAD models into a computer aided machining application to generate numeric code (NC) that will be interpreted by computer numerically controlled (CNC) mill to produce the device.
- Acquire 5 sheets of 30.5 cm × 30.5 cm sacrificial layer materials that are a minimum of 3.2 mm thick.
- Acquire 1 sheet each of COC, PEEK, aluminum, and poly(methylmethacrylate) that are 30.5 cm × 30.5 cm and 3.2 mm thick.
- Acquire 1 sheet of aluminum that is 30.5 cm × 30.5 cm and 9.5 mm thick.
- Affix each of the sheets in steps 2.4-2.5 to a sheet of sacrificial stock from step 2.3 with double-sided adhesive. Ensure that at a maximum an outer un-taped border of 2.5 cm exists. The tape serves to hold the work material in place while being milled and to protect it once the milled part is cut away from the stock material at the end of the mill cycle.
- Fasten the COC + sacrificial stock to the table of the CNC Mill, load the tools quoted in the numeric code (NC), and calibrate the tools and stock (work) materials in x, y, and z.
- Load the NC code and mill the COC layer.
- Remove the sheet of material from the mill and carefully remove the machined part from the substrate. During this process, mill coolant will saturate the part and the stock. Rinse thoroughly before gently removing the part. Wash with a mild detergent, followed by washing with a 70% isopropyl alcohol. The mild detergent will remove oily residues, and the alcohol will remove residual adhesive. If burrs are trapped in the microarchitectures, sonication may be necessary to dislodge them.
- Repeat steps 2.7 and 2.9 for each of the other layers that will be used to create the sheath flow device.
- With the exception of the PMMA layer, each of the layers that have been prepared to this point will be used in the device directly. The PMMA will be used to prepare a PDMS layer by combining 10 parts Sylgard 184 base with 1 part curing agent and mixing thoroughly by stirring. This information is provided in case one would rather replace one of the COC layers with the gasket-like PDMS material.
- Pour the Sylgard 184 into the PMMA mold cavity prepared earlier, ensuring that air bubbles are eliminated. If necessary, bubbles can be removed in a vacuum. The PDMS can be cured at room temperature for 48 hr, 45 min at 100 °C, 20 min at 125 °C, or 10 min at 150 °C.
3. Sheath Flow Apparatus Assembly
- Assemble the sheath flow device from the bottom up by placing one fastening plate at the bottom, then the COC layer followed by the other COC layer, and the remaining fastening plate (Figure 2). Ensure that the shaping grooves align with each other along the edges of the channel and that the fluid shaping geometries in the COC layers perfectly overlap. A dissection microscope can be used to aid in the alignment.
- Insert bolts across the center of the device, and hand tighten the nuts and bolts to clamp the device together.
- Alternating from left to right of center, repeat step 3.2 from the center out to lock in the alignment and to prevent leaks. Add on the inlet chuck when its mounting holes are reached and continue mounting the screws in an alternating fashion.
- Use standard HPLC fittings to interface the sheath flow device to the tubing and syringes that contain sheath fluid and prepolymer solution. Hand tightening is sufficient for all connections.
- Mount the device vertically using a ring stand and clamp. Ensure that the device is vertical using a level on the top-most portion. If the sheath flow device is not vertical, the fiber may touch the microchannel wall and cause clogging.
- Position the UV source perpendicularly ~1 cm from the COC face of the sheath flow device such that the last 3-5 cm of the microchannel is irradiated. The UV source should be calibrated to deliver ~200 mW/cm2.
4. Solution Preparation
As indicated earlier, many materials can be used to create microfibers using analogous protocols and sheath flow systems, but thiol-yne chemistry is used here. Prepare the prepolymer solution immediately before beginning the fiber extrusion process to avoid the increase in viscosity that may occur over time in storage.
- Prepare an aliquot of polyethylene glycol 400 (PEG 400) to serve as the sheath fluid.
- Fill a 1 ml Luer-tipped syringe with PEG 400 to serve as a nonpolymerizable core fluid, and fill a 30 ml Luer-tipped syringe with PEG 400 to serve as the sheath fluid.
