Unraveling Entropic Rate Acceleration Induced by Solvent Dynamics in Membrane Enzymes
Summary
Channels for the transportation of water molecules in enzymes influence active site solvation and catalysis. Herein we present a protocol for the engineering of these additional catalytic motifs based on in silico computer modeling and experiments. This will enhance our understanding of the influence of solvent dynamics on enzyme catalysis.
Abstract
Enzyme catalysis evolved in an aqueous environment. The influence of solvent dynamics on catalysis is, however, currently poorly understood and usually neglected. The study of water dynamics in enzymes and the associated thermodynamical consequences is highly complex and has involved computer simulations, nuclear magnetic resonance (NMR) experiments, and calorimetry. Water tunnels that connect the active site with the surrounding solvent are key to solvent displacement and dynamics. The protocol herein allows for the engineering of these motifs for water transport, which affects specificity, activity and thermodynamics. By providing a biophysical framework founded on theory and experiments, the method presented herein can be used by researchers without previous expertise in computer modeling or biophysical chemistry. The method will advance our understanding of enzyme catalysis on the molecular level by measuring the enthalpic and entropic changes associated with catalysis by enzyme variants with obstructed water tunnels. The protocol can be used for the study of membrane-bound enzymes and other complex systems. This will enhance our understanding of the importance of solvent reorganization in catalysis as well as provide new catalytic strategies in protein design and engineering.
Introduction
Water constitutes a cornerstone for the chemistry of life1. Water patterns and solvation of enzyme active sites affect both the enthalpy and entropy of ligand binding1,2 and catalysis3 in a highly complex fashion extending beyond the hydrophobic effect2,3. NMR4, calorimetry2 and molecular modeling of solvated proteins have been used to shed light on the role of explicit water molecules in providing a driving force for ligand association5-8, specificity and activity2,9,10. Herein we present a unique methodology for the experimental assessment of the thermodynamical impact of solvent displacement on enzyme catalysis (Figure 1). Our combined strategy is based on using computer simulations in concert with enzyme engineering and thermodynamical analysis (Figure 1). This allows for shedding additional light on the impact of solvent dynamics on catalysis, which is currently poorly understood.
Stabilizing enthalpic interactions, provided by water-mediated hydrogen bonds in solvated enzyme active sites, can be offset by entropic penalties1. These entropic costs are associated with the decrease in the degrees of freedom displayed by water molecules confined within protein cavities, as compared to water in bulk5. The release of ordered water molecules can thus provide an entropic driving force for ligand association1 and catalysis3. A key aspect of solvent dynamics is the displacement of water molecules between the interior of proteins and the exterior solvent4. The accompanying changes in activation energy, enthalpy, and entropy11 are not completely understood on the molecular level. By obstructing individual tunnels responsible for the transport of water in enzymes, the importance of solvent dynamics and its contribution to the activation energy can be evaluated (Figure 1). Moreover, by performing one-pot kinetic experiments at different temperatures, the relative thermodynamical activation parameters of several substrates can be extracted from a reduced number of experiments (Figure 1, right). Our interdisciplinary method is validated for complex membrane-bound triterpene cyclase enzymes that generate polycyclic terpenes of high importance for life12. The protocol allows for the recovery of high amounts of membrane protein (10-20 mg/L) using a standard centrifuge.
Although enzymes evolved in water, the role of the solvent in promoting catalysis is usually neglected. In addition to protein dynamics13,14 that shape pre-organized active sites with electrostatic complementarity to the transition state15, water dynamics could be of high importance for efficient enzyme catalysis. By forging several interdisciplinary techniques, our aim is to facilitate the highly complex study of water dynamics and thermodynamics. Making these tools more accessible to the scientific community will lead to the development of new strategies in enzyme engineering and protein design for altered activities and specificities.
Protocol
1. In Silico Computer Modeling
- Download a PDB structure of the protein of interest from the protein data bank (PDB). Prepare the structure by removing excess subunits and then adding missing hydrogen atoms and explicit solvent using the "Cell Neutralization and pKa prediction" experiment in YASARA16. For membrane bound triterpene cyclases, suitable dimensions of the water box are 91x67x77 Å. Adjust the protonation state of the catalytically active amino acids manually.
