Visualizing Neuroblast Cytokinesis During C. elegans Embryogenesis
Summary
This protocol describes how to image dividing cells within a tissue in Caenorhabditis elegans embryos. While several protocols describe how to image cell division in the early embryo, this protocol describes how to image cell division within a developing tissue during mid late embryogenesis.
Abstract
This protocol describes the use of fluorescence microscopy to image dividing cells within developing Caenorhabditis elegans embryos. In particular, this protocol focuses on how to image dividing neuroblasts, which are found underneath the epidermal cells and may be important for epidermal morphogenesis. Tissue formation is crucial for metazoan development and relies on external cues from neighboring tissues. C. elegans is an excellent model organism to study tissue morphogenesis in vivo due to its transparency and simple organization, making its tissues easy to study via microscopy. Ventral enclosure is the process where the ventral surface of the embryo is covered by a single layer of epithelial cells. This event is thought to be facilitated by the underlying neuroblasts, which provide chemical guidance cues to mediate migration of the overlying epithelial cells. However, the neuroblasts are highly proliferative and also may act as a mechanical substrate for the ventral epidermal cells. Studies using this experimental protocol could uncover the importance of intercellular communication during tissue formation, and could be used to reveal the roles of genes involved in cell division within developing tissues.
Introduction
While there are protocols describing how to image cell division in the early C. elegans embryo, this protocol describes how to image cell division within a tissue during mid embryogenesis. One of the major challenges in imaging organisms during development has been their sensitivity to phototoxicity. However, increased accessibility to spinning disk confocal microscopes or swept field microscopes has permitted more widespread imaging applications. Both systems use solid state lasers and scattered light, limiting the levels of UV that the organisms are exposed to. However, widefield stands can still be used for imaging in vivo, particularly if they are outfitted with cameras with high sensitivity (e.g. EMCCD), aperture control and light control (e.g. LEDs or adjustable mercury bulbs). This protocol describes how to use either a confocal based system or a widefield system to image cell division within developing C. elegans embryos. As an example, we describe how to image neuroblast cell division. Neuroblasts may facilitate epidermal morphogenesis by providing chemical or mechanical cues to the overlying epidermal cells, and provide an excellent example of the importance of intercellular communication in the formation of tissues.
Caenorhabditis elegans is an ideal model organism for microscopy based studies due to its transparency and simple tissue organization1. Furthermore, C. elegans is amenable to genetic methods and RNAi, and since many of its genes have human homologues, it can be used to identify conserved mechanisms for tissue formation2-5. In C. elegans, formation of the epidermis occurs during mid embryogenesis, when the embryo has >300 cells. Epidermal morphogenesis consists of several major phases, during which the embryo is enclosed in a layer of epidermal cells that constrict and extend to transform the embryo from an ovoid form into the elongated shape of a worm6. Ventral enclosure describes one of these morphogenetic events, when the ventral epidermal cells migrate towards the ventral midline to cover the ventral surface of the embryo (Figure 1). First, two pairs of anterior located leading edge cells migrate towards the ventral midline, where they adhere and fuse with their contralateral neighbors6. This is followed by migration of the posterior located pocket cells, which form wedge like shapes creating a ventral pocket6-7. The mechanism that closes the pocket is not well understood. One possibility is that a supracellular actin myosin contractile structure ties the pocket cells together in a purse string like fashion, similar to wound healing8. Interestingly, migration of some of the pocket cells is mediated by specific subsets of underlying neuroblasts9 (neuronal precursors that are found underneath the epidermis; Figure 1B).
Previous studies showed that the neuroblasts regulate ventral epidermal cell migration and ventral enclosure. VAB-1 (Ephrin receptor) and VAB-2 (Ephrin ligand) are highly expressed in the neuroblasts and facilitate the sorting of anterior and posterior neuroblasts from one another, and mutations in vab-1 or vab-2 cause ventral enclosure phenotypes10-13. However, promoter rescue experiments showed that vab-1 is also required in the overlying ventral epidermal cells and receptors for other guidance cues secreted by the neuroblasts are expressed in the ventral epidermal cells9. Although mutations in any of these receptors cause ventral enclosure phenotypes, it is not clear if defects arise due to problems in neuroblast positioning or due to failure of the ventral epidermal cells to respond to guidance cues14. Altering the division of neuroblasts without affecting their ability to secrete guidance cues could shed light on the role of neuroblasts and their ability to provide mechanical input during epidermal morphogenesis. Recently, it was found that a cell division gene, ani-1 (anillin) is highly expressed in neuroblasts (Figure 2A) and its depletion causes neuroblast division defects. Interestingly, these embryos display ventral enclosure phenotypes (Fotopoulos, Wernike and Piekny, unpublished observations).
Anillin is required for cell division, and particularly for cytokinesis, which describes the process where a mother cell physically divides into two daughter cells. Cytokinesis is driven by the formation of an actomyosin contractile ring, which needs to be tightly controlled in space and time to ensure that it is properly coupled with sister chromatid segregation. The master regulator of metazoan cytokinesis is RhoA (RHO-1 in C. elegans), a small GTPase that is active in its GTP-bound form. The GEF Ect2/ECT-2 activates RhoA, after which RhoA-GTP interacts with downstream effectors that form the contractile ring and mediate its ingression15. Anillin is a multi domain protein that binds to RhoA via its C-terminus and to actin and myosin via its N-terminus. Anillin is required to stabilize the position of the contractile ring in mammalian or Drosophila S2 cells16. Anillin depletion causes contractile rings to undergo lateral oscillations, and cytokinesis eventually fails forming multinucleate cells17-19. Interestingly, although C. elegans ani-1 coordinates actomyosin contractility in the early embryo, it is not essential for cytokinesis. However, as described above, ani-1 is required for neuroblast cytokinesis during mid-embryogenesis (Fotopoulos, Wernike and Piekny, unpublished observations). Cytokinesis failure would alter the number and position of neuroblasts and could affect the location of chemical guidance cues, or it could alter the mechanical properties of the tissue. Both models highlight the nonautonomous role of neuroblasts for ventral enclosure, and the importance of tissue-tissue communication during embryonic development.
This experimental protocol describes how to image cell division during C. elegans mid embryogenesis using fluorescence microscopy. The majority of experiments studying the mechanisms of cell division were performed in single cells within culture dishes (e.g. HeLa or S2 cells) or in early embryos with a limited number of cells (e.g. C. elegans one-cell embryo, Xenopus, or echinoderm embryos). However, it is important to also study cell division within tissues, as there are external cues that can influence the timing and placement of the division plane. Furthermore, cells may provide chemical or mechanical cues to influence the development of neighboring tissues, and it is important to understand how intercellular communication helps tissues to form during development.
Protocol
1. Preparation of Plates for Maintaining Worm Strains and Performing RNAi
- Nematode Growth Media Plates
- Plates
- Prepare Nematode Growth Media (NGM) plates to maintain worm strains and to perform genetic crosses. Combine 3 g NaCl, 17 g Agar and 2.5 g BactoPeptone with 1 L of distilled water in a 2 L flask and add a metal stirring bar.
- Autoclave the flask to dissolve the agar and to sterilize the media. Then place the flask on a stir plate and allow the media to cool while stirring.
