High-throughput Identification of Bacteria Repellent Polymers for Medical Devices
Summary
A high-throughput microarray method for the identification of polymers which reduce bacterial surface binding on medical devices is described.
Abstract
Medical devices are often associated with hospital-acquired infections, which place enormous strain on patients and the healthcare system as well as contributing to antimicrobial resistance. One possible avenue for the reduction of device-associated infections is the identification of bacteria-repellent polymer coatings for these devices, which would prevent bacterial binding at the initial attachment step. A method for the identification of such repellent polymers, based on the parallel screening of hundreds of polymers using a microarray, is described here. This high-throughput method resulted in the identification of a range of promising polymers that resisted binding of various clinically relevant bacterial species individually and also as multi-species communities. One polymer, PA13 (poly(methylmethacrylate-co-dimethylacrylamide)), demonstrated significant reduction in attachment of a number of hospital isolates when coated onto two commercially available central venous catheters. The method described could be applied to identify polymers for a wide range of applications in which modification of bacterial attachment is important.
Introduction
Polymer microarrays are miniaturized high-throughput platforms in which up to 7,000 polymers1 are printed onto glass slides for parallel analysis with prokaryotic or eukaryotic cells2. The method presented here builds on that which we first described in 20103. This screening system has been applied to numerous cell types including human hepatocytes4, stem cells5, renal tubular epithelial cells2, bacteria3,6 and protozoan pathogens7. In each case, polymers that promote or resist binding of the cells under study were identified8. Complexes of DNA with synthetic polycationic polymers have also been used in the microarray format for high-throughput screening of gene transfection candidates9. As well as screening for cell-substrate interactions, polymer microarrays have also been used for evaluating material properties10.
The ability of synthetic polymers to modulate attachment of bacteria to a surface is well established3,6,11. Numerous factors including charge, hydrophobicity and surface roughness of the polymer surface are known to affect bacterial binding. The conventional approaches of discovering biomaterials that resist binding of bacteria through sequentially or empirically designing and testing one material at a time are labor-intensive, costly and time-consuming processes. Polymer microarrays offer an attractive alternative for circumventing such limitations.
Surface-associated bacteria grow as a complex population termed a biofilm — such biofilms are highly resistant to many environmental stresses and antibiotics. This is in part due to their dense extracellular matrix (composed of proteins, polysaccharides and nucleic acids)12 and in part due to the increased presence of robust "persistor" cells in biofilms13. Although the precise mechanisms of surface association and subsequent biofilm formation are difficult to characterize, it is generally believed that there are three different stages of surface growth14–16. Initial, reversible attachment is followed by stronger adhesion of cells, the establishment of a biofilm by production of an extracellular protein and polysaccharide matrix and cell proliferation. Finally, the mature biofilm releases free-living planktonic cells, which can initiate new infections elsewhere. Bacteria-repelling polymers that prevent the initial attachment of bacteria, and hence prevent early stages of biofilm formation, potentially represent an excellent solution for minimizing infections. Given the rise of antibiotic resistance (and also the intrinsically greater resistance of surface-associated bacteria12), antibiotic-free means of reducing infections are of particular interest. In a hospital setting, bacteria-repelling polymer coatings can have a direct medical application in the reduction of nosocomial infections, which commonly form around implanted devices17.
Here, a high-throughput method for the screening of 381 polymers for repellent activity against a range of pathogenic bacteria associated with nosocomial infections, followed by hit validation and subsequent coating and assay of central venous catheter materials, is described (Figure 1). Briefly, the polymers were spotted onto agarose-coated glass slides by contact printing and, after drying and sterilization, the miniaturized arrays were incubated with clinically important bacterial cultures. After incubation, the microarrays were gently washed and adherent bacterial cells were stained and visualized by fluorescence. Subsequently, polymers which inhibited bacterial binding were investigated on a larger scale by coating onto glass cover slips and visualized by electron microscopy. Selected repellent polymers were then coated onto commercial catheters and shown to reduce attachment of bacteria by almost 100 fold.
Protocol
1. Preparation of Agarose Coated Slides
NOTE: Before fabricating the polymer microarrays, aminoalkylsilane-coated glass slides are coated with agarose I-B to minimize non-specific background binding and allow evaluation of polymers that bind or repel bacteria6. Silane coating facilitates the binding of agarose to the slides.