Note: If necessary, a substrate analog in the PDB file can be modified to a real substrate by using the "Edit|Swap|Atom" command. - Minimize the structure by the "Energy Minimization" command using the AMBER force field suite17. Use the particle mesh Ewald approach (PME)18 to account for long-range electrostatic interactions. Set the cut-off for van der Waals interactions according to standard settings.
Note: The "Energy Minimization" command removes conformational stress by a short steepest descent minimization, then a simulated annealing (timestep 2 fsec, atom velocities scaled down by 0.9 every 10th step) is performed until convergence is reached (i.e., improvement of energy by less than 0.012 kcal/mol per atom during 200 steps).
2. Model of Active Site Solvation and Water Access
- After the simulated annealing, run a 20 nsec molecular dynamics (MD) trajectory and generate at least 10 snapshots by the "File|Save As|Simulation Snapshot" command followed by "Simulation On". Run the simulations on a standard computer with periodic boundary conditions under the canonical ensemble. Maintain the temperature at 298 K using a Berendsen thermostat.
- Prepare the snapshots obtained from 2.1 by deleting all water molecules and eventual ligands/substrates using the "Edit|Delete|Residue" command. Superpose each snapshot individually on the original PDB-structure by the "Superpose|Object" command to ensure that all structures share the same spatial position. Save each snapshot prepared in this way as a new PDB-file (using the "File|Save as|PDB" command).
Note: For experienced modelers: Write a script to automate this process according to the template given in Supplementary Code File 1. - Use the prepared snapshots (PDB) from 2.2 that have been aligned in 3D-space as inputs to the software CAVER 3.019. For the analysis of a limited number of snapshots, run CAVER on a standard computer. Use a probe radius of 0.7 Å19 to assure the detection of tunnels that are specific for the transport of water molecules.
- Visualize the tunnels generated by CAVER 3.0 in a molecular graphics software. For easy visualization of tunnels, use the macro shown in Supplementary Code File 2.
3. In Silico Enzyme Engineering to Modify Water Patterns and Water Dynamics
- Identify the highest ranked tunnels according to the header "ID" listed in the output file "summary.txt" generated from the CAVER 3.0 software.
- Identify amino acids lining the highest ranked tunnels (3.1) and that display a small side chain pointing towards the channel interior by visual inspection (using the model obtained from 2.4). Identify single amino acid substitutions that will block the tunnel by increased steric hindrance using the "Swap Residue" command. Verify that the tunnel is obstructed by visual inspection, and then by repeating steps 1.2-3.1 of the protocol.
4. Expression of Mutated Genes
- Introduce mutations in the wild-type gene by Mutagenesis PCR using non-overlapping primers20.
- Add plasmid DNA containing the gene of interest to tubes with competent cells of a suitable cloning strain and incubate on ice for 30 min. Perform a heat shock transformation for 45 sec in a water bath with the temperature set to 42 °C. Add 500 µl of a fresh rich medium (2YT, 16 g/L tryptone, 10 g/L yeast extract, 5 g/L NaCl) and incubate at 37 °C, 200 rpm for 45 min. Make a selection by plating the transformed cells on agar plates supplemented with appropriate antibiotics.
- After verifying the DNA sequence of the mutated gene, transform the plasmid DNA into an expression strain using heat shock as described above. For the expression of membrane bound triterpene cyclases, use BL21(DE3).
- Start a 5 ml O/N culture at 37 °C of the expression strain in 2YT-media supplemented with the appropriate antibiotics.
- Inoculate a 2 L baffled shake flask, containing 300 ml 2YT-media supplemented with appropriate antibiotics, to a final OD of 0.05-0.09 (measured at 600 nm). After induction at an OD of 0.6-0.8 and protein expression, freeze the cell pellet and store at -80 °C.
- For membrane-bound triterpene cyclases in a pD861 vector, induce with 0.5 to 2 mM rhamnose and 4 hr of expression at 37 °C to achieve high yields of protein21.