- Once the media has cooled down and is still somewhat warm to touch (45-50 °C), add 1 ml 1 M CaCl2, 1 ml 1 M MgSO4, 1 ml of 5 mg/ml cholesterol solution and 25 ml 1 M potassium phosphate buffer (PPB; recipe in table of reagents/materials; filter sterilize stock solutions) and immediately pour the media into small Petri dishes (60 mm x 15 mm; fill the dishes ~3/4 to the top or 13 ml/plate using an automated dispenser). Note: For long term maintenance of some strains tetracycline can be added to the plates (12.5 μg/ml) to allow Tn10 transposon inactivation of the bacterial RNAse III gene.
- Let the media solidify overnight at room temperature. Note: Plates should be kept in a biological hood or in an area of the lab that is less prone to contamination. Also, remove any condensation that has built up on the lids (e.g. clean with tissue paper) to limit fungal contamination. If Tetracycline is also added to the plates, then cover them with aluminum foil as this antibiotic is light sensitive.
- The next day, seed the dry NGM plates with a suspension of E. coli (OP50 strain; see Section 1.1.2) that has been gently vortexed. To make plates for mating, add ∼50 μl of OP50 to the center of each plate (to form a small circle). To make plates for maintaining worm strains, seed each NGM plate with ∼100-200 μl of OP50 (to ensure that it covers a greater area of the plate).
- Let the seeded plates dry O/N at room temperature.
- The next day, turn the dried NGM plates upside down and stack them in plastic storage containers at 4 °C. Note: Plates are usable up to ∼3-4 weeks. Plates containing Tetracycline should be kept in the dark.
- Food
- Use E. coli OP50 [from the Caenorhabditis Genetics Center (CGC; http://www.cbs.umn.edu/cgc)] as a food source for C. elegans. Note: OP50 can be stored as glycerol stocks at -80 °C (ratio 1:1 in 50% v/v glycerol).
- To make OP50 for the NGM plates, make a streak plate using a small aliquot of the glycerol stock (<10 μl; use a sterile pipette tip). Spread the bacteria on an LB agar plate (recipe in table of reagents/materials) and leave it at 37 °C for ~16 hr. Note: If there are problems with contamination, streptomycin resistant OP50 can be used, and streptomycin can be added to the LB agar plates (50 μg/ml).
- The next day, pick a single colony and inoculate 100 ml of liquid LB media (recipe in table of reagents/materials) in a 500 ml flask.
- Let the culture grow O/N at 37 °C with shaking.
- The next day, use the bacterial suspension for seeding NGM plates. Note: OP50 can be aliquoted in 50 ml conical tubes and stored at 4 °C for ∼4-6 weeks.
- Plates
- RNAi
Feeding is one of the main ways to perform RNA mediated interference (RNAi; to knockdown a specific gene product) in C. elegans. C. elegans hermaphrodites can be fed E. coli strain HT115 transformed with a feeding vector that expresses dsRNA upon induction. This dsRNA (or its fragments) is transmitted to the gonad, usually resulting in substantial knockdown of the specific gene target.- RNAi Plates
- Prepare NGM plates as described above (see Section 1.1.1), but also add 1 ml of 1 M IPTG (isopropylthio-beta-D-galactoside; to induce the expression of dsRNA) and 500 μl of 50 mg/ml ampicillin (Amp; the vectors expressing dsRNA are Amp-resistant) to the warm media (45-50 °C).
- After the plates have dried, seed them with ~50-100 μl of E. coli HT115 (see Section 1.2.2). Note: Unseeded plates can be stored at 4 °C for ~4-5 weeks.
- Let the seeded plates dry overnight at room temperature.
- The next day, turn the dried RNAi plates upside down and store them in a plastic storage container at 15-20 °C. Note: Seeded plates retain RNAi efficiency for up to ∼2-3 weeks.
- RNAi Food
- Use E. coli HT115 (from the CGC; see Section 1.1.2) transformed with the feeding vector L4440, or L4440 containing cDNA to a gene of interest. Note: Feeding libraries that target the majority of open reading frames in the genome have already been made and are available (clones in L4440 transformed into HT115; e.g. containing a fragment from Y49E10.19 for ani-120-21; http://www.gurdon.cam.ac.uk/~ahringerlab/pages/rnai.html). Note: HT115 can be kept as glycerol stocks at -80 °C (ratio 1:1 in 50% v/v glycerol).
- To generate a new construct targeting a gene of interest, see the L4440 vector map and clone cDNA between the flanking T7 promoters, which are inducible by IPTG.
- Transform HT115 with the L4440 construct using a standard protocol for transformation (e.g. make chemically competent bacteria and perform heat shock transformation).
- As described for OP50 (see Section 1.1.2), streak bacteria from the glycerol stock on an LB agar plate, but the plate should also contain 50 μg/ml of Ampicillin. Note: If using a pre existing L4440 construct, skip Sections 1.2.2.2-1.2.2.3.
- Pick a single colony and inoculate 5 ml of LB media containing 5 μl of ampicillin from a 50 mg/ml stock (LB Amp), and place it at 37 °C with shaking.
- After ~16 hr, inoculate 5 ml of fresh LB Amp media with 50 μl of the O/N culture and grow the culture for 7-8 hr with shaking at 37 °C.
- Centrifuge the bacteria for 1 min at 4,000-8,000 x g, discard the supernatant and resuspend the pellet in 500 μl of fresh LB Amp media.
- Use this bacterial solution to seed the RNAi plates as described above (see Section 1.2.1). Note: HT115 should be used immediately and should not be stored.
- RNAi Plates
2. Cultivating Worm Strains
Maintain C. elegans strains ordered from the CGC on NGM plates according to standard protocol1. Strains used for this protocol include: C. elegans var Bristol, wild-type (strain ID at CGC N2); unc-119(tm4063); wgIs102 [ham-1::TY1::EGFP::3xFLAG + unc-119(+)] (strain ID at CGC OP102); unc-119(ed3); ltIs44pAA173 [pie-1p-mCherry::PH(PLC1delta1) + unc-119(+)] (strain ID at CGC OD70); ajm-1(ok160); jcEx44 [ajm-1::GFP + rol-6(su1006)] (strain ID at CGC SU159); unc-119(ed3); xnIs96 [pJN455(hmr-1p::hmr-1::GFP::unc-54 3’UTR + unc-119(+)] (strain ID at CGC FT250).
- Pick ~3 young adult hermaphrodites that display the correct phenotype to a fresh NGM plate. Note: N2 (var. Bristol; wild-type) can be maintained between 15-25 °C, but they will reach density faster at higher temperatures. New stocks should be made when the worm density is high.
- Keep all fluorescent strains (e.g. GFP and/or mCherry-expressing worms) at 20-25 °C for optimal expression of fluorescent proteins. New stocks should be made when the worm density is high and food is low.
- To pick worms, use a pick made from a pulled glass pipette with a platinum wire fused at the pulled end (~3-5 cm in length). Flatten the wire with a smooth edge. Use the pick to ‘scoop’ up hermaphrodites and transfer them onto new plates. Flame the wire in between use to avoid cross contamination of strains.
3. RNAi
- Performing RNAi
- First, place ~8 L4 hermaphrodites on an unseeded NGM plate for ~30-60 min to remove the OP50 bacteria from the worms.
