Environmental Modulations of the Number of Midbrain Dopamine Neurons in Adult Mice
Summary
This protocol describes two different environmental manipulations and a concurrent brain infusion protocol to study environmentally-induced brain changes underlying adaptive behavior and brain repair in adult mice.
Abstract
Long-lasting changes in the brain or ‘brain plasticity’ underlie adaptive behavior and brain repair following disease or injury. Furthermore, interactions with our environment can induce brain plasticity. Increasingly, research is trying to identify which environments stimulate brain plasticity beneficial for treating brain and behavioral disorders. Two environmental manipulations are described which increase or decrease the number of tyrosine hydroxylase immunopositive (TH+, the rate-limiting enzyme in dopamine (DA) synthesis) neurons in the adult mouse midbrain. The first comprises pairing male and female mice together continuously for 1 week, which increases midbrain TH+ neurons by approximately 12% in males, but decreases midbrain TH+ neurons by approximately 12% in females. The second comprises housing mice continuously for 2 weeks in ‘enriched environments’ (EE) containing running wheels, toys, ropes, nesting material, etc., which increases midbrain TH+ neurons by approximately 14% in males. Additionally, a protocol is described for concurrently infusing drugs directly into the midbrain during these environmental manipulations to help identify mechanisms underlying environmentally-induced brain plasticity. For example, EE-induction of more midbrain TH+ neurons is abolished by concurrent blockade of synaptic input onto midbrain neurons. Together, these data indicate that information about the environment is relayed via synaptic input to midbrain neurons to switch on or off expression of ‘DA’ genes. Thus, appropriate environmental stimulation, or drug targeting of the underlying mechanisms, might be helpful for treating brain and behavioral disorders associated with imbalances in midbrain DA (e.g. Parkinson’s disease, attention deficit and hyperactivity disorder, schizophrenia, and drug addiction).
Introduction
DArgic signaling by neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) of the midbrain is thought to be important for reward-motivated cognitive, emotive and motor behaviors. However, too much or too little midbrain DA signaling causes many disabling symptoms in a variety of neurological disorders (e.g. Parkinson’s disease, attention deficit and hyperactivity disorder, schizophrenia, and drug addiction). Drugs that increase or decrease DA signaling alleviate these symptoms, however they also produce side-effects attributable to dysregulated signaling and off-target effects. Drug efficacy also declines over time due to compensatory responses of the brain. The challenge therefore is to restore normal midbrain DA signaling in a more targeted and physiological way, and a favored approach is by increasing or decreasing the number of midbrain DA neurons.
Evidence has been accumulating for several decades that the expression of genes and proteins involved in metabolizing and trafficking DA and other catecholamines in mature adult cells is modifiable (reviewed in1). In midbrain, the number of tyrosine hydroxylase immunopositive (TH+, the rate-limiting enzyme in DA synthesis) neurons decreases then increases following neurotoxin administration2,3, while the number of TH immunonegative (TH-) neurons shows the opposite pattern (i.e. increases then decreases3). This is consistent with loss then gain of the ‘DA phenotype’ by some cells. The number of TH+ and TH- SNc neurons has also been shown to change in equal but opposite directions following various treatments that alter the electrical activity of these cells4,5. For example, infusion of the small-conductance, calcium-activated potassium (SK) channel antagonist apamin into midbrain for 2 weeks decreases the number of TH+ and increases (by the same amount) the number of TH- SNc neurons4,5. In contrast, infusion of the SK channel agonist 1-EBIO increases the number of TH+ and decreases (by the same amount) the number of TH- SNc neurons4,5. Similar changes were seen following a variety of treatments targeting SNc neuronal activity, including some which targeted afferent inputs4. This apparent regulation of the number of SNc DArgic neurons by neuronal activity and afferent input raises the possibility that the environment or behavior can influence the number of SNc neurons. Indeed adult mice exposed to different environments have more or less midbrain (SNc and VTA) TH+ neurons, and at least some of these environment-induced changes are abolished by concurrent blockade of synaptic input in midbrain6. The aims of this communication are to: (1) provide further details about how to implement our environmental manipulations and drug infusions; and (2) provide further data supporting our contention that the environment regulates the number of midbrain DA neurons, via afferent input.
Protocol
NOTE: All experimental procedures on animals were approved by the Florey Institute of Neuroscience & Mental Health Animal Ethics Committee and conform to Australia’s National Health and Medical Research Council published code of practice for the care and use of animals for scientific purposes (7th edition, 2004).
1. Environmental Manipulations
- Gender Pairing
- Use sexually mature (>8 week old), age-matched male and female mice.