- Prepare a prepolymer solution that contains 0.01 mol pentaerythritol tetrakis 3-mercaptopropionate (PETMP) and 0.01 mol 1,7-octadiyne (ODY). Be sure that the two components are well mixed throughout the experiment, minimize exposure of all prepolymer reagents to sources of UV light, including ambient light (e.g. wrap syringes with foil).
- Supplement the PETMP/ODY solution with 4 x 10-4 mol 2,2-dimethoxy-2-phenylacetophenone (DMPA) photoinitiator. Continue to ensure that the solutions are well mixed, and that they are not exposed to UV light by covering the containers with aluminum foil.
- Load a 5 ml aluminum foil-wrapped, Luer-tipped syringe with the prepolymer solution.
5. Microfiber Production (Focus of Video)
- Ensure that the outlet of the microfluidic channel is in contact with a solution in the collection bath (Figure 3). For complex structures, the solution in the collection bath should be viscosity-matched to the core and sheath fluids, but for the simple hollow fibers, water is sufficient.
- Set the core, cladding, and sheath fluid syringe pumps to infuse at 1, 30, and 120 µl/min, respectively. Ensure that the respective syringe diameters have been properly entered into the syringe pumps.
- Mount the syringes into their corresponding syringe pumps and connect them to the sheath flow device with UV protective Tygon tubing.
- Start the sheath fluid to prime the sheath flow device and eliminate air from the system. Visually inspect the microchannel, ensuring that no air bubbles remain in the microchannel before going on to the next step. Pay particular attention to the stripes. A dissection microscope may be used to aid in microchannel inspection. If air bubbles are present, agitate the device by rotating and/or tapping gently while under flow to flush air bubbles out of the device.
- Start the cladding fluid, also allowing the flow to stabilize. Ensure that no air bubbles remain in the microchannel before going on to the next step. Pay particular attention to the shaping grooves. If air bubbles exist, agitate the device while under flow to flush the air bubbles out of the device.
- Finally, start the core fluid; again, ensure that bubbles aren’t present in the system.
- Turn on the UV source and observe the collection bath for continuous production of the hollow microfiber (Figure 4A) as it is ejected with the sheath fluid. Retrieve the fiber from the collection bath using a modified spatula or an inoculating loop, and allow the continuous fiber to be collected on a motorized spool (Figure 3).
Representative Results
A simple 2-stage design, using shaping grooves and three solution inputs, was used to create hollow fibers (Figure 1). COMSOL simulations were used to determine the appropriate flow-rate ratios to obtain the desired cross sectional size (Figure 1, ESI Video). A combination of milling and molding produced the components for the sheath flow assembly to fabricate the fibers (Figure 2). The complete assembly included the sheath flow device, fiber optic-coupled UV laser, three syringe pumps, a collection bath (beaker), and a fiber collection spool (Figure 3).
Polymerization of the cladding material was initiated by the UV light source, and hollow fibers were extruded from the microchannel into the collection bath. The fiber formed and was collected continuously until the UV light was turned off. The production of fibers continued for minutes and generated a single fiber over a meter in length. Fibers made under these conditions were approximately 200 µm in diameter. The structure of the fibers was visualized using optical and electron microscopy. The fibers had an oval shape with a hollow core. Capillary action was used to introduce liquid and bubbles into the interior of the fiber and confirmed that the hollow structure was continuous over the length of the fiber (Figure 4A).
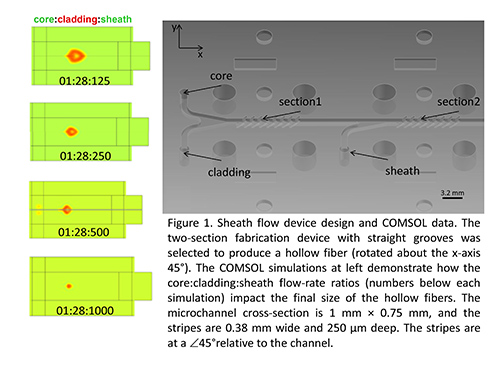
Figure 1. Sheath flow device design and COMSOL data. The two-section fabrication device with straight grooves was selected to produce a hollow fiber (rotated about the x-axis 45°). The COMSOL simulations at left demonstrate how the core:cladding:sheath flow-rate ratios (numbers below each simulation) impact the final size of the hollow fibers. The microchannel cross-section is 1 mm x 0.75 mm, and the stripes are 0.38 mm wide and 250 µm deep. The stripes are at a ∠45° relative to the channel.