- Then place the 8 hermaphrodites onto an NGM Amp IPTG plate seeded with HT115 that carries the L4440 plasmid expressing dsRNA to a gene of interest (e.g. ani-1; see 1.2) for ~24 hr. Note: Depending on the gene of interest, or the desired phenotypes (which may depend on the strength of knockdown), worms can be left on the RNAi plates for shorter or longer periods of time (after 24 hr, place the hermaphrodites on fresh RNAi plates).
- For a negative control, place worms on an RNAi plate seeded with HT115 carrying the empty L4440 vector. Note: These embryos should have no phenotypes.
- For a positive control, place worms on an RNAi plate expressing dsRNA that targets a gene with well-characterized phenotypes. Note: For rho-1 (Y51H4A.3), embryos are 100% lethal after 24 hr and hermaphrodites are sterile after 48 hr.
- RNAi Efficacy
The levels of knockdown by RNAi should be tested, particularly since some tissues may be more resistant than others. During early mid embryogenesis, most tissues are RNAi-sensitive, but in the late embryo or larva, neurons are RNAi-resistant. Different strains can be used to improve RNAi sensitivity (e.g. rrf-3), or to generate tissue-specific RNAi (e.g. sid-1 in combination with a transgene expressed by the neural promoter unc-119).- Western Blotting
Immunoblotting using C. elegans proteins can be performed according to standard protocol22.- After performing RNAi, collect ~10 gravid (filled with embryos) hermaphrodites in a microcentrifuge tube, add 20-30 μl 1x SDS-PAGE sample buffer and lyse by boiling for ~ 2 min. Similarly, collect N2 worms in a separate tube for the control sample. Note: The number of hermaphrodites may vary depending on the sensitivity of the primary antibodies.
- Create dilutions of the control sample (e.g. 3-100%) in sample buffer. Load the control and RNAi samples and run by SDS-PAGE as per standard protocol (the % gel will vary depending on the size of the protein of interest).
- Transfer the gel to a membrane and perform immunoblotting as per standard protocol. Note: Use different primary and secondary antibody dilutions as recommended for each antibody.
- Develop (e.g. if using chemiluminescence) or scan (e.g. if using fluorescence) the immunoblot and compare the density of bands in the N2 lanes vs. the RNAi lane, and determine the level of knockdown (e.g. the density of the RNAi lane matches the 12% control lane, this suggests that the protein is reduced by 88%). Note: To see the efficiency of ANI-1 knockdown, see Maddox et al.23
- Immunostaining
Immunostaining in C. elegans can be performed according to standard protocol22.- After performing RNAi, collect gravid hermaphrodites and harvest embryos using one of two methods. For one of the methods, place hermaphrodites in M9 buffer (recipe in table of reagents/materials) in 1.5 ml siliconized microcentrifuge tubes and pellet via centrifugation (1,000-4,000 x g). Note: Similarly collect N2 gravid hermaphrodites.
- Remove the M9 solution.
- Expel embryos by adding 1 ml of bleaching solution (1 M NaOH, 5% bleach) to the microcentrifuge tube for 3 min. Similarly, bleach N2 hermaphrodites to make control slides.
- Vortex gently, then immediately pellet the embryos via centrifugation (4,000-8,000 x g) and remove the bleaching solution. Note: The total time that the worms are incubated with bleach should not exceed 5 min.
- Wash the embryos 3x with Tris Buffered Saline (TBS; 50 mM Tris pH 7.5, 150 mM NaCl) and transfer embryos using a glass Pasteur pipette onto a poly-L-lysine-coated microscope slide. Note: To coat a glass microscope slide with poly-L-lysine, first wipe the slide, then add a drop (~20-30 μl) of poly-L-lysine and spread it evenly across the surface and let dry. For optimal results, coat the slides two more times. An alternative method to collect embryos (Sections 3.2.2.1-3.2.2.5) is to place a drop of M9 onto a poly-L-lysine-coated microscope slide and pick ~10 gravid hermaphrodites into the drop.
- Add a coverslip on top of each microscope slide (to the area that contains most of the embryos) and place the slides in liquid nitrogen. Note: For hermaphrodites placed directly into the drop, put pressure onto the coverslip to break open the hermaphrodites and expel the embryos.
- Freeze-crack the embryos by flicking off the coverslips immediately after freezing them in liquid nitrogen.
- Place the slides in ice cold methanol for 20 min to fix the embryos. Note: For some antigens, a crosslinking fixative such as paraformaldehyde may be preferable. See the protocol from Duerr22.
- Rehydrate and permeabilize the embryos by washing the slides 4x with Tris Buffered Saline containing Tween-20 (TBST; 50 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 10 min each.
- Dry each slide around the area that contains most of the embryos and draw a circle around the embryos using a liquid blocking pen (e.g. PAP pen).
- Add ~100 μl of TBST with 5% normal donkey serum (NDS; block) to each slide (circled area) and incubate for 20 min at RT. Note: Place the slides in a ‘wet chamber’ (e.g. a dish with a lid containing wet paper towels).
- Remove the block, add ~50-100 μl of primary antibodies (diluted in TBST) to each slide (circled area) and incubate for 2 hr at RT. Note: Depending on the antibody, the dilution and incubation time may vary. To detect GFP, we use an anti-mouse anti-GFP primary antibody in a 1/200 dilution. To detect ANI-1, we use an anti-rabbit anti-ANI-1 primary antibody in a 1/1600 dilution (kindly provided by Amy Shaub Maddox, UNC Chapel Hill). For longer incubation times, place slides at 4 °C.
- Remove primary antibodies (if possible, keep for future use) and wash slides 3x with TBS for 5 min each.
- Remove the last wash and add ~50-100 μl of secondary antibodies (diluted in TBST) to each slide (circled area) and incubate for 2 hr at RT. Note: Depending on the antibody, dilution may vary. To detect GFP, we use an anti-mouse Alexa Fluor 488 secondary antibody in a 1/250 dilution. To detect ANI-1, we use an anti-rabbit Alexa Fluor 568 secondary antibody in a 1/250 dilution.
- Remove secondary antibodies and wash slides 3x with TBS for 5 min each. Note: To stain DNA, add ~50-100 μl of DAPI (4’,6-diamidino-2-phenylindole; 1 mg/ml) at 1/500 dilution in TBST for 5 min.
- Remove DAPI and wash slides 2x with TBS for 5 min each. Note: If DAPI staining is not performed, skip this extra washing step.
- Perform one quick wash with 0.1M Tris (pH 9), remove and add ~15 μl of mounting media prewarmed to 37 °C (4% n-propyl 3.4.5-trihydroxybenzoate (propyl gallate), 50 mM Tris, pH 9 in 50% glycerol) to each slide (circled area).
- Add a coverslip to each slide (on top of the mounting media in the circled area), wick off the excess liquid and seal the slides with nail polish. Note: Slides can be stored at -20 °C.
- Observe the embryos on the slides using a widefield fluorescent microscope or laser scanning confocal microscope. Importantly, adjust the settings to collect images of optimal pixel intensity using control (N2 or non-RNAi) embryos. Then, collect images from RNAi embryos using the same settings. Examples of images collected for control vs. ani-1 RNAi embryos are shown in Figure 2B.
- Western Blotting
4. Preparation of Slides for Live Imaging and Harvesting Embryos
- Preparation of agarose pads for live imaging
- Place a clean, pre warmed microscope slide between two other slides, each with 1-2 strips of tape on them.
- Using a glass Pasteur pipette, add one drop of 2% w/v agarose (dissolved in heated distilled water) to the slide.