NOTE: We typically use C57BL/6 mice, but have had the same outcomes using Swiss mice in this protocol. We typically use n = 8 males and n = 8 females for each experiment, divided into n = 2 male-male pairs (n = 4 males), n = 2 female-female pairs (n = 4 females), and n = 4 male-female pairs (n = 4 males and n = 4 females). - Upon arrival into the animal holding facility, group-house the mice by gender for >3 days to acclimatize. Here and throughout the gender pairing, keep extraneous environmental stimuli (e.g. ambient temperature, light:dark cycle, food, water, handling and cleaning) constant or distribute equally to all mice.
- Randomly assign each mouse to a male-male pair, a female-female pair, or a male-female pair. Place each pair into a clean cage in isolation (2 mice/cage) with ad libitum access to food and water, and simply house them in this way continuously for 7 days.
NOTE: Routine animal husbandry is OK during this period but must be distributed equally to all mice. Take care to avoid pairing of male and female littermates.
- Use sexually mature (>8 week old), age-matched male and female mice.
- Environmental Enrichment
- Use sexually mature (>8 week old), age-matched male or female mice.
NOTE: We typically use n = 18 mice for each experiment, divided into n = 6/treatment group. - Upon arrival into the animal holding facility keep them group-housed in identical standard housed (SH) (e.g. see below) conditions at this stage for >3 days to acclimatize. Here and throughout the experiment, keep extraneous environmental stimuli (e.g. ambient temperature, light:dark cycle, food, water, handling and cleaning) constant or distribute equally to all mice.
- To begin environmental enrichment, randomly assign each mouse to one of 3 groups: SH; running wheel (RW), or environment enriched (EE) and place each group of mice together into an identical clean cage with ad libitum access to food and water.
NOTE: Here, larger rat-holding cages for all groups, measuring 27 cm wide, 42 cm long, and 16 cm deep were used.- In addition, provide the following environmental conditions to each group: SH comprising only litter (paper pellets or sawdust) on the floor; RW comprising SH plus 2 running wheels; EE comprising RW plus toys (ropes, ladders, tunnels, and objects such as empty paper towel rolls and pieces of tissue paper) with which to explore, play, climb, hide, and nest.
- House them in this way continuously for 14 days.
NOTE: Routine animal husbandry is OK during this period but must be distributed equally to all mice.
- Subject EE mice to additional ‘super-enrichment’ (SE) by placing them together into a larger cage containing novel toys for 1hour/day (same hour each day), 5days/week.
NOTE: Here, a 46 cm wide, 69 cm long, and 40 cm deep plastic tub was used.- Maintain novelty by presenting a different set of toys each session. Clean toys that are to be re-presented to mice with soapy water and 80% ethanol to remove scents.
- Following each SE session, return the mice to their EE cage. Handle SH and RW mice the same as SE mice (except for SE itself) throughout this period (e.g. remove and return each SH and RW mouse from and to its cage).
- Use sexually mature (>8 week old), age-matched male or female mice.
2. Osmotic Pump and Brain Infusion Cannula Implants for Drug Infusion
- Preparation
- Use sterile osmotic pumps and brain infusion kits, which are available commercially. The day before implantation, using aseptic technique, prime the implants by filling each pump, connecting tube and cannula with sterile drug or vehicle solution (as described in the instructions supplied with the pumps). Connect them together and incubate in sterile saline overnight at 37 °C. Incubate drug- and vehicle-filled implants separately to avoid cross-contamination.
- Surgery.
NOTE: Depending on the country of the experimenter, special animal experimentation permits or allowances are required to pursue such types of surgery and post-operative experiments.- Sterilize all surgical equipment and employ aseptic technique throughout. Anesthetize a mouse (e.g. using 1-2% isofluorane in air) and place it in a stereotaxic headframe. Check the depth of anesthesia throughout the procedure and administer further anesthetic as necessary (e.g. absence of limb withdrawal to noxious paw pinch is indicative of adequate anesthesia). Ensure the eyes are protected from desiccation by lubricant eye ointment, and that the mouse’s body temperature remains normal throughout the procedure.
- Make a midline incision through the skin starting from between the eyes and finishing 2 cm posterior to the back edge of the skull. Blunt dissect the skin away from the underlying fascia down the back of the mouse to create a subcutaneous ‘pocket’ large enough to comfortably fit the pump and connecting tube once the cannula is implanted (the connecting tube should not be bent upon completion). Scrape clear the fascia from over the dorsal aspect of the skull.
- Using an approximately 1.5 mm diameter dental burr, drill down into the skull at the appropriate stereotaxic coordinates until a thin, flexible layer of bone remains. Peel this layer away using fine forceps (this avoids damaging the underlying dura mater and brain with the dental burr). Ensure the skull is cleaned of any blood and bone fragments, and that there is no further bleeding.
- With the cannula held by a cannula holder, place the pump and connecting tube into the subcutaneous ‘pocket’ overlying the mouse’s back. Next, position the tip of the cannula at the appropriate stereotaxic coordinates and on the surface of the brain. Carefully lower the cannula into the brain but stop approximately 1 mm short of the required depth.