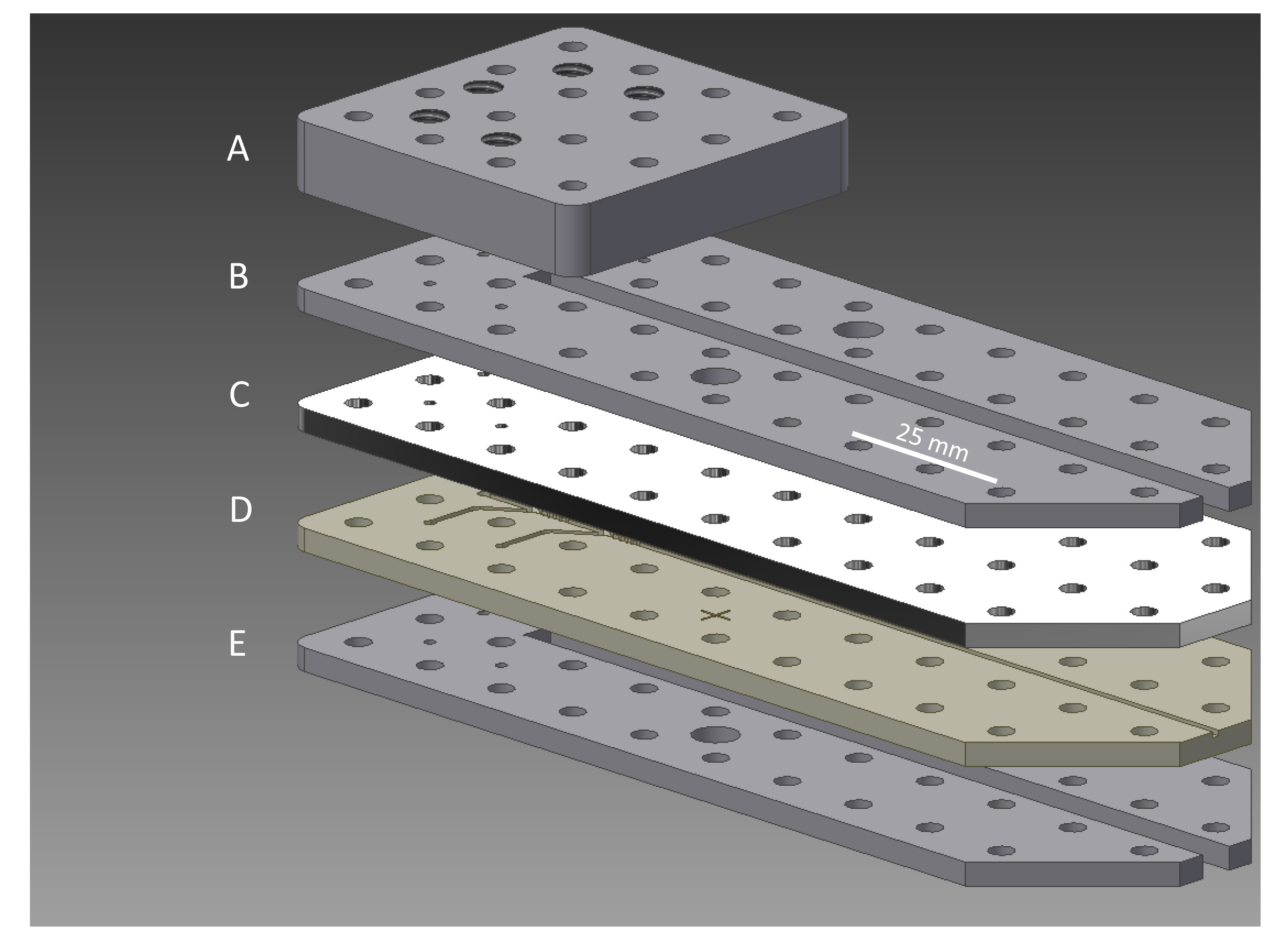
Figure 2. Exploded view of sheath flow assembly. From top to bottom, (A) inlet chuck, (B) fastening plate, (C) microchannel cover, (D) microchannel base, (E) fastening plate. The components are fabricated from aluminum, aluminum, COC (or PDMS), COC (or PEEK) and aluminum, respectively. The regularly spaced holes accommodate assembly screws.