- Quickly place a second, clean microscope slide on top of the first slide in a perpendicular manner to flatten the agarose drop. Note: The tape on the neighboring slides provides the thickness for the pad.
- Let the agarose pad dry for a 1-2 min before removing the top slide. Slide off the top slide carefully to avoid breaking the agarose pad underneath. Note: Sometimes the pad is transferred to the top slide and is still usable, as long as it remains intact.
- Transfer the embryos onto the agarose pad within a few minutes after its preparation as described below (see step 4.2). Note: The pad should have an opaque appearance; once it is clear, it is too dry to use.
- Harvesting embryos
- Use the following protocol for collecting embryos for live imaging, which is derived from Sulston et al.24
- Pick approximately 4-6 gravid adults (not starved) from NGM or RNAi plates and place them in 20-30 μl of M9 buffer in a well on a depression slide.
- Use a sterilized scalpel to cut the worms on either side of the spermatheca (or alternatively near the vulva) to release embryos into the solution.
- Use a rubber hose with a mouth piece connected to a glass capillary/pipette pulled to have a small bore and suction the embryos by mouth pipetting. Alternatively, collect embryos with a capillary glass tube that is attached to a Pasteur pipette rubber bulb.
- Transfer the suctioned embryos to a second well also containing M9 buffer, to help separate them from the worm debris.
- Then, suction the embryos onto a freshly prepared agarose pad (see step 4.1).
- Clump embryos together using an eyelash (glued to a tooth pick) to increase the number of embryos that can be filmed in one x, y plane. Note: To avoid oxygen deprivation, do not clump more than 10 embryos together.
- Next, cover the slide with a coverslip and partially seal it with pre heated liquid VALAP (petroleum jelly, lanolin and paraffin; melted and combined 1:1:1) to prevent dehydration of the embryos during imaging. Note: Additional M9 buffer can be added to the pad to ensure that embryos have sufficient liquid for imaging. In place of VALAP, petroleum jelly can be used to partially seal coverslips, but it is less rigid causing the coverslips to slip and could get onto the microscope objectives.
5. Live Imaging
- To visualize neuroblasts, obtain a worm strain that expresses a neuroblast-specific protein attached to a fluorescent protein (e.g. HAM-1:GFP, strain ID at CGC OP102). To detect cell membranes, use a worm strain that also expresses a protein domain that recognizes lipids attached to a different fluorescent protein (e.g. mCherry:PH, strain ID at CGC OD70).
- Harvest embryos from fluorescent hermaphrodites kept on control or ani-1 RNAi plates (see Protocols 3 and 4), and prepare slides as described above (see Protocol 4).
- Image embryos in 4D (x, y, z and time-lapse) by epifluorescence microscopy. Use either a widefield system [automated fluorescent microscope with filters for GFP and TexasRed, objectives up to 60X or 100X, a high resolution CCD (charge coupled device) camera, highly motorized stage (e.g. Piezo Z) and specialized acquisition software] or a livescan swept field confocal microscope [automated fluorescent scanning microscope with 488 nm and 561 nm lasers, objectives up to 100X, an EMCCD (electron multiplying CCD) camera, highly motorized stage (e.g. Piezo Z) and specialized acquisition software]. Note: For the widefield system, using LEDs and an EMCCD camera will limit phototoxicity. Also, a spinning disk confocal microscope can be used in place of the swept field system.
- To image neuroblast cell division on either system, find x, y frames of embryos using the 10X or 20X objectives, and choose an optimal x, y frame where the embryos are undergoing dorsal intercalation (step prior to ventral enclosure; Figure 1A) or are at early stages of ventral enclosure (∼350 min after the first cell division; Figure 1A).
- On the swept field microscope, use the 488 nm and 561 nm 100 mW lasers at 40% power with a slit of 50. On the widefield system, use the GFP and TexasRed filters with low-medium light intensity (if controllable). Note: For the widefield system, LEDs have low phototoxicity and are preferable.
- On either system, use the 60X or 100X oil immersion objectives and collect 20 Z-stacks of 0.2 μm every 2 min for a total of 10 min. Note: Although the HAM-1:GFP and mCherry:PH probes express at relatively high levels, the exposure times for each probe will have to be optimized for different microscopes. Note: For the widefield system, fewer Z-stacks are recommended to limit phototoxicity and for imaging over longer periods of time, use fewer time points.
- To minimize phototoxicity caused by the exposure of embryos to UV light on the widefield system (if using a mercury bulb), close the aperture to 30% and decrease the light intensity (using an adjustable illumination system). Note: Although gain is not needed using either system, other microscopes may have light sources with lower intensities or objectives with different numerical apertures, which could require the use of gain to obtain images of optimal pixel intensity. Test the conditions using a control embryo to ensure that any observed phenotypes are not due to artifactual phototoxicity.
6. Analysis of Microscopy Data
- To analyze the images, export files as TIFFs and open the TIFFs as a stack using specialized software such as a Java based image processing program (e.g. ImageJ, NIH, http://rsbweb.nih.gov/ij/). Note: Depending on the acquisition software used to collect the images, the files can be kept in their original form and opened in ImageJ. This is preferable as the metadata is kept with the files.
- Separate the stack into ‘images’ and select time points and Z-planes as desired. Note: Although a stack of 20 Z-planes is taken, many of the neuroblasts are <1 μm thick during mid late embryogenesis and only a few Z-planes are needed.
- Restack the selected Z planes for each time point and make separate Z-stack projections. Then, stack the projections for each time point together to make a time sequence.
- Make adjustments to the brightness/and or contrast for each channel.
- Then, select a region of interest, crop (and rotate if necessary) the region.
- Create merged images using the ‘merged channels’ function and choose a different color for each channel. Convert merged images to RGB.
- Invert grayscale images for each channel from steps 6.3 or 6.4 (use ‘invert’ function under ‘edit’) to visualize images more clearly.
- Save all final images as 8-bit TIFFs to make figures in vector based software programs or as Quicktime files to make movies.
- If measurements of pixel size are needed (e.g. to include a scale bar with the images), use ImageJ, or other image processing software to measure the size of the embryo. Note: Images collected using different objectives will have different pixel sizes.
Representative Results
This experimental protocol describes how to image cell division in C. elegans embryos during mid embryogenesis. In particular, it describes how to image neuroblasts, which may facilitate epidermal morphogenesis. Epidermal morphogenesis occurs due to a combination of epidermal cell shape changes, migration and adhesion, but also relies on chemical or mechanical cues from the underlying neuroblasts (Figure 1B). The neuroblasts secrete guidance cues that are received by receptors on the surface of ventral epidermal cells, which regulates their migration10,14,25. Furthermore, the neuroblasts are highly proliferative, which may provide mechanical forces that contribute to the migration of ventral epidermal cells26. This protocol can be used to study neuroblast cell division during mid embryogenesis. First, embryos coexpressing markers to visualize neuroblasts (HAM-1:GFP) undergoing cytokinesis (mCherry:PH) are generated. HAM-1:GFP expresses GFP (green fluorescent protein) tagged protein that localizes to neuroblast nuclei27, and is necessary to use since there are many other cell types present during mid embryogenesis (>300 cells; Figure 3). This also acts as a good marker for dividing cells, since DNA condenses into chromosomes during mitosis. mCherry:PH is an mCherry-tagged fluorescent protein that is expressed by a maternal promoter (pie-1), and contains the lipid binding domain from PLC1∂1 28, which localizes to cell membranes. This protein is needed to visualize cytokinesis, when the membrane and cytosol pinch in to separate the mother cell into two daughter cells; Figure 3; Video 1). Although this protocol describes how to image neuroblast division, (shown by HAM-1:GFP expression), other markers could be used to visualize other cell types.