5. Membrane Extraction Using a Normal Centrifuge and Protein Purification
- Prepare the following buffers: Resuspension buffer (100 mM potassium phosphate, pH 7.5 supplemented with Protease Inhibitor Tablets), Detergent buffer (1% (v/v) Triton X-100, 50 mM potassium phosphate, pH 7.5) and Reaction buffer (0.2% Triton X-100, 50 mM potassium phosphate, pH 7.5).
- For squalene-hopene cyclases or other membrane-bound enzymes with a lower pH optimum, replace the potassium phosphate in the resuspension buffer with citrate. For such enzymes use the following buffers: Detergent buffer (1% Triton X-100, 60 mM citrate, pH 6.0) and Reaction buffer (0.2% Triton-X 100, 60 mM citrate, pH 6.0).
Note: If a His-tag is used for purification, supplement the resuspension buffer with 20 mM imidazole (final concentration).
- For squalene-hopene cyclases or other membrane-bound enzymes with a lower pH optimum, replace the potassium phosphate in the resuspension buffer with citrate. For such enzymes use the following buffers: Detergent buffer (1% Triton X-100, 60 mM citrate, pH 6.0) and Reaction buffer (0.2% Triton-X 100, 60 mM citrate, pH 6.0).
- Weigh a frozen cell pellet in a glass beaker. Resuspend the cells in resuspension buffer using a homogenizer to a final concentration of 0.3 g cells/ml. Lyse the resuspended cells by ultrasonication with an amplitude of 80% and a pulse of 1 sec on and 1 sec off. Use three repeating cycles of 50 sec each.
- Add detergent buffer to a final concentration of 0.2% (v/v) detergent. Equilibrate by end-over-end mixing at 4 °C for 1 hr. Recover the membrane protein fraction by saving the supernatant after a single centrifugation step at 39,000 x g for 50 min at 4 °C.
Note: If His-tag purification is used, add approximately 1.5 ml Ni-NTA agarose/g cells and incubate for 1 hr. - Perform a first purification step by either ion exchange or His-tag purification21. Supplement the equilibration and elution buffers with 0.2% Triton X-100. Concentrate the purified protein five times using Centrifugal Filter Units with a cut-off of 10 kDa.
Note: The size of the Triton X-100 micelles results in a concentration of detergent to a final concentration of approximately 1%. - Finalize purification by a gel filtration polishing step using the concentrated protein and a matrix compatible with a fractionation range for globular proteins of 2 × 104-8 × 106. Use the reaction buffer as running buffer. Measure the amount of purified protein by using the Bradford Ultra kit according to the manufacturer's protocol.
6. Kinetics
- Make a fresh 5 mM stock solution of substrate in reaction buffer. Obtain a homogenous emulsion by ultrasonication at 30% amplitude for 2-5 min.
- Equilibrate a thermomixer at the desired reaction temperature. Verify the temperature inside a glass vial containing water by an external thermometer.
- For single-substrate kinetics, dilute the emulsified substrate into at least five glass vials to the same final substrate concentration.
- For one-pot relative kinetics with multiple substrates, prepare at least five identical glass vials by mixing all substrates in each vial. For hydrophobic triterpenes, use final substrate concentrations in the range of 10-200 µM.
- Preincubate the glass vials in the thermomixer for 10 min. Start the reaction by adding the enzyme. A total reaction volume of 1 ml is suitable. Use a shaking speed of at least 1,200 rpm.
- Stop the reactions at different time points by adding 500 µl ethylacetate spiked with 100 µM decanol as an integration standard.
7. Extraction and Thermodynamic Analysis
- Extract the reaction mixtures by vortexing and manually shaking the glass vials vigorously for 1 min. Centrifuge the glass vials at 9,600 x g in a table top centrifuge for 10 min at RT. Transfer the top layer (organic phase) to an empty tube. Add an additional 500 µl of extraction solvent to the reaction tubes and repeat the extraction procedure.
- Dry the extracted reaction mixtures with Na2SO4. Vortex for 5 sec and let the tubes rest for 10 min. Perform a final centrifugation step at 9,600 x g for 1 min at RT using a table top centrifuge. Transfer the extracted samples to gas chromatography (GC) vials.