NOTE: The remaining depth will be negotiated following application of the first layer of dental acrylic. - Ensure again the surface of the skull is clean and dry, then prepare a small volume of dental acrylic and use a toothpick to smooth it over the entire exposed area of skull, including into the burr-hole through the skull around the cannula. Before the acrylic hardens, lower the cannula tip the remaining depth into the target.
- Prepare another small volume of dental acrylic and layer this over the first, as well as over the white plastic support for the cannula, to fix the cannula in place. Repeat with additional layers of acrylic as necessary.
- Once the acrylic has hardened, carefully remove the cannula holder and cut off the white plastic removable cannula tab using a scalpel blade heated with a butane flame. Lastly, suture the skin closed over the entire implant.
- Post-surgical Treatment of Animals
- Apply antiseptic ointment to the skin margins, and administer an anti-inflammatory to the mouse (e.g. Meloxicam, 3 mg/kg s.c.). Remove the mouse from the frame, place it under a heat lamp, and observe until it regains consciousness. Do not return a mouse that has undergone surgery to the company of other animals until fully recovered.
NOTE: Experimental manipulations such as gender pairing and environment enrichment can be initiated or resumed the day following surgery at the earliest. - Monitor their body weight, general behavior and appearance daily. Treat signs of infection (e.g. swelling, redness, pus) around surgical incisions with topical antiseptic ointment. Treat any signs sickness, pain, stress or discomfort (e.g. loss of >10% body weight, social withdrawal, lack of grooming, “fluffing”, movement dysfunction, seizures) with systemic analgesics and/or antibiotics as necessary.
- Euthanize mice if >15% weight loss or symptoms of sickness, pain, stress or discomfort are non-recoverable within 1 week of remedial treatment.
- Apply antiseptic ointment to the skin margins, and administer an anti-inflammatory to the mouse (e.g. Meloxicam, 3 mg/kg s.c.). Remove the mouse from the frame, place it under a heat lamp, and observe until it regains consciousness. Do not return a mouse that has undergone surgery to the company of other animals until fully recovered.
3. Brain Tissue Preparation, Immunohistochemical Processing, and Stereology
- Perfusion
- Immediately following environmental/drug manipulations, prepare the brain for study.
NOTE: In the experiments reported here the number of SNc TH+ neurons were measured and compared in each of the different treatment groups. - First administer an overdose of anesthetic to kill the mice (e.g. 100 mg/kg i.p. sodium pentobarbitone). Once anesthetized but before the heart stops beating, lay the mouse on its back and tie or pin down both forelimbs.
- Using a scalpel cut away the skin overlying the thorax then incise through the muscle just below the rib cage into the abdominal cavity. Place a clamp on the xiphoid process of the ribcage and lift the ribcage up and away from the liver exposing the diaphragm.
- Cut through the diaphragm and through the ribs laterally on both sides using large scissors until the ribcage can be folded back over the head to expose the lungs and heart. Using fine scissors make a small incision in the right atrium as an exit point for blood and perfused solutions, then cut through the base of the left ventricle and place a cannula up through the left ventricle, the left atrium, and into the aorta.
- Clamp the cannula in place then pump warm (37 °C) heparinized and 0.1 M phosphate buffered physiological saline (PBS) through the vasculature until the solution exiting the right atrium is entirely free of blood. Next, pump cold (4 °C) fixative solution (such as 4% paraformaldehyde in PBS) through the vasculature until the entire mouse is well fixed. Remove the brain and place in PBS with 30% sucrose for 2-3 days until the brain sinks.
NOTE: Other types of tissue preparation might be applied depending on the expertise in the corresponding laboratory.
- Immediately following environmental/drug manipulations, prepare the brain for study.
- Preparation of Free-floating Cryosections
- Cut serial sections through the brain regions of interest and collect these in PBS.
NOTE: We cut 40 µm thick sections using a cryostat. Other types of tissue sectioning might be applied depending on the expertise in the corresponding laboratory.
- Cut serial sections through the brain regions of interest and collect these in PBS.
- Immunohistochemistry
- Perform standard immunohistochemistry for proteins of interest. Incubate sections in 5% normal goat serum and 0.3% triton X-100 in PBS at room temperature for 30 min, then immunoreact with polyclonal rabbit anti-TH (1:400) at 4 °C for 48 hr, then polyclonal biotinylated goat anti-rabbit (1:1,000) at room temperature for 2 hr.
- Next, incubate in avidin-peroxidase (1:500) at room temperature for 1hr, then in cobalt- and nickel-intensified diamino-benzidine (0.5 mg/ml) at room temperature for 18-20 min; for the last 3-5 min of the diamino-benzidine incubation add hydrogen peroxide (0.01%) to catalyze chromagen precipitation. Wash sections three times for 10min each in PBS before, after, and between each of the above steps.