To visualize dividing neuroblasts, embryos coexpressing HAM-1:GFP and mCherry:PH are imaged every 2 min (for a total of 10 min). Each neuroblast is only ~1-4 μm in diameter, and is <1 μm thick, and it is best to use an objective with high magnification (e.g. 100X). Furthermore, numerous Z-stacks (~20) are collected with a small step size (0.2 μm) to ensure that the various angles of each cell (spherical in shape) are imaged. To collect this many images without compromising time points, a highly motorized stage (e.g. Piezo Z Stage) is recommended. After collecting a series of images, they are analyzed to find cells within a few Z-stacks that are dividing , the DNA is condensed, the membrane is ingressed and the new daughter cells are forming (time points of the projected stack of merged channels are shown in Figure 3A, and selected stacks of merged channels are shown in Figure 3B; Video 1). To better visualize the cells, it is recommended to keep each channel in grayscale and invert the images (e.g. Figure 4).
The image resolution is different for the widefield system using a high resolution CCD camera (1344 x 1024 pixels) vs. the swept field system using a high speed EMCCD camera with high sensitivity (~35 frames/sec and 512 x 512 pixels). Inverted images of dividing neuroblasts from control embryos taken with both systems are presented for comparison (Figure 4 vs. 5). As shown in Figure 4A (widefield system; Video 2), the images are clearer vs. Figure 5A (swept field system; Video 4). However, when using the widefield system, embryos are susceptible to phototoxicity and faster time points are not possible with the high resolution camera. For example, to examine changes in the localization of various proteins (e.g. to study changes in their dynamics), time points might need to be collected more often. Also, it may not be possible to image for longer periods of time without reducing the number of Z-stacks and time points. Using an EMCCD camera on the widefield system may permit a wider range of imaging applications. Cameras that have both high resolution and high speed have been developed, but the drivers may not be available depending on the acquisition software being used, and they still may not be as sensitive as EMCCD cameras. Another system that is commonly used for imaging C. elegans is the spinning disk confocal, which also has lower phototoxicity and can be outfitted with either EMCCD or CCD cameras (see Discussion).
To study genes that are required for cell division, hermaphrodites are treated with RNAi against a gene of interest (e.g. ani-1; see Sections 1.2 and 3 of the protocol) and their embryos are imaged in the same way as control embryos. To compare cells from RNAi embryos to control embryos, it is best to image at similar stages of embryonic development (to help match cell shapes and positions). For example, there is a larger cell visible in the center of the embryo during ventral enclosure that can act as a good reference point for imaging neuroblasts (Figures 4 and 5). The gene studied here, ani-1 (anillin), organizes actomyosin contractility, but is not required for cytokinesis in the early embryo23,29-30. Anillin’s homologues, however, are required for cytokinesis in higher eukaryotes16, and ani-1 is required for neuroblast cytokinesis (Fotopoulos, Wernike and Piekny, unpublished observations). As shown in Figures 4B (Video 3) and 5B (Video 5), several neuroblasts initiate cytokinesis and their membranes pinch in, but instead of forming two separate daughter cells, their membranes regress and the cells become multinucleate (>1 nucleus/cell). It must be noted that ani-1 may be required for the division or shape change of other cell types, which are not revealed by this protocol. Furthermore, this protocol does not show results from tissue specific RNAi (see Discussion).
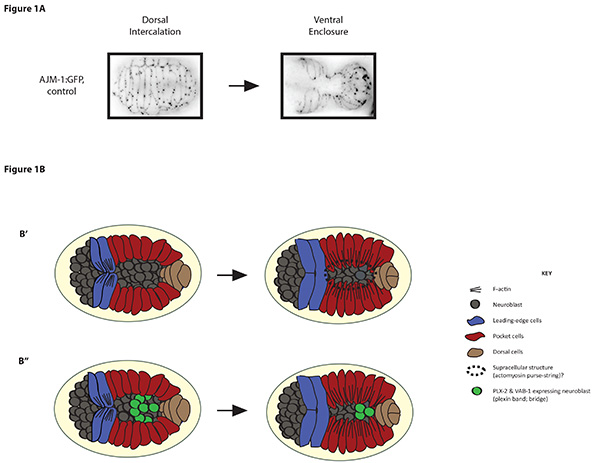
Figure 1. Ventral enclosure during C. elegans epidermal morphogenesis. A) Z-stack projections (3 Z-stacks of 0.5 μm in thickness) of a control AJM-1:GFP (adherens junction marker to visualize epidermal cell boundaries; strain ID at CGC SU159) embryo undergoing dorsal intercalation (left, dorsal view) and a control AJM-1:GFP embryo undergoing ventral enclosure (right, ventral view). Images are inverted to better visualize cell boundaries. B) Cartoons show ventral views of C. elegans embryos undergoing ventral enclosure. First, two pairs of leading edge cells (blue) use actin rich filopodia (black lines) to migrate toward the ventral midline (embryo on the left). Next, the posterior ventral pocket cells (red) migrate toward the midline creating a hole on the ventral surface that may close by a purse string like mechanism (B’; black dotted line/circle; reminiscent of wound healing)25. Also shown are the underlying neuroblasts (as grey circles). The second embryo (B”) shows how the migration of specific subsets of epidermal cells are mediated by a ‘bridge’ formed from the rearrangement of PLX-2/plexin and VAB-1/Ephrin receptor-expressing ‘plexin band’ cells (green circles). Click here to view larger image.
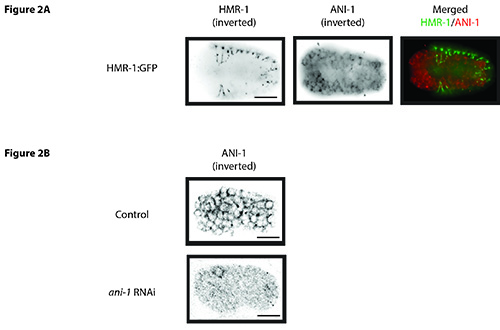
Figure 2. ANI-1 localization in C. elegans embryos. A) A fixed C. elegans embryo expressing HMR-1:GFP (E-cadherin; marker of epidermal cell boundaries) costained for GFP (green) and ANI-1 (red). Confocal images of the outer cell layers (4 Z-stacks of 0.2 μm in thickness) show the overlying epidermal cells and underlying neuroblasts, which are ANI-1 positive. B) Fixed N2 and N2; ani-1 RNAi embryos are co stained for ANI-1. ANI-1 is greatly reduced in the ani-1-depleted embryo. Scale bars: 10 μm. Click here to view larger image.