- Repeat the steps under 6.1-7.2 for each enzyme variant of interest, using at least four different initial concentration of the substrate ( for single substrate kinetics) and four different temperatures (for both single substrate and competitive kinetics).
- Perform GC-analysis as previously described3. Use a non-polar column for the analysis of hydrophobic pentacyclic products. Set the initial oven temperature to 120 °C and use a temperature gradient of 5 °C/min, depending on the products and the column.
- Integrate the peak areas of the product(s) and transform product peak areas into the corresponding product concentrations by utilizing the area of the integration standard (corresponding to 100 µM). Plot the concentration of each product ([P]) versus the reaction time (t). Perform linear regression of the data points corresponding to less than 10% conversion. The initial reaction speed (V0) is given by the slope of the fitted line according to:
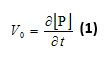
Note: For one-pot relative kinetics with multiple substrates, V0 is the initial rate of a particular substrate under the influence of other substrates. - For single substrate kinetics, plot V0/[E] against the corresponding substrate concentrations. The apparent kcat/KM value is given by the slope of the linear part of the graph according to:
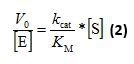
[S] is the concentration of substrate and [E] the concentration of enzyme used in the transformation. kcat/KM equates to the catalytic constant over the Michaelis constant. Repeat the analysis for each temperature and each enzyme variant. - For single substrate kinetics, perform a thermodynamic analysis according to transition state theory22
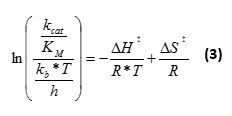
kb is the Boltzmann constant, h the Planck's constant, T the temperature in Kelvin, and R the gas constant. Perform a linear regression analysis of the plot of ln((kcat/KM)/((kb*T)/h))) versus 1/T. The activation enthalpy (ΔH‡) is given by (-slope)*R and the activation entropy (ΔS‡) is given by (intercept)*R.
Note: Likewise, an analysis under saturating substrate concentration can be performed by using kcat as rate constant in equation 3. - For one-pot relative kinetics with multiple substrates, obtain the relative apparent
kcat/KM values from23:
(kcat/KM)A/(kcat/KM)B = V0,A/V0,B*[B]/[A] (4)
for which V0,A refers to the initial rate for substrate A in competition with substrate B. - For one-pot relative kinetics with multiple substrates, obtain the relative thermodynamic parameters of activation for B, compared to A as reference, by nonlinear regression:
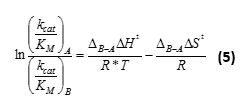
- Calculate the absolute activation parameters for substrate B from the activation enthalpy and entropy of reference compound A:
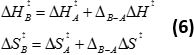
Representative Results
The importance of water dynamics in enzymatic polycyclization catalysis is implicated by in silico analysis and subsequent visualization of detected tunnels (using the script in Supplementary Code File 2). Following section 3 of the protocol, S168 is found to be a "hot spot" amino acid residue lining one of the tunnels in the triterpene cyclase enzyme from Alicyclobacillus acidocaldarius (Figure 1, middle left). By introducing the mutation S168F in silico, the tunnel is obstructed as seen by visual inspection (Figure 1, bottom left).
The reaction rate for the formation of polycyclic products displayed by the triterpene cyclase enzyme is highly sensitive to temperature (Figure 2A). Following the protocol (section 4-7), it is found that the apparent kcat/KM displayed by wild-type enzyme for the formation of pentacyclic products increases fiftyfold when the temperature is raised by 25 °C (Figure 2A). Blocking individual tunnels by introducing a single point mutation has a significant effect on the experimentally determined absolute apparent kcat/KM values, as well as on their temperature dependence (Figure 2A).
From the experimentally determined kinetic parameters, linear thermodynamical plots are generated by equation 3 and by following section 6-7 of the protocol for single substrate kinetics (Figure 2B). The very large changes in activation entropy and enthalpy observed for variants harboring blocked tunnels implies a key role for water dynamics in promoting the polycyclization cascade (Figure 2C, calculations based on Figure 2B). Moreover, variants with altered water tunnels display an altered Gibbs free energy of activation (ΔG‡=ΔH‡–T*ΔS‡, Figure 2B-C). For instance, at 303 K the S168F tunnel variant displays a Gibbs free energy of activation of 14 kcal/mol compared to 16 kcal/mol for the wild-type enzyme.