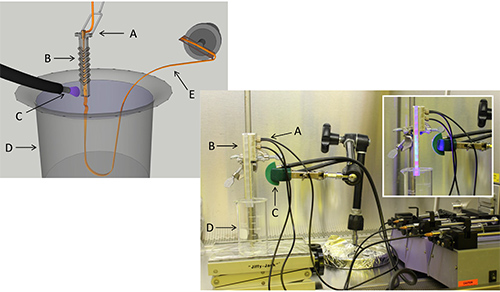
Figure 3. Photo of layout and schematic overview. Setup includes sheath flow assembly secured vertically over beaker containing water bath, fiber optic laser for photopolymerization, three syringe pumps, and spindle for collecting polymer fibers. Inset shows fabrication assembly with UV illumination. (A) Sheath and core inlets, (B) microfluidic channel, (C) UV light, (D) collection reservoir, (E) polymerized fiber being collected.
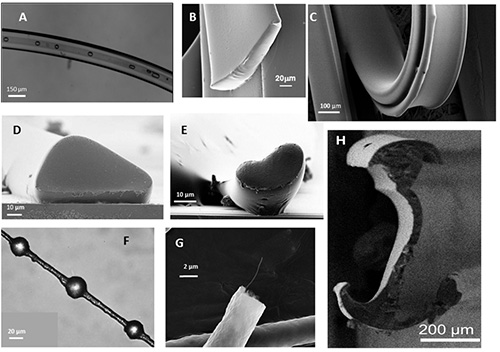
Figure 4. Optical and scanning electron micrograph images of fibers made using hydrodynamic focusing. Fibers have been fabricated in the following shapes using hydrodynamic focusing: (A) Hollow tubes, (B) Rectangular ribbons, (C) Thin elastic ribbons, (D) Triangles, (E) Kidney beans, (F) String of pearls, (G) Round fiber with embedded carbon nanofiber, and (H) Double anchor shaped. The fibers are made of various materials including acrylates, methacrylates and thiol-enes.
ESI Video. Slice plot produced in COMSOL Multiphysics depicting one half of microchannel with core, cladding, and sheath fluids entering the device and traversing the two-stage flow-altering diagonal stripe grooves. The core, cladding, and sheath flow rates simulated are 1, 28, and 256 µl/min, respectively. The video represents ~6 sec in real-time, slowed down 6-fold for illustrative purposes.
Discussion
Fabrication of polymer fibers using the sheath flow approach has multiple advantages in comparison to other fiber fabrication techniques. One of those advantages is the ability to fabricate fibers using various reagent combinations. Although a specific thiol-yne combination was presented here, several other thiol click (including thiol-ene) chemistry combinations work equally well. A wide variety of other combinations can be employed to produce fibers as long as the sheath solution is miscible with the core material to be polymerized. Inclusions such as nanofibers, particles, and cells are also possible as long as the contributions of these additives to the viscosity of the prepolymer solution are taken into account.
Thiol click chemistry is a subset of the click chemistry family in which a complex with a thiol group can be covalently attached to a complex with either an alkene (double bond) or alkyne (triple bond) functional group by UV light photopolymerization. Reactions involving alkenes are termed thiol–ene reactions, and reactions involving alkynes are termed thiol–yne reactions. One pi bond (from an alkene or alkyne) will attach to one thiol group upon UV light irradiation. The process fits well within the click family of reactions and has been effectively used in our microfluidic channel to produce fibers of various shapes (e.g. round, ribbon-shaped, double anchor) from numerous thiol click starting components.
A specific advantage to the method outlined here in comparison to most other similar processes is the ability to control both the shape and the size of the fibers produced (Figures 4A-H). By designing a channel to have stripes, chevrons, or herringbones, the fiber produced will have a different cross-section shape. In general, the stripes are useful for producing round shapes or for introduction of additional sheath streams to completely encircle previously shaped streams and move them away from the channel walls prior to polymerization. The chevrons reduce the vertical dimension in the center of the shaped stream, maintaining the horizontal symmetry. The herringbones reduce the vertical dimension of one side of the shaped stream, producing asymmetry. These shaping tools can be mixed in innumerable combinations. The number of equivalent features (i.e. 7 chevrons versus 10 chevrons) can also be used to produce fibers with different cross-sectional profiles.