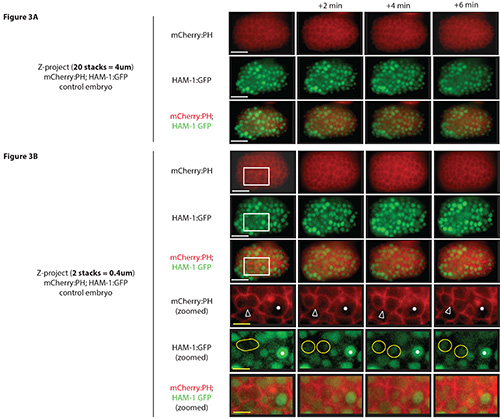
Figure 3. Visualizing dividing neuroblast during C. elegans embryogenesis. (A) Z-stack projections (20 Z-stacks of 0.2 μm in thickness) are shown from a control embryo coexpressing HAM-1:GFP (to visualize neuroblast nuclei; green) and mCherry:PH (to visualize cell membranes; red). Many neuroblasts and other cell types are visible in the projections. (B) Z-stack projections using a smaller number of Z planes are shown from another control embryo coexpressing HAM-1:GFP (green) and mCherry:PH (red). An area is zoomed in (white box) to more clearly show an individual dividing neuroblast. White arrow heads point to the ingressing plasma membrane of a dividing cell and green circles highlight the condensed DNA during mitosis and newly forming daughter nuclei towards the end of mitosis. The large cell serves as a reference point (white asterisk) to determine developmental stage and location within the embryo. Scale bars: 10 μm (white) and 3.5 μm (yellow). Click here to view larger image.
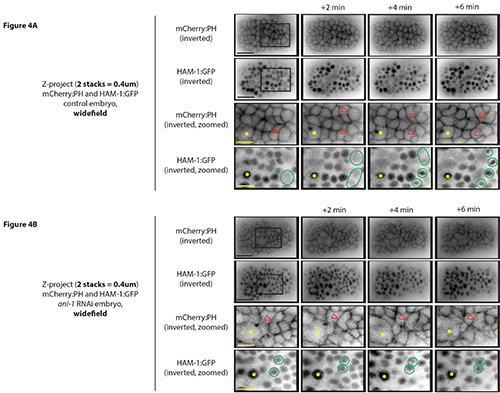
Figure 4. Visualizing neuroblast cytokinesis during C. elegans embryogenesis using a widefield system. A) Inverted Z-stack projections (2 Z-stacks of 0.2 μm in thickness) are shown from a control embryo coexpressing HAM-1:GFP and mCherry:PH collected from the widefield system using a CCD camera with high-resolution. Red arrowheads point to dividing neuroblasts with ingressing cell membranes. Green circles highlight condensed DNA during early phases of mitosis or newly forming daughter nuclei towards the end of mitosis/cytokinesis. The large cell serves as a reference point (yellow asterisk) to determine developmental stage. B) Images shown are similar to A), but are from an embryo treated with ani-1 RNAi. The cell membrane ingresses in as the cell proceeds through mitosis, but relaxes shortly after, leaving a multinucleate cell. Scale bar: 10 μm (black), 3.5 μm (yellow). Click here to view larger image.
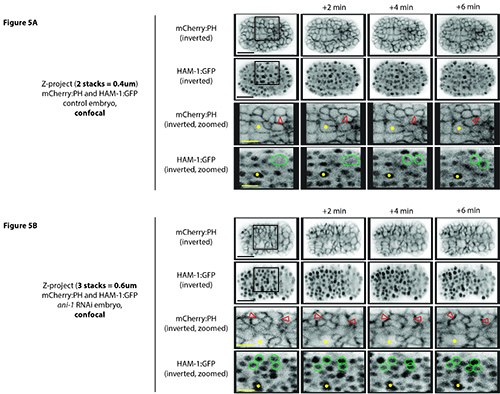
Figure 5. Visualizing neuroblast cytokinesis during C. elegans embryogenesis using a swept field system. A) Inverted Z-stack projections of 2 Z-stacks of 0.2 μm (total 0.4 μm) are shown from an embryo coexpressing HAM-1:GFP and mCherry:PH collected from the swept field system using an EMCCD camera with high sensitivity, but lower resolution. The red arrowhead points to a neuroblast undergoing cytokinesis. Green circles highlight condensed DNA during mitosis and the newly forming daughter nuclei after cytokinesis. The large cell serves as a reference point (yellow asterisk) to determine developmental stage. B) Images shown are similar to A), but are from an embryo treated with ani-1 RNAi, and 3 Z-stacks are used (total 0.6 μm). As described above, the membrane ingresses in as the cell proceeds through mitosis, but then relaxes causing the cell to become multinucleate. Scale bar: 10 μm (black), 3.5 μm (yellow). Click here to view larger image.
Video 1. Visualizing dividing neuroblasts during C. elegans embryogenesis. Z-stack projections of two Z planes (0.4 μm/plane) from a control embryo coexpressing HAM-1:GFP (green) and mCherry:PH (red). Images collected from the widefield microscope are shown for both channels. The video corresponds to the embryo shown in the third panel of Figure 3B. Click here to watch video.
Video 2. Visualizing dividing neuroblasts during C. elegans embryogenesis. Z-stack projections of two Z planes (0.4 μm/plane) from a control embryo coexpressing HAM-1:GFP (green) and mCherry:PH (red). The video corresponds to inverted images collected from the widefield microscope for the mCherry:PH channel only (embryo in Figure 4A). Click here to watch video.
Video 3. Visualizing dividing neuroblasts during C. elegans embryogenesis. Z-stack projections of two Z planes (0.4 μm/plane) from an ani-1 RNAi treated embryo coexpressing HAM-1:GFP (green) and mCherry:PH (red). The video corresponds to inverted images collected from the widefield microscope for the mCherry:PH channel only (embryo in Figure 4B). Click here to watch video.
Video 4. Visualizing dividing neuroblast during C. elegans embryogenesis. Z-stack projections of two Z planes (0.4 μm/plane) from a control embryo coexpressing HAM-1:GFP (green) and mCherry:PH (red). The video corresponds to inverted images collected from the swept field confocal microscope for the mCherry:PH channel only (embryo in Figure 5A). Click here to watch video.
Video 5. Visualizing dividing neuroblast during C. elegans embryogenesis. Z-stack projections of three Z planes (0.4 μm/plane) from an ani-1 RNAi treated embryo coexpressing HAM-1:GFP (green) and mCherry:PH (red). The video corresponds to inverted images collected from the swept field confocal microscope for the mCherry:PH channel only (embryo in Figure 5B). Click here to watch video.
Discussion
This protocol describes the use of various types of microscopy to image cell divisions during mid embryogenesis. In particular, this protocol highlights how to image the division of neuroblasts, cells that may facilitate epidermal morphogenesis. Cell-cell communication is important for tissue formation during metazoan development and C. elegans is an excellent model to study tissue formation in vivo. One event that nicely portrays the interplay of tissues is epidermal morphogenesis, which covers the embryo in a layer of epithelial cells. In particular, ventral enclosure is the process where the ventral epidermal cells migrate to enclose the ventral surface of the embryo, and relies on the underlying neuroblasts. The neuroblasts provide chemical or mechanical cues, and ventral enclosure is compromised if the position of neuroblasts is altered. The number (or polarity) of neuroblasts also may be essential for ventral enclosure to occur properly, and it is important to understand the mechanisms that regulate their division. This protocol describes how to image cell division within a living tissue using C. elegans embryos coexpressing two fluorescent probes (mCherry:PH to outline plasma membranes and HAM-1:GFP to label neuroblast nuclei).