Following the protocol in section 7, equation 4 allows for the calculation of apparent kcat/KM values for additional substrates from one-pot kinetic experiments (Figure 3A). Moreover, a linear thermodynamic plot (Figure 3B) can be constructed by running the competition essay at different temperatures (equation 5). In analogy to single substrate kinetics, the relative activation enthalpy and entropy for additional substrates compared to a reference compound are readily accessible from the intercept and slope of the linear fits to the experimental data. Absolute values of thermodynamic parameters of activation can be assessed from arithmetic addition by using thermodynamic data associated with the reference compound (equation 6). Using the protocol herein, generated results clearly show that membrane enzyme variants with blocked water tunnels display fundamentally different thermodynamic parameters of activation for substrates of different sizes (Figure 3C).
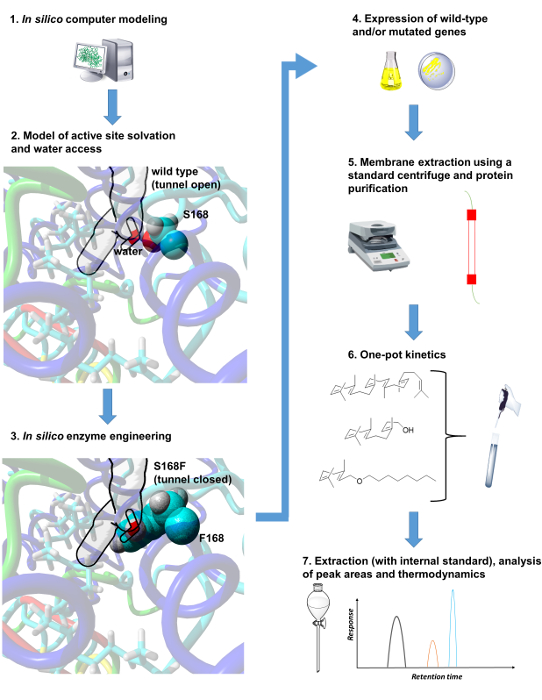
Figure 1. Protocol summary: in silico computer modeling in concert with experimental protein design and thermodynamic analysis allows for an enhanced understanding of the influence of solvent dynamics on enzyme catalysis. Please click here to view a larger version of this figure.
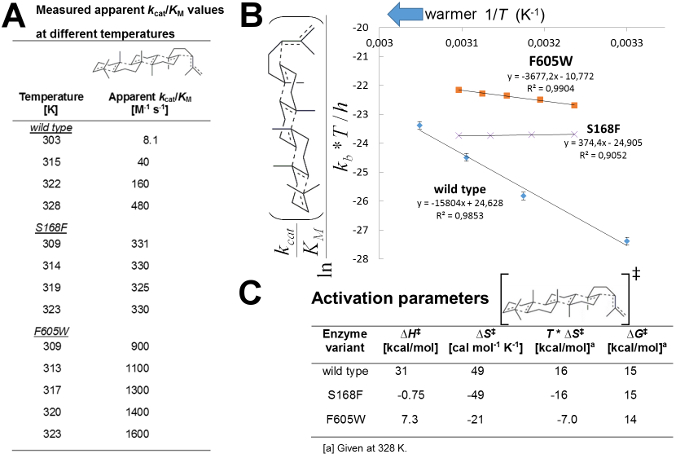
Figure 2. Thermodynamic parameters of activation of wild-type and tunnel variants from single substrate kinetics. (A) Apparent kcat/KM values obtained from experimental data and by using equations 1 and 2. (B) Thermodynamical analysis using transition state theory and linear fit of the experimental data to equation 3. (C) Thermodynamic parameters of activation calculated from the plots in (B). The prefolded squalene substrate is shown with bonds formed/broken as dotted lines. Please click here to view a larger version of this figure.