- Make up a 2% (w/v) solution of agarose I-B in distilled water in a 250 ml bottle.
- After capping the bottle loosely, heat the suspension in a microwave oven in 30 sec periods until dissolved. Ensure that the cap is not tightly closed to avoid any potential pressure build up. Remove the bottle regularly and swirl gently.
- Ensure that the solid has dissolved and the solution is clear. Pour this agarose solution into a 100 ml beaker, and place in a 65 °C water bath to maintain a liquid solution.
- Dip the silane-coated slides into the agarose solution, ensuring uniform coating, and wipe the back of the slide with a tissue paper.
- Dry the slides, with the coated side up, in a dust-free environment (e.g., inside a cupboard or fume hood) for a minimum of 24 hr.
- Confirm a uniform coating by visual inspection. Coating can be easily assessed by breathing onto the slide — condensation will form on uncoated regions. Only slides coated entirely and evenly should be used for microarray printing.
2. Preparation of Polymer Solutions for Printing
- Prepare solutions (1% w/v) of a selection of preformed polymers consisting of polyacrylates, polyacrylamides and polyurethanes in N-methylpyrrolidone (NMP) in glass vials. (prepare 1 ml of each polymer). Vortex until the polymer is fully dissolved. Synthesis of polymers is described elsewhere6.
- Using a micro-pipette, fill each well of a 384 microwell plate with 25 µl polymer solution, ensuring that there is no cross contamination and each well contains a unique polymer. Use NMP as a negative control in at least 2 wells. Record the identity of polymer solution in each well on a well plate template or spreadsheet file.
3. Printing Polymer Microarrays Using a Contact Printer
NOTE: Printing of microarrays was performed using a contact printer. Specific instructions regarding polymer microarray printing are given below. For general guidelines on using the printer and safety recommendations, follow the user manual from the manufacturer. Although we use a contact printer, a suitable manual stamping device could also be used.
- As advised in the printer user manual, create a routine (see step 3.3) that allows the printing of 384 liquids (the polymer solutions or NMP) in quadruplicates, using a 32-pin microarraying head (arranged as 8 rows and 4 columns).
- Print 1,536 spots arranged as 48 rows and 32 columns. Program the routine to print in 12 sections, i.e., 32 solutions at a time, so that the printer can be stopped after printing each section to allow for cleaning of pins.
- To create a printing routine:
- Open the software and from the available options choose 'Create a new routine'. Select the 'Description' tab to input details of the experiment. Select the 'Head' tab and choose '32-pin microarraying head'.
- Select the 'Source' tab and choose plate holder (source plate holder (1×5)), plate type (plate 384 x 6,000), total plates (1) and source order (by columns).
- In the tab 'Slide design', choose '3×1'' slide' and choose arraying by 'Field number'. Then select 'Arraying pattern'. Input 'Spot view' as 'layout' and 'Pattern dimensions' as 'Row count' (6), 'Column count' (8), 'Row pitch' (750) and 'Column pitch' (560). Choose one section to print first.
- In the 'Slide layout' tab input the number of slides used for printing. Select the 'Print' tab to input the printing parameters (number of stamps per inking: 1, number of stamps per spot: 5, stamping time: 200 msec, inking time: 100 msec).
NOTE: A routine is now created for printing 384 liquids (the polymer solutions or NMP) in quadruplicates, using a 32-pin microarraying head (arranged as 8 rows and 4 columns). In total the routine should print 1,536 spots arranged as 48 rows and 32 columns with a row pitch of 750 µm and column pitch of 560 µm.
- Place the agarose-coated slides on the platform and ensure a good vacuum seal to hold the slides in position.
- Adjust the print-head so that all the pins are at the same height. Check this by manually lowering the head on to a glass slide and confirming that the pins move up at the same time. If there are any discrepancies with the height, adjust by using the sleeve on the pin up or down.
- Place the well plate on the plate holder in the correct orientation, i.e., well A1 is to the top right of the holder and ensure the well plate is fixed firmly.
- Using the height adjustor, ensure that the height of the plate holder is such that when picking the polymer solutions, the pins do not touch the bottom of the wells.