- Mount the sections on gelatinized microscope slides, air dry them, then Nissl stain (neutral red), dehydrate in alcohol, clear (X-3B), and coverslip.
- Stereology
- Estimate the total number of TH+ and TH- SNc (and VTA and LC) neurons using unbiased stereological methods. Ensure that the stereologist is blind to the treatment received. Exclude glia on the basis of soma diameter <5 µm and count only those cells with a visible nucleus.
- Identify the SNc (and VTA and LC) by the spatial locations of TH+ cells and anatomical landmarks/boundaries according to the brain atlas of Paxinos and Watson7. Count TH+ cells within a counting frame (55 x 55 µm = 3025 µm2) at regular pre-determined intervals (x = 140 µm, y = 140 µm for SNc; x = 100 µm, y = 100 µm for VTA and LC) throughout each nucleus in every fourth section.
Representative Results
Adult mice subjected to these environmental manipulations have altered numbers of midbrain (SNc and VTA), but not LC, TH+ neurons, and EE plus concurrent midbrain infusion of either picrotoxin or bicuculline (GABAA receptor antagonists) abolishes EE-induction of more SNc TH+ neurons. These data were previously published in6. The present data were compiled in replicate experiments performed as part of that previous study, but have not been published elsewhere.
Specifically, adult male mice that have been paired with adult female mice have more TH+ SNc and VTA (midbrain) neurons than males paired with males, whereas females paired with males have fewer TH+ SNc and VTA neurons than females paired with females (Figure 1 in6). Another example of this is provided here in Figure 1, which shows the mean ± SE number of TH+ SNc neurons in male and female mice immediately following gender pairing. Males paired with females for 7 days have approximately 12% more TH+ SNc neurons than males paired with males (two blue columns in Figure 1). By contrast, females paired with males have approximately 12% fewer TH+ SNc neurons than females paired with females (two red columns in Figure 1).
The number of TH+ SNc and VTA neurons also increases in adult male mice subject to EE (Figure 2 in6), and EE-induction of more SNc TH+ neurons is abolished by concurrent blockade of midbrain GABAA receptors (Figure 3 in6). Another example of this is provided here in Figure 2, where EE with vehicle infusion resulted in approximately 14% more TH+ SNc neurons than SH with vehicle infusion (left two columns in Figure 2). However, EE with GABAA receptor antagonist infusion (either 10 µM picrotoxin or 5 µM bicuculline) completely abolished this increase (right two columns in Figure 2). Note, these data are from the SNc contralateral to the infusion cannula, and are almost identical to the effects observed in the ipsilateral SNc which were reported in6.
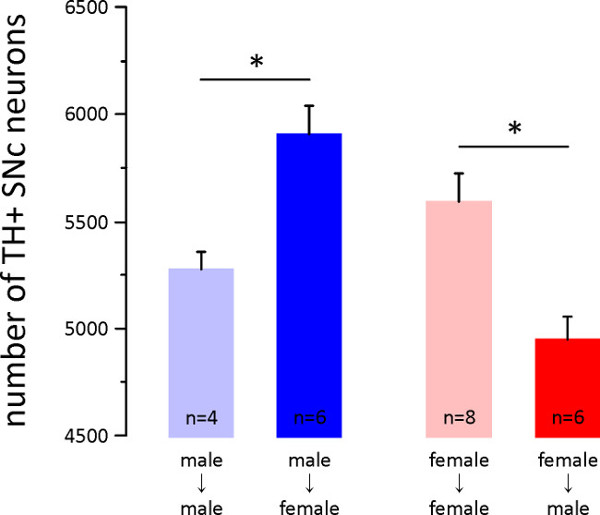
Figure 1: Effects of gender pairing on the number of SNc TH+ neurons. Immediately following 7 days of gender pairing (protocol 1.1) adult mice were perfused (3.1), their midbrain was sectioned (3.2), sections were processed for tyrosine hydroxylase (TH, the rate limiting enzyme in DA synthesis) immunohistochemistry (3.3), and the numbers of TH+ SNc neurons were estimated using stereology (3.4). Plotted here are the mean ± SE number of SNc TH+ neurons in male mice paired with male mice (n = 4 male mice from n = 2 male-male pairs, light blue column), males paired with females (n = 6 male mice from 6 male-female pairs, dark blue column), females paired with females (n = 8 female mice from n = 4 female-female pairs, light red column), and females paired with males (n = 6 female mice from n = 6 male-female pairs, dark red column). Pairing with the opposite gender resulted in an increase in number of SNc TH+ neurons in males but a decrease in number of SNc TH+ neurons in females (p <0.001 One-way ANOVA; *p <0.05 Tukey pairwise multiple comparisons).