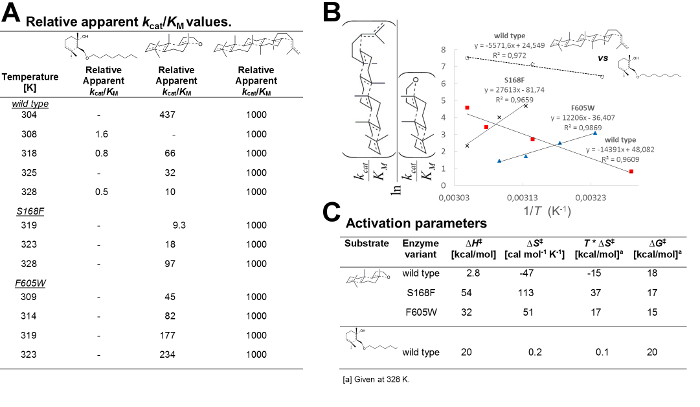
Figure 3. Thermodynamic parameters of activation of wild-type and tunnel variants for different substrates. (A) Relative kcat/KM values obtained from one-pot kinetics by using equation 4. (B) Thermodynamical plots of the kinetic data at different temperatures using equation 5. (C) Absolute thermodynamic parameters of activation calculated by equation 6 and by using the substrate squalene in Figure 2 as reference. Bonds formed/broken in the substrates are shown as dotted lines. Please click here to view a larger version of this figure.
Discussion
The most critical steps in achieving high quality experimental thermodynamical data for wild-type membrane enzyme and tunnel variants are: 1) generation of the computer model; 2) homogeneously purified proteins; 3) emulsified substrate stocks; 4) control of temperature during kinetics; 5) extraction of reaction mixtures using internal standard.
The generation of the computer model is greatly facilitated by the use of a software with a user-friendly interface that supports a variety of platforms. Hence, this protocol is premised on the YASARA modeling suite16 to make our strategy accessible even to modeling non-experts. A computer model for water tunnel identification should ideally be based on a crystal structure of the enzyme of interest24. For this purpose, the wealth of crystal structures available in the protein data bank is very beneficial. In our experience, a key aspect in the successful preparation of templates for tunnel identification is to keep crystallographic waters. It is of equal importance to use a solvated enzyme box when performing molecular dynamics simulations, which can be run on a normal computer. The triterpene cyclase from Alicyclobacillus acidocaldarius is stable in water during MD simulations3. However, keeping crystallographic detergents and/or using cell membrane mimics would perhaps be required for potentially unstable enzymes to allow for extended MD simulations. It is envisioned that the minimized crystal structure of highly challenging targets could provide important mechanistic insight using the protocol, although this would not capture dynamical aspects of tunnel organization.
CAVER19 in the basic mode, with a single or a limited number of snapshots as input, can be used by non-experts on a standard laptop. Based on our experience3, tunnels with a bottleneck radius (i.e., the radius at the most narrow point) smaller than 1 Å can be highly relevant for water, especially if crystallographic water molecules reside within the predicted tunnel (Figure 1, middle left). On the other hand, a larger bottleneck radius could imply a tunnel for the transport of the substrate in and out of the active site10. The script in Supplementary Code File 2 can be used by non-experts for the visualization of predicted tunnels. Future experiments will reveal whether homology models will be of sufficiently high resolution to allow for the atomistic study of water networks and dynamics. Shedding light on how performing molecular dynamics simulations, with and without a ligand present in the active site, influences the process of tunnel identification would also be of importance.
Kinetics of membrane proteins can constitute a formidable challenge25. The protocol herein is based on a simple membrane extraction protocol to obtain the membrane enzyme without the use of expensive equipment, such as an ultracentrifuge. The use of gel filtration as a final polishing step removes potential residual membrane particles and allows for defining an appropriate detergent environment25.