In addition to the ability to control fiber shape, the fiber fabrication methodology presented also affords the ability to control the size of the fibers fabricated, even using a single sheath flow assembly (e.g. Figure 1). Adjusting the sheath:core flow-rate ratio is one means of fabricating fibers with different cross-sectional areas. It is also possible to control the size of the fiber by adjusting the channel design to have additional sheathing stages. Whether the shaping occurs in one or more stages, a simple final stage can be used to reduce the size of the core without changing the shape.
The ease with which a multitude of reagent combinations can be used to produce fibers of various shapes and sizes using this microfluidic channel design will prove useful in a wide range of applications, from tissue engineering to optical communications to smart textiles.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Darryl A. Boyd and Michael A. Daniele are National Research Council Postdoctoral Fellows. The work was supported by ONR/NRL Work Units 4286 and 9899. The views are those of the authors and do not represent the opinion or policy of the US Navy or Department of Defense.
Materials
| Pentaerythritol tetrakis 3-mercaptopropionate | Sigma-Aldrich | 381462 | See references |
| 1.7-Octadiyne | Sigma-Aldrich | 161292 | See references |
| 2,2-Dimethoxy-2-phenylacetophenone | Sigma-Aldrich | 196118 | See references |
| Polyethylene glycol 400 | Sigma-Aldrich | 202398 | Polyethylene glycol 200 or 600, dextrose, or glycerol may be substituted |
| Sylgard 184 | Sigma-Aldrich | 761036 | QSIL 216, OptiTec 7020, or GS RTV 615 may be substituted |
| Table of Specific Equipment | |||
| Equipment | Company | Catalogue number | Comments |
| MiniMill | Haas | MINIMILL | Any NC code interpreting 2.5 axis (or higher) mill may be substituted |
| Syringe pumps (3) | Harvard Apparatus | 702212 | Syringe pumps that can be programmed to deliver the desired volume flow rates may be substituted |
| Tygon tubing (3 m) | Fisher Scientific | 14-169-13A | NA |
| PEEK tubing | Upchurch Scientific | 1435 | NA |
| HPLC fittings | Upchurch Scientific | 1457 | NA |
| BlueWave 200 UV lamp with stand and light guides | Dymax | 38905; 38477; 39700 | Any guided UV source that delivers 300-450 nm, >200 mW/cm2 may be substituted |
| 500 ml beaker | Fisher Scientific | FB-100-600 | Any vessel of approximately the same size and shape may be substituted |
| Ring stand | Fisher Scientific | S47807 | Any ring stand capable of mounting a clamped sheath flow apparatus above the level of the syringe pumps may be substituted |
| Ring stand clamp holder (2) | Fisher Scientific | S02625 | Any ring stand clamp holder capable of holding the clamps may be substituted |
| Ring stand clamps (2) | Fisher Scientific | 02-216-352 | Any ring stand clamp capable of holding the clamped sheath flow apparatus and light guides may be substituted |
| 1, 5, and 60 ml Syringes | Fisher Scientific | 14-823-16H; 14-823-16D; 14-820-11 | Any syringe with known inner diameter and sufficient volume may be substituted |
| Poly(methylmethacrylate) (3.2 mm) | McMaster-Carr | 8560K239 | Polycarbonate and cyclic olefin copolymer may be substituted |
| Polyether ether ketone (3.2 mm) | McMaster-Carr | 8504K25 | Solvent resistant machinable materials may be substituted |
| Aluminum (3.2, 9.5 mm) | McMaster-Carr | 1651T41; 9246K23 | Substitute other materials as needed |
References
- Khademhosseini, A., Langer, R., Borenstein, J., Vacanti, J. P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. U.S.A. 103, 2480-2487 (2006).
- Blond, D., McCarthy, D. N., Blau, W. J., Coleman, J. N. Toughening of artificial silk by incorporation of carbon nanotubes. Biomacromolecules. 8, 3973-3976 (2007).