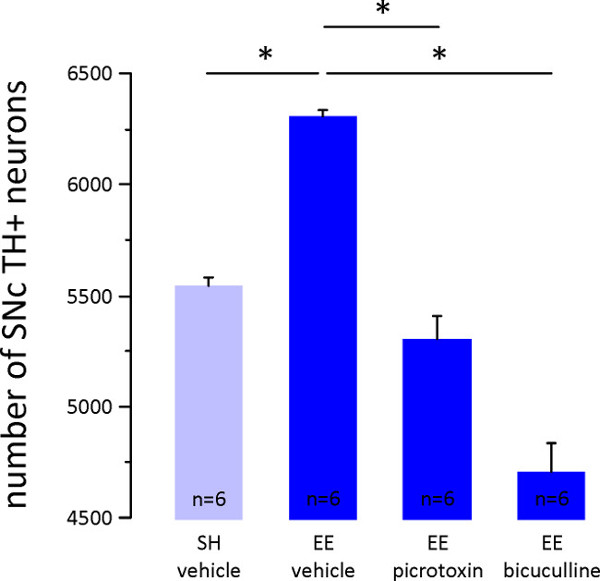
Figure 2: Effects of environment enrichment with midbrain GABAA receptor blockade on the number of SNc TH+ neurons. Immediately following 14 days of environment enrichment (protocol 1.2) with concurrent infusion of either vehicle, the GABAA receptor antagonist picrotoxin (10 µM), or the GABAA receptor antagonist bicuculline (5 µM) into the left midbrain (protocol 2), adult male mice were perfused (3.1), their midbrain was sectioned (3.2), sections were processed for tyrosine hydroxylase (TH, the rate limiting enzyme in DA synthesis) immunohistochemistry (3.3), and the numbers of TH+ neurons in the right (contralateral) SNc were estimated using stereology (3.4). Plotted here are the mean ± SE number of SNc TH+ neurons in standard housed (SH) male mice with vehicle infusion (n = 6, light blue column), environment enriched (EE) males with vehicle infusion (n = 6, first dark blue column), EE males with picrotoxin infusion (n = 6, second dark blue column), and EE males with bicuculline infusion (n = 6, third dark blue column). EE resulted in an increase in number of SNc TH+ neurons, but this was abolished by infusion of picrotoxin or bicuculline into the contralateral midbrain (p <0.001 One-way ANOVA; *p <0.001 Tukey pairwise multiple comparisons).
Discussion
Environmental manipulations
The motivation behind the design of these environmental manipulations (gender pairing and environmental enrichment) was to determine whether the environment, and/or behavior prompted by the environment, is associated with changes in the number of midbrain DA neurons. The focus was therefore on providing environments and stimulating behaviors that are likely to engage midbrain DA signaling. These included pairing with the opposite gender, and environmental enrichment comprising access to running wheels, ropes, ladders, tunnels, and objects to explore, play, climb, hide, and nest. Both of these environments ought to foster reward-motivated cognitive, emotive and motor behaviors, which have been associated with acute increases in midbrain DA neuronal discharge and DA release (e.g.8). Our hypothesis was they are also associated with longer-lasting changes in midbrain DA signaling brought about by changes in the number of midbrain DA neurons. Here and elsewhere6, evidence is presented that this is indeed the case.
Our investigations began using the gender pairing protocol (1.1) because it comprises a core primal and essential reward-motivated behavior (i.e. copulation). Also, reproductive, pair-bonding, and social behaviors are known to induce analogous changes in other neurochemical systems (e.g. changes in density of oxytocin, vasopressin and corticotrophin-releasing hormone cells in hypothalamus9). Indeed, after 7 days of continuous pairing with the opposite gender, we show here and elsewhere6 that males have approximately 10% more TH+ midbrain neurons whereas females have approximately 10% fewer TH+ midbrain neurons [Note: These effects comprise SNc (present study and6) and VTA6, but not the locus coeruleus6, where TH helps synthesize noradrenaline]. This supports the notion that the number of midbrain DA neurons in the adult brain is not fixed, but changes depending on the environment, behavior, or environment/behavior interactions. Other evidence argues these changes are brought about by changes in expression of the TH gene and protein in extant midbrain neurons, as opposed to DA neurogenesis or degeneration. (1) DA neurogenesis in the adult mouse midbrain occurs either not at all10-14, or at rates much lower than can account for the addition of approximately 500 new SNc DA neurons in a week15,16. (2) Studies quantifying changes in number of SNc neurons following 2 week infusions of various drugs targeting the electrical activity of midbrain neurons have consistently revealed equal (again approximately 10%) but opposite changes TH+ and TH- cells, resulting in no net change in the total cell number4,5. This is consistent with regulation of TH protein levels above or below immunohistochemically detectable levels without addition or subtraction of neurons. (3) Many studies have reported activity-dependent changes in TH gene and protein expression within cells over a timescale of several hours17-23, and the same has been reported in midbrain4.