A key aspect in achieving reproducible kinetic results from the protocol is to emulsify the substrate stock solution by ultrasonication. Simple vortexing of hydrophobic substrates diluted in the reaction buffer gives inhomogeneous substrate-detergent mixtures. The pipetting of non-emulsified substrate solutions leads to irreproducible concentrations (confirmed by quantitative GC), which prevents accurate determination of initial rates. In contrast, the pipetting of properly emulsified stock solutions should result in linear regression analysis of initial rates with R2 in the range of 0.98-0.99. Another important aspect of hydrophobic substrates is the apparent substrate solubility and availability in the substrate-detergent mixtures. In fact, it was not possible to saturate the triterpene cyclase with the reference substrate squalene. For this reason apparent kcat/KM values are presented herein which could contain contributions from both binding and chemistry. However, it has been shown that chemistry is rate limiting for kcat/KM for the polycyclization cascade conducted by triterpene cyclases3.
It is of high importance to verify the actual temperature inside a reaction glass vial with an external thermometer. Still, linear fits can be poorer for variants with essential constant apparent kcat/KM values at different temperatures. This is stressed for the S168F variant herein (Figure 2A) with an activation enthalpy close to zero (Figure 2B and 2C). Very small temperature-dependent changes in apparent kcat/KM, could induce uncertainty in the observed activation entropy ΔS‡ (i.e., the intercept in the linear plots in Figure 2B). In principle, the observed activation entropy could also be affected by a different abundance of active enzymes for different variants, which would not be detected by measuring the protein concentration. It is expected that experimental errors are reduced when mixing several substrates in one pot. This is because all the different substrates interact with the same amount of enzyme under these circumstances (equation 4). The use of an extraction solvent spiked with an internal standard is important to account for differences during extraction and/or GC-injection.
Transition state theory has been successfully used in enzymology26. This important theoretical framework was originally developed for unimolecular reactions in the gas phase. However, it has been shown that enzymes mainly work by lowering the classical activation energy barrier26. The transmission coefficient assumed to be one herein could affect the measured activation enthalpy and/or entropy. The contribution of tunneling, and other non-classical effects such as recrossing of the transition state, can roughly contribute 1,000-fold to catalysis26 corresponding to about 4 kcal/mol in energy. It can be seen that the activation entropy displayed by the wild-type enzyme (16 kcal/mol at 328 K, Figure 2C) is much larger than such non-classical effects caused by a non-uniform transmission coefficient. The impact of the transmission coefficient should decrease when comparing thermodynamic parameters of activation for wild type and tunnel variants using the protocol.
The Gibbs free energy of activation (ΔG‡) is composed of both an enthalpic (ΔH‡) and an entropic (-T*ΔS‡) term. Solvent reorganization in enzymes during catalysis can influence both parameters. The present protocol is expected to facilitate the study of these phenomena by assembling a toolbox of relevant and user friendly in silico computational tools with the necessary biophysical experimental framework. The method is envisaged to be useful for studying a plethora of enzymatic processes, including catalysis by membrane-bound enzymes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The Swedish Research Council (VR) is greatly acknowledged for financial support of this work by a young investigator grant #621-2013-5138. The PDC Center for High Performance Computing at the KTH Royal Institute of Technology is acknowledged for providing computational support.
Materials
| YASARA | YASARA Biosciences | http://www.yasara.org/ | Molecular modeling and simulation program |
| CAVER | CaverSoft | http://caver.cz/ | Tool for analysis of tunnels in proteins, free license for academic use |
| Bradford Ultra | Expedeon | BFU1L, BFU05L | Protein quantitation in solutions containing up to 1% detergent |
| Potter-Elvehjem homogenizer | VWR | 432-0205, 432-0217 | Homogenization of frozen cell pellet |
| Protease Inhibitor Cocktail Tablets | Roche | 4693159001 | Protease inhibitor |
| Centrifugal Filter Units | Millipore | UFC901008 | Centrifugal filter units for the concentration of proteins, MWCO 10 kDa |
| Thermomixer | Eppendorf | 5382000015 | Thermomixer for sample incubation |
References
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 108 (1), 74-108 (2008).
- Snyder, P. W., et al. Mechanism of the hydrophobic effect in the biomolecular recognition of arylsulfonamides by carbonic anhydrase. Proc. Natl. Acad. Sci. U. S. A. 108 (44), 17889-17894 (2011).