- Aykut, Y., Saquing, C. D., Pourdeyhimi, B., Parsons, G. N., Khan, S. A. Templating quantum dot to phase-transformed electrospun TiO(2) nanofibers for enhanced photo-excited electron injection. ACS Appl. Mater. Interfaces. 4 (2), 3837-3845 (2012).
- Puigmarti-Luis, J., Schaffhauser, D., Burg, B. R., Dittrich, P. S. A Microfluidic Approach for the Formation of Conductive Nanowires and Hollow Hybrid Structures. Adv. Mater. 22, 2255-22 (2010).
- Edie, D. D., Fox, N. K., Barnett, B. C., Fain, C. C. Melt-spun noncircular carbon-fibers. Carbon. 24, 477-482 (1986).
- Park, S. J., Seo, M. K., Shim, H. B. Effect of fiber shapes on physical characteristics of non-circular carbon fibers-reinforced composites. Mater. Sci. Eng. A Struct. 352, 34-39 (2003).
- Haile, W. A., Phillips, B. M. Deep grooved polyester fiber for wet lay applications. Tappi. 78, 139-142 (1995).
- Yamada, J. Radiative properties of fibers with non-circular cross sectional shapes. J. Quant. Spectrosc. Ra. 73, 261-272 (2002).
- Kopp, V. I., et al. Chiral fiber gratings. Science. 305, 74-75 (2004).
- Thangawng, A. L., Howell, P. B., Richards, J. J., Erickson, J. S., Ligler, F. S. A simple sheath-flow microfluidic device for micro/nanomanufacturing: fabrication of hydrodynamically shaped polymer fibers. Lab Chip. 9, 3126-3130 (2009).
- Thangawng, A. L., Howell, P. B., Spillmann, C. M., Naciri, J., Ligler, F. S. UV polymerization of hydrodynamically shaped fibers. Lab Chip. 11, 1157-1160 (2011).
- Thangawng, A. L., et al. A hard microflow cytometer using groove-generated sheath flow for multiplexed bead and cell assays. Anal. Bioanal. Chem. 398, 1871-1881 (2010).
- Mott, D. R., Howell Jr, ., B, P., Obenschain, K. S., Oran, E. S. The Numerical Toolbox: An approach for modeling and optimizing microfluidic components. Mech. Res. Commun. 36, 104-109 (2009).
- Mott, D. R., et al. Toolbox for the design of optimized microfluidic components. Lab Chip. 6, 540-549 (2006).
- Howell Jr, ., B, P., Ligler, F. S., Shields, A. R. Sheath fow device and method. United States patent US20110193259. , (2011).
- Howell, P. B., Ligler, F. S., Shields, A. R. Creating sheathed flow for applications e.g. particle counting, by introducing sheath and core streams at proximal end of channel that creates multiple sheathed flows, and polymerizing multiple sheathed flows to form multiple fibers. United States patent US2011193259-A1. , (2009).
- Mott, D., Howell Jr, ., B, P., Ligler, F. S., Fertig, S., Bobrowski, A. Sheath flow device and method. United States patent US20090208372. , (2009).
- Daniele, M. A., et al. Rapid and continuous hydrodynamically controlled fabrication of biohybrid microfibers. Adv. Funct. Mater. 23, 698-704 (2012).
- Howell, P. B., Mott, D., Golden, J. P. Numerical toolbox for design of fluidic components and systems. United States patent US20080221844. , (2008).
- Shields, A. R., et al. Hydrodynamically directed multiscale assembly of shaped polymer fibers. Soft Matter. 8, 6656-6660 (2012).
- Boyd, D. A., Shields, A. R., Naciri, J., Ligler, F. S. Hydrodynamic shaping, polymerization, and subsequent modification of thiol click fibers. ACS Appl. Mater. Inter. 5, 114-119 (2012).
- Daniele, M. A., et al. Rapid and Continuous Hydrodynamically Controlled Fabrication of Biohybrid Microfibers. Adv. Funct. Mater. 23, 698-704 (2013).
- Boyd, D. A., Shields, A. R., Howell, P. B., Ligler, F. S. Design and fabrication of uniquely shaped thiol-ene microfibers using a two-stage hydrodynamic focusing design. Lab Chip. 13, 3105-3110 (2013).