The question thus arises what are the mechanisms underlying changes in expression of the TH gene and protein in extant midbrain neurons. Possibilities include sex steroids, which may underlie both the baseline differences in number of TH+ midbrain neurons in males and females, as well gender-specific changes (increases in males and decreases in females) following pairing reported here and previously6. Another possibility is activity-dependent regulation of TH expression. SNc and VTA neurons (both DA and non-DA) receive synaptic input predominantly from the striatum, hypothalamus, subthalamic nucleus, and frontal cortices24. In the context of gender pairing, these inputs could be regulated by sensorimotor behavior, olfaction, pheromones, stress, metabolism, or reproduction. Activity-dependent regulation of TH expression could be mediated via calcium or neuropeptide signaling pathways affecting gene and protein expression (e.g.1).
To help distinguish between sex steroid versus activity-dependent mechanisms underlying the effects of gender pairing on the number of midbrain DA neurons we employed the environment enrichment protocol (1.2), which better controls against the potential influence of sex steroids and perhaps also other hormones (e.g. pheromones, stress, reproduction), and for the potential influence of afferent-driven neuronal activity. The relevant factor here is the different groups were much more similar in terms of gender (all males), hormones, social status and stress. Although social status and stress may still differ between groups (e.g. a more dominant and aggressive male in one group), it is unlikely this would always be the same group (e.g. EE), and the effects of EE have now been replicated multiple times6. In EE, the magnitude of the increase in midbrain TH+ neurons was the same as in the gender pairing protocol (compare males in Figures 1 & 2 present study, and Figures 1 & 2 in6). Furthermore, the EE-induced increase in SNc TH+ neurons induced by environmental enrichment was completely abolished by concurrent blockade of midbrain GABAA receptors (Figure 2 present study and Figure 3 in6), which provide around 70% of all afferent input to midbrain DA neurons25. Together, these data indicate afferent-driven neural activity is at least as potent an influence over the number of midbrain DA neurons as hormones.
Further insight into the environmental factors regulating the number of midbrain TH+ neurons comes from comparing the effects of RW and EE. RW comprises a well-learned or banal motor activity (running on the wheels), whereas EE comprises a similar amount of such activity, albeit of greater variety (i.e. various toys but unchanged throughout the 14 days), plus ongoing exposure to novel toys in the SE component (1.2.4). Our previously published data6 revealed either no or small increases in SNc and VTA TH+ neurons in RW compared to SH mice, but much larger increases in EE (+SE) mice. This highlights sensory stimulation, cognition, novelty and/or learning and memory as more potent regulators of the number of midbrain DA neurons than banal physical activity alone.
EE has been used extensively to induce therapeutic effects and enhance brain repair in various animal models of brain disorders, such as Alzheimer’s, Huntington’s and Parkinson’s disease26. The nature of such environmental manipulations is that they vary extensively between laboratories (as do ‘standard-housed’ conditions), however there are key aspects of EE protocols that are noteworthy and have been previously discussed27. EE provides increased environmental novelty and complexity relative to standard housing, so as to enhance levels of sensory stimulation, cognitive activity and physical exercise. In designing EE experiments, one needs to consider ethological factors, including the sensory and cognitive abilities of the animals and their instinctive drives. As mice (and rats) are nocturnal, with inferior visual abilities to humans but excellent somatosensory and olfactory acuities, the enrichment objects should reflect the innate instincts and capacities of the experimental animals. Therefore, it is the novelty, shape, texture and smell of the objects that may be particularly salient to mice. Assessments of the relative strength of rodents’ motivations for a variety of enrichment objects have led to recommendations for the inclusion of chewable objects to allow the opportunity to exercise fundamental, species-typical behaviors28,29. The provision of nesting material is considered to be a fundamental component of the enrichment protocol and mice will instinctively tear any objects made of paper or cardboard, thus providing opportunity for sensory, cognitive and motor activities30,31. It is important also that in an experiment with multiple EE cages, the equivalent novelty and complexity of objects is provided, to minimize variance within the EE group, and similar control of environmental parameters should be exercised for the SH group. Ideally, the numbers of mice per cage should be the same between EE and SH groups (unless the effects of social group size are being specifically investigated), and no ‘food treats’ should be used in EE, as any dietary differences between groups could confound the interpretation of the effects of the cognitive activity and physical exercise that EE enhances12. Other key considerations are the duration of exposure to an enriched environment and the age at which enrichment commences. While differential expression of genes has been shown after only 3 hr in an enriched environment32, longer periods of exposure may be required for structural and functional changes to occur33,34. Furthermore, enrichment-induced plasticity may be enhanced during postnatal critical developmental periods35.