- Syren, P. O., Hammer, S. C., Claasen, B., Hauer, B. Entropy is Key to the Formation of Pentacyclic Terpenoids by Enzyme-Catalyzed Polycyclization. Angew. Chem., Int. Ed. 53 (19), 4845-4849 (2014).
- Persson, F., Halle, B. Transient Access to the Protein Interior: Simulation versus NMR. J. Am. Chem. Soc. 135 (23), 8735-8748 (2013).
- Abel, R., Young, T., Farid, R., Berne, B. J., Friesner, R. A. Role of the Active-Site Solvent in the Thermodynamics of Factor Xa Ligand Binding. J. Am. Chem. Soc. 130 (9), 2817-2831 (2008).
- Michel, J., Tirado-Rives, J., Jorgensen, W. L. Energetics of Displacing Water Molecules from Protein Binding Sites: Consequences for Ligand Optimization. J. Am. Chem. Soc. 131 (42), 15403-15411 (2009).
- Pearlstein, R. A., Sherman, W., Abel, R. Contributions of water transfer energy to protein-ligand association and dissociation barriers: Watermap analysis of a series of p38α MAP kinase inhibitors. Proteins: Struct., Funct., Bioinf. 81 (9), 1509-1526 (2013).
- Young, T., Abel, R., Kim, B., Berne, B. J., Friesner, R. A. Motifs for molecular recognition exploiting hydrophobic enclosure in protein-ligand binding. Proc. Natl. Acad. Sci. U. S. A. 104 (3), 808-813 (2007).
- Matsuoka, S., et al. Water-Mediated Recognition of Simple Alkyl Chains by Heart-Type Fatty-Acid-Binding Protein. Angew. Chem., Int. Ed. 54 (5), 1508-1511 (2014).
- Pavlova, M., et al. Redesigning dehalogenase access tunnels as a strategy for degrading an anthropogenic substrate. Nat. Chem. Biol. 5 (10), 727-733 (2009).
- Breiten, B., et al. Water Networks Contribute to Enthalpy/Entropy Compensation in Protein-Ligand Binding. J. Am. Chem. Soc. 135 (41), 15579-15584 (2013).
- Oldfield, E., Lin, F. Y. Terpene biosynthesis: Modularity rules. Angew. Chem., Int. Ed. 51 (5), 1124-1137 (2012).
- Henzler-Wildman, K. A., et al. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 450 (7171), 913-916 (2007).
- Tzeng, S. R., Kalodimos, C. G. Protein activity regulation by conformational entropy. Nature. 488 (7410), 236-240 (2012).
- Warshel, A. Electrostatic origin of the catalytic power of enzymes and the role of preorganized active sites. J. Biol. Chem. 273, 27035-27038 (1998).
- Krieger, E., Darden, T., Nabuurs, S. B., Finkelstein, A., Vriend, G. Making optimal use of empirical energy functions: Force-field parameterization in crystal space. Proteins: Struct., Funct., Bioinf. 57 (4), 678-683 (2004).
- Duan, Y., et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 24 (16), 1999-2012 (2003).
- Essmann, U., et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577-8593 (1995).
- Chovancova, E., et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 8 (10), e1002708 (2012).
- Zheng, L., Baumann, U., Reymond, J. L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32 (14), e115/1-e115/5 (2004).
- Kürten, C., Uhlen, M., Syren, P. O. Overexpression of functional human oxidosqualene cyclase in Escherichia coli. Protein Express. Purif. , (2015).
- Eyring, H., Stearn, A. E. The application of the theory of absolute reaction rates to proteins. Chem. Rev. 24 (2), 253-270 (1939).
- Fersht, A. Structure and mechanism in protein science: a guide to enzyme catalysis and protein. , (1999).
- Wendt, K. U., Poralla, K., Schulz, G. E. Structure and function of a squalene cyclase. Science. 277 (5333), 1811-1815 (1997).
- Seddon, A. M., Curnow, P., Booth, P. J. Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta, Biomembr. 1666 (1-2), 105-117 (2004).
- Garcia-Viloca, M., Gao, J., Karplus, M., Truhlar, D. G. How enzymes work: analysis by modern rate theory and computer simulations. Science. 303 (5655), 186-195 (2004).