The most essential steps to image living C. elegans embryos expressing fluorescent probes are: i) use healthy, young adult hermaphrodites expressing the desired fluorescent markers (maintained at 20-25 °C), ii) use mutants or RNAi to study gene requirements during tissue formation, iii) collect embryos at the right stage for microscopy (e.g. mid embryogenesis), and iv) perform fluorescent microscopy using settings optimized to keep phototoxicity low, but to maintain high image quality.
To identify specific cells and/or intracellular compartments within a tissue of interest, use worms expressing markers tagged with fluorescent probes. The Caenorhabditis Genetics Center (CGC; http://www.cbs.umn.edu/cgc) offers a variety of strains that coexpress multiple markers. However, two strains (each expressing a single marker of interest) may have to be crossed and screened with a fluorescent microscope over several generations to generate a strain stably expressing both probes. For example, to carry out this experimental protocol, a strain expressing a marker to visualize cell membranes (mCherry:PH; OP70) was crossed with a strain expressing a marker to visualize neuroblast nuclei (HAM-1:GFP; OP102) to generate a strain coexpressing both.
The best way to study gene requirements during embryonic development is to perform loss of function experiments, where the gene product is knocked down or mutated. This experimental protocol describes performing RNAi against anillin (ani-1) to reduce endogenous ANI-1 levels in all embryonic tissues. There are no well characterized hypomorphic alleles of ani-1. Therefore, RNAi is the best option to examine ani-1’s loss of function phenotypes and determine its function during embryonic development. Typically, it is better to use mutants instead of RNAi, as the efficiency of knockdown can be variable and is rarely null. One of the most common problems encountered with feeding RNAi protocols is contamination, which will reduce RNAi efficiency. To decrease the risk of contamination, prepare the RNAi plates and food in aseptic conditions. Contamination can be detected by screening a few of the plates before use, and plates with milky/opaque looking bacteria should be discarded. Another problem that can reduce RNAi efficiency is by not choosing hermaphrodites at the right developmental stage. It is best to use L4-staged or young adult worms (the developing vulva appears as a whitish circle/hole on the ventral side of the worm), which have no/few fertilized embryos. Furthermore, the RNAi conditions and treatments may vary for different gene products. Different ways to increase or decrease the strength of the RNAi is by resuspending the pelleted HT115 bacteria in different amounts of LB Amp media (see Section 1.2.2), and by altering the duration of RNAi treatment (length of time that worms are kept on RNAi plates; see Protocol 3). To yield optimal ani-1 RNAi phenotypes for this protocol, bacteria were resuspended in 500 μl of LB Amp media and worms were kept on RNAi plates for 2 days (ani-1 RNAi was previously characterized by Maddox et al.23). RNAi resistant or sensitive strains (e.g. rde-1, sid-1 or rrf-3) can also be used to alter the strength of RNAi. More importantly, these strains can be coupled with the tissue specific expression of their wild type genes to create tissue sensitive strains. For example, to perform neuroblast specific RNAi, one could use sid-1 mutant embryos (RNAi-resistant) expressing wt sid-1 under the unc-119 promoter (strain ID at CGC TU3401)31.
This protocol describes how to image neuroblast cell division during mid embryogenesis. There are several aspects of the protocol that require careful consideration, such as the developmental stage of the embryos, collecting the appropriate Z-stacks and optimizing the imaging conditions to minimize phototoxicity without compromising image quality. To capture time points at the right developmental stage (e.g. ventral enclosure), embryos should be filmed from dorsal intercalation (pairs of dorsal epidermal cells interdigitate and fuse to form a single layer of epidermis on the dorsal side of the embryo; Figure 1A6). At this time, the neuroblasts are rapidly dividing. Since staging the embryos can be difficult under low magnification (e.g. using a dissecting microscope), it helps to collect many embryos of varying stages and embryos at the right stage can be selected using higher magnification.
Neuroblasts are relatively small and thin, and are found throughout the middle of the embryo. It is essential to optimize the number and size of Z-stacks to ensure that the entire cell is captured during division. For example, collecting too many images will expose embryos to more light and make them prone to phototoxicity. However, collecting too few Z-stacks or using thick Z sections could make it difficult to properly image an entire dividing neuroblast cell.
This protocol describes the use of a swept field confocal microscope or widefield system to image neuroblast cell division. As described earlier, one of the main limitations of using a widefield epifluorescent microscope is the potential for phototoxicity. While it is highly recommended to use a spinning disk confocal or swept field confocal for imaging C. elegans embryos, some labs may have limited access to these systems. This protocol gives some suggestions to limit phototoxicity when using widefield systems, such as closing the aperture to 30%, lowering the intensity of light from the mercury bulb (or preferably using LEDs), using an EMCCD camera, and increasing the gain or binning to permit a decrease in exposure time. The number of Z-stacks and time points also will be limited using a widefield system. Although it was not described in this protocol, the spinning disk confocal microscope is commonly used for live imaging as it causes lower phototoxicity in comparison to a widefield microscope. Similar to the swept field system, it uses solid state lasers and scatters light (although in a different manner vs. the swept field). Either CCD or EMCCD cameras can be used with spinning disk system, which often has multiple camera ports to permit dual imaging. Similar to the swept field microscope, images can be captured with exposure times 50-90% lower vs. the widefield system.
This protocol can be used to study the function of various genes that regulate mitosis during tissue formation. The time between each image could be shortened (e.g. every 15-30 sec vs. 2-3 min) to better visualize mitotic phenotypes. Different probes could be used (e.g. instead of using HAM-1:GFP, fluorescent-tagged H2B could be used to visualize DNA, and/or fluorescent tagged tubulin could be used to visualize mitotic spindles). The number of Z-stacks and the thickness for each stack could also be altered (e.g. more stacks of a smaller step size). As described above, choosing the appropriate settings is crucial for limiting phototoxicity and optimizing image quality. Even though the mCherry:PH and HAM-1:GFP probes express at relatively high levels, collecting faster time points, increasing the number of Z-stacks or imaging for longer periods of time vs. what is described in this protocol may not be possible on a widefield system.
A Java-based image processing program (ImageJ, NIH) can be used to process images collected by the different microscopes and images can be exported as TIFFs. However, depending on the software used for acquisition, the files could already be compatible with the processing software. It is better to open original files vs. exported images (e.g. TIFFs), since the metadata is kept with the original files. Processing software can be used to manipulate images for making figures or movies. In addition, quantitative analyses also can be performed, such as measuring pixel intensities in selected regions. Optimal software should be selected depending on the analysis, such as Image Processing Toolbox (MathWorks, Matlab) or 3D and 4D Real-Time Interactive Data Visualization (Imaris, Bitplane).
Using this protocol, ani-1 was shown to be required for neuroblast cytokinesis, even though it is not required for cytokinesis in the early embryo. Interestingly, by controlling the number and position of neuroblasts, ani-1 may regulate the migration of ventral epidermal cells for ventral enclosure, because the neuroblasts provide chemical or mechanical cues to the overlying epithelial cells. However, it is not known if ani-1 is required for the division or shape change of other cell types during mid late embryogenesis. Showing how the overall composition of a tissue affects neighboring tissues emphasizes the importance of tissue communication during metazoan development.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge that this work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) grant.