Osmotic pump and brain infusion cannula implants for drug infusions
The osmotic pump and cannula implants are very effective for delivering drugs directly into brain. With experience, the implant surgery takes approximately 1 hr per mouse. Dental acrylic alone is adequate to fix the cannula in place for extended periods (Note: We have gone up to 28 days in other experiments); there is no need for additional attachment hardware such as bone screws. It is important to ensure there is plenty of slack in the tube connecting the pump and cannula to allow for maximal head and neck flexion without putting too much stress on the connections. This is particularly so for longer-term infusions, where growth of connective tissue around the pump can anchor it in place thereby reducing the flexibility of the implant. Where possible, mice are also single-housed following surgery to prevent housemates damaging the implant through scratching, pulling or biting. The dose of drug is largely determined by the amount added to the pump. However, in this respect attention should also be paid to the pump flow-rate, the chemical stability of the drug at 37 °C, and the rate of diffusion of the drug from the cannula through the brain. Currently available pumps from our supplier can maintain infusion for up to 6 weeks at a flow-rate of 0.15 µl/hr; the fastest available flow-rate is 10 µl/hr but lasting for only 1 week. Longer infusion times can be achieved by performing additional minor surgeries to replace depleted pumps with full pumps on the back of the connecting tube (i.e. without disturbing the implanted cannula). The spatial extent of methylene blue (1% methylene blue, MW 320, in 0.9% NaCl) diffusion from a cannula tip implanted 0.3 mm above the left SNc midway along its mediolateral aspect was measured to extend from the lateral edge of the midbrain to just across the midline mediolaterally, and throughout the entire dorsoventral extent of the midbrain (after 2 weeks of infusion). This is much more widespread than a small nucleus like SNc, and the spread of drug may be larger (or smaller) depending on its MW, chemical stability, buffering, etc. Indeed, one can infer from the present data (Figure 2) that both picrotoxin and bicuculline reached the contralateral SNc in biologically active concentrations.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Health and Medical Research Council of Australia (NHMRC) Project grant 1022839. AJH is an Australian Research Council (ARC) FT3 Future Fellow (FT100100835). The Florey Institute of Neuroscience and Mental Health acknowledges support from the Victorian Government’s Operational Infrastructure Support Grant.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Isofluorane | Baxter Healthcare Pty Ltd, Baxter Drive, NSW 2146, Australia | AHN3640 | |
| ALZET Osmotic pump 1002 | DURECT Corporation, PO Box 530 Cupertino, CA 95015-0530 | 0004317 | |
| ALZET Brain infusion kit 1 | DURECT Corporation, PO Box 530 Cupertino, CA 95015-0530 | 0004760 | |
| ALZET cannula holder 1 | DURECT Corporation, PO Box 530 Cupertino, CA 95015-0530 | 0008860 | |
| Vertex Monomer Self-curing (dental acrylic solvent) | Vertex Dental, Postbus 10, 3700 AA ZEIST, The Netherlands | n/a | |
| Vertex Self Curing (dental acrylic powder) | Vertex Dental, Postbus 10, 3700 AA ZEIST, The Netherlands | n/a | |
| METACAM (Meloxicam) | Troy Laboratories, 98 long Street, smithfield NSW 2164 Australia | L10100 | |
| Sodium Pentobarbitone | Lethabarb, Virbac, Milperra, NSW, Australia | 571177 | |
| Normal goat serum | chemicon-temecula, CA | S26-Litre | |
| Triton X-100 | Merck Millipore Headquarters , 290 Concord road, Billerica, MA 01821 | 1.08603.1000 | |
| Polyclonal rabbit anti-tyrosine hydroxylase | Merck Millipore Headquarters , 290 Concord road, Billerica, MA 01821 | AB152 | |
| Polyclonal biotinylated goat anti-rabbit | Dako Australia Pty. Ltd., Suite 4, Level 4, 56 Berry street, North Sydney, NSW, Australia 2060 | EO432 | |
| Avidin peroxidase | Sigma-aldrich, Castle Hill, NSW 1765 AU | A3151-1mg | |
| Diamino-benzidine | Sigma-aldrich, Castle Hill, NSW 1765 AU | D-5637 | |
| Stereo Investigator | MicroBrightField Bioscience, 185 Allen Brook Lane, Suite 101, Williston, VT 05495 | n/a |
References
- Aumann, T., Horne, M. Activity-dependent regulation of the dopamine phenotype in substantia nigra neurons. Journal of neurochemistry. 121, 497-515 (2012).
- Sauer, H., Oertel, W. H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. 神经科学. 59, 401-415 (1994).
- Stanic, D., Finkelstein, D. I., Bourke, D. W., Drago, J., Horne, M. K. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. The European journal of neuroscience. 18, 1175-1188 (2003).
- Aumann, T. D., et al. Neuronal activity regulates expression of tyrosine hydroxylase in adult mouse substantia nigra pars compacta neurons. Journal of neurochemistry. 116, 646-658 (2011).