Materials
| Agar | BioShop Canada Inc. | #AGR001.1 | For making C. elegans NGM and RNAi plates |
| Agar | Bio Basic Inc. | #9002-18-0 | For making bacteria LB agar plates |
| Agarose | BioShop Canada Inc. | #AGA001.500 | |
| Anti-mouse Alexa 488 antibody | Life Technologies Corporation (Invitrogen) | #A11029 | |
| Anti-mouse anti-GFP antibody | Roche Applied Science | #11814460001 | |
| Anti-rabbit Alexa 568 antibody | Life Technologies Corporation (Invitrogen) | #A11011 | |
| Ampicillin | BioShop Canada Inc. | #AMP201.5 | Store powder at 4 °C and dissolved ampicillin at -20 °C |
| Bactopetone (peptone-A) | Bio Basic Inc. | #G213 | |
| CaCl2 (calcium chloride) | BioShop Canada Inc. | #C302.1 | |
| Cholesterol | BioShop Canada Inc. | #CHL380.25 | Dissolve in ethanol |
| DAPI | Sigma-Aldrich | #D9542 | Use to stain nucleic acids (DNA) |
| Glycerol | BioShop Canada Inc. | #GLY001.1 | |
| IPTG (isopropylthio-β-galactoside) | Bio Basic Inc. | #367-93-1 | Store powder and dissolved IPTG at -20 °C |
| KH2PO4 (potassium phosphate, monobasic) | BioShop Canada Inc. | #PPM666.1 | |
| K2HPO4 (potassium phosphate, dibasic) | BioShop Canada Inc. | #PPD303.1 | |
| L4440 (feeding vector) | Addgene | #1654 | Keep as glycerol stock at -80 °C |
| MgSO4 (magnesium sulfate) | BioShop Canada Inc. | #MAG511.500 | |
| NaCl (sodium chloride) | Bio Basic Inc. | #7647-14-5 | |
| Na2HPO4 (sodium phosphate, dibasic) | Bio Basic Inc. | #7558-79-4 | |
| Normal Donkey Serum (NDS) | Wisent Bioproducts | #035-110 | |
| n-propyl 3.4.5-trihydroxybenzoate (propyl gallate) | Alfa Aesar | #A10877 | |
| Poly-L-lysine | Sigma-Aldrich | #P8920 | For optimal results coat microscope slides three times |
| Streptomycin | BioShop Canada Inc. | #STP101.50 | Store powder at 4 °C and dissolved streptomycin at -20 °C |
| Tetracyclin | BioShop Canada Inc. | #TET701.10 | Store powder at 4 °C and dissolved tetracycline at -20 °C |
| Tween-20 | Bio Basic Inc. | CAS#9005-64-5 | |
| Tryptone | BioShop Canada Inc. | #TRP402.500 | |
| Yeast Extract | Bio Basic Inc. | #8013-01-2 |
References
- Brenner, S. The genetics of Caenorhabditis elegans. 遗传学. 77, 71-94 (1974).
- Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E., Mello, C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391, 806-811 (1998).
- . C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 282, 2012-2018 (1998).
- Pennisi, E. Worming secrets from the C. elegans genome. Science. 282, 1972-1974 (1998).
- Tabara, H., et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 99, 123-132 (1999).
- Chisholm, A. D., Hardin, J. . Epidermal morphogenesis. WormBook. The C. elegans Research Community. , 1-22 (2005).
- Zhang, H., Gally, C., Labouesse, M. Tissue morphogenesis: how multiple cells cooperate to generate a tissue. Curr. Opin. Cell Biol. 22, 575-582 (2010).
- Kiehart, D. P. Wound healing: the power of the purse string. Curr. Biol. 9, 602-605 (1999).
- Ikegami, R., et al. Semaphorin and Eph receptor signaling guide a series of cell movements for ventral enclosure in C. elegans. Curr. Biol. 22, 1-11 (2012).
- Chin-Sang, I. D., George, S. E., Ding, M., Moseley, S. L., Lynch, A. S., Chisholm, A. D. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. C. elegans. Cell. 99, 781-790 (1999).
- Wang, X., Roy, P. J., Holland, S. J., Zhang, L. W., Culotti, J. G., Pawson, T. Multiple Ephrins Control Cell Organization in C. elegans Using Kinase-Dependent and -Independent Functions of the VAB-1 Eph. Mol. Cell. 4, 903-913 (1999).
- Chin-Sang, I. D., Moseley, S. L., Ding, M., Harrington, R. J., George, S. E., Chisholm, A. D. The divergent C. elegans ephrin EFN-4 functions in embryonic morphogenesis in a pathway independent of the VAB-1 Eph receptor. Development. 129, 5499-5510 (2002).
- Ghenea, S., Boudreau, J. R., Lague, N. P., Chin-Sang, I. D. The VAB-1 Eph receptor tyrosine kinase and SAX-3/Robo neuronal receptors function together during C. elegans embryonic morphogenesis. Development. 132, 3679-3690 (2005).
- Bernadskaya, Y. Y., Wallace, A., Nguyen, J., Mohler, W. A., Soto, M. C. UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph polarize F-actin during embryonic morphogenesis by regulating the WAVE/SCAR actin nucleation complex. PLoS Genet. 8, (2012).
- Piekny, A. J., Werner, M., Glotzer, M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 15, 651-658 (2005).
- Piekny, A. J., Maddox, A. S. The myriad roles of Anillin during cytokinesis. Sem. Cell Dev. Biol. 21, 881-891 (2010).
- Zhao, W. M., Fang, G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J. Biol. Chem. 280, 33516-33524 (2005).
- Piekny, A. J., Glotzer, M. Anillin is a scaffold protein that links RhoA, actin and myosin during cytokinesis. Curr. Biol. 18, 30-36 (2008).
- Hickson, G. R., O’Farrell, P. H. Rho-dependent control of anillin behavior during cytokinesis. J. Cell Biol. 180, 285-294 (2008).
- Fraser, A. G., Kamath, R. S., Zipperlen, P., Martinez-Campos, M., Sohrmann, M., Ahringer, J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 408, 325-330 (2000).
- Kamath, R. S., et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 421, 231-237 (2003).
- Duerr, J. S. . Immunohistochemistry. WormBook, The C. elegans Research Community. , 1-61 (2006).
- Maddox, A. S., Habermann, B., Desai, A., Oegema, K. Distinct roles for two C. elegans anillins in the gonad and early embryo. Development. 132, 2837-2848 (2005).
- Sulston, J. E., Schierenberg, E., White, J. G., Thomson, J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119 (1983).
- Chin-Sang, I. D., Chisholm, A. D. Form of the worm: genetics of epidermal morphogenesis in C. elegans. Trends Genet. 16, 544-551 (2000).
- Zhang, H., Labouesse, M. Signaling through mechanical inputs – a coordinated process. J. Cell Sci. 125, 3039-3049 (2012).
- Guenther, C., Garriga, G. Asymmetric distribution of the C. elegans HAM-1 protein in neuroblasts enables daughter cells to adopt distinct fates. Development. 122, 3509-3518 (1996).
- Audhya, A., et al. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J. Cell Biol. 171, 267-279 (2005).
- Maddox, A. S., Lewellyn , L., Desai, A., Oegema, K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell. 12, 827-835 (2007).
- Dorn, J. F., et al. Actomyosin tube formation in polar body cytokinesis requires Anillin in C. elegans. Curr. Biol. 20, 2046-2051 (2010).
- Calixto, A., Ma, C., Chalfie, M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods. 7, 554-559 (2010).