- Aumann, T. D., et al. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Experimental neurology. 213, 419-430 (2008).
- Aumann, T. D., Tomas, D., Horne, M. K. Environmental and behavioral modulation of the number of substantia nigra dopamine neurons in adult mice. Brain and behavior. 3, 617-625 (2013).
- Paxinos, G., Franklin, K. B. J. . The mouse brain in stereotaxic coordinates. , (2001).
- Schultz, W. Behavioral dopamine signals. Trends in neurosciences. 30, 203-210 (2007).
- Sun, P., Smith, A. S., Lei, K., Liu, Y., Wang, Z. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural brain research. 265, 22-31 (2014).
- Aponso, P. M., Faull, R. L., Connor, B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. 神经科学. 151, 1142-1153 (2008).
- Frielingsdorf, H., Schwarz, K., Brundin, P., Mohapel, P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 101, 10177-10182 (2004).
- Lie, D. C., et al. The adult substantia nigra contains progenitor cells with neurogenic potential. The Journal of neuroscience : the official journal of the Society for Neuroscience. 22, 6639-6649 (2002).
- Chen, Y., Ai, Y., Slevin, J. R., Maley, B. E., Gash, D. M. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Experimental neurology. 196, 87-95 (2005).
- Yoshimi, K., et al. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol. 58, 31-40 (2005).
- Shan, X., et al. Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease-like mice. Stem Cells. 24, 1280-1287 (2006).
- Zhao, M., et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 100, 7925-7930 (2003).
- Baker, H., Kawano, T., Margolis, F. L., Joh, T. H. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 3, 69-78 (1983).
- Black, I. B., Chikaraishi, D. M., Lewis, E. J. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain research. 339, 151-153 (1985).
- Zigmond, R. E., Chalazonitis, A., Joh, T. Preganglionic nerve stimulation increases the amount of tyrosine hydroxylase in the rat superior cervical ganglion. Neuroscience letters. 20, 61-65 (1980).
- Biguet, N. F., Rittenhouse, A. R., Mallet, J., Zigmond, R. E. Preganglionic nerve stimulation increases mRNA levels for tyrosine hydroxylase in the rat superior cervical ganglion. Neuroscience letters. 104, 189-194 (1989).
- Richard, F., et al. Modulation of tyrosine hydroxylase gene expression in rat brain and adrenals by exposure to cold. Journal of neuroscience research. 20, 32-37 (1988).
- Schalling, M., Stieg, P. E., Lindquist, C., Goldstein, M., Hokfelt, T. Rapid increase in enzyme and peptide mRNA in sympathetic ganglia after electrical stimulation in humans. Proc Natl Acad Sci U S A. 86, 4302-4305 (1989).
- Liaw, J. J., He, J. R., Barraclough, C. A. Temporal changes in tyrosine hydroxylase mRNA levels in A1, A2 and locus ceruleus neurons following electrical stimulation of A1 noradrenergic neurons. Brain research. Molecular brain research. 13, 171-174 (1992).
- Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A., Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 74, 858-873 (2012).
- Tepper, J. M., Lee, C. R. GABAergic control of substantia nigra dopaminergic neurons. Progress in brain research. 160, 189-208 (2007).
- Hannan, A. J. Environmental enrichment and brain repair: harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathology and applied neurobiology. 40, 13-25 (2014).
- Nithianantharajah, J., Hannan, A. J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature reviews. Neuroscience. 7, 697-709 (2006).
- Chmiel, D. J., Noonan, M. Preference of laboratory rats for potentially enriching stimulus objects. Laboratory animals. 30, 97-101 (1996).
- Hanmer, L. A., Riddell, P. M., Williams, C. M. Using a runway paradigm to assess the relative strength of rats’ motivations for enrichment objects. Behavior research methods. 42, 517-524 (2010).
- Burrows, E. L., McOmish, C. E., Hannan, A. J. Gene-environment interactions and construct validity in preclinical models of psychiatric disorders. Progress in neuro-psychopharmacology & biological psychiatry. 35, 1376-1382 (2011).
- Van de Weerd, H. A., Van Loo, P. L. P., Van Zutphen, L. F. M., Koolhaas, J. M., Baumans, V. Strength of preference for nesting material as environmental enrichment for laboratory mice. Applied Animal Behaviour Science. 55, 369-382 (1998).
- Rampon, C., et al. Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Sciences of the United States of America. 97, 12880-12884 (2000).
- Eckert, M. J., Abraham, W. C. Effects of environmental enrichment exposure on synaptic transmission and plasticity in the hippocampus. Current topics in behavioral neurosciences. 15, 165-187 (2013).
- Sztainberg, Y., Chen, A. An environmental enrichment model for mice. Nature protocols. 5, 1535-1539 (2010).
- Sale, A., Berardi, N., Maffei, L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiological reviews. 94, 189-234 (2014).