NOTE: This is important for uniform polymer spotting and also to avoid spilling of polymer solutions into adjoining wells, thereby causing cross-contamination. - Select the 'Start' tab and choose the 'Run type' as 'Normal'. Click 'Run', and confirm that slide, well plate, etc., are in position when prompted.
- Print 1 section at a time (32 solutions at a time; 12 sections), allowing for cleaning of pins between sections. Clean the pins thoroughly with paper towel dipped in acetone, followed by dry tissue paper to ensure the pins are fully dry.
- On completion of printing of the microarrays, place them in a slide holder and dry them overnight in a vacuum oven set at 45 °C to remove the NMP in the polymer spots. After sterilization with UV light for 30 min, the microarrays are ready for inoculation of bacteria.
- Before inoculating slides with bacteria, take a measurement of background fluorescence (as described in section 5). Use this measurement in the calculation of bacterial binding.
4. Inoculation of Polymer Microarrays with Bacteria
NOTE: Ensure good aseptic technique. All handling of cultures should be performed in a sterile environment: either using a Bunsen burner or in a flow hood. Cultures should be grown with oxygen availability, growth medium, and temperature adjusted to the requirements of each species.
- Prepare overnight cultures by inoculating 5 ml Luria-Bertani broth (LB: 10 g L-1 bacto-tryptone, 5 g L-1 NaCl, and 10 g L-1 yeast extract) with a colony from a plate. Incubate overnight at 37 °C, shaking at 200 rpm.
- Prepare freezer stocks of overnight cultures by addition of glycerol (10% concentration overall) and store the stock at -80 °C.
- In order to determine the cell numbers from samples of the thawed bacterial stocks, perform serial dilutions of each stock and grow the bacteria overnight on solid media. Accurate determination of cell numbers in stocks ensures appropriate inoculation of each species6.
- Prepare inocula for microarray. Single species cultures are grown as above and applied directly to the microarrays. BacMix-1 is a bacterial mixture of Klebsiella pneumoniae (K. pneumoniae), Staphylococcus saprophyticus (S. saprophyticus), and Staphylococcus aureus (S. aureus). BacMix-2 is a bacterial mixture consisting of Streptococcus mutans (S. mutans), S. aureus, K. pneumoniae and Enterococcus faecalis (E. faecalis).
- To produce either mixed culture, mix overnights in equal volumes (3 ml each) and dilute four times with fresh LB (to around 50 ml final volume).
- Place UV-sterilized polymer microarrays in rectangular 4-well plates and incubate them with 6 ml of mixed bacterial cultures at 37 °C with gentle agitation (30 rpm) for 5 days.
- After incubation, gently rinse the microarrays twice with phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) and incubate with a solution of 1 µg ml-1 of 4',6-diamidino-2-phenylindole (DAPI) stain in PBS for 30 min.
- Wash the stained microarray slides in a fresh well plate by covering with PBS and swirling gently, changing the PBS once. Dry under a flow of air. Apply a glass cover slip to the microarray slide and seal in place with glue. Once dry, sterilize the outer surface with 70% (v/v) ethanol.
- Safely dispose of cultures according to their containment level.
5. Microarray Imaging and Analysis
- Capture single images for each polymer spot in brightfield and DAPI (Ex/Em = 358 nm / 361 nm) channels. Perform this manually with a fluorescent microscope or using an automated fluorescence microscope (with an X-Y-Z stage controlled by an image acquisition software) fitted with a 20X objective.
NOTE: Refer to the manual18 for operating the software and adjusting the microscope to obtain images using brightfield and DAPI channels. Ensure that the gain and exposure time for image capture for all the microarrays are the same. - Obtain the average fluorescence intensity of each spot in the DAPI channel by choosing the spot area and quantifying the fluorescence with an image processing software.
- For the background fluorescence of each spot, obtain images of the polymer spots on a replicate microarray incubated only with media with no bacteria. Deduct the background fluorescence from the intensity of the spot to obtain fluorescence from bacteria.
- Calculate average normalized value from the four spots, representing each polymer, used for comparing bacteria binding of various polymers6.
- Identify the polymers that exhibit the least bacterial binding (lowest fluorescence) as 'hit' polymers6.
6. Coating of Cover Slips for 'Hit' Validation
- To Coat Coverslips with Polymers:
- Prepare solutions of 'hit' polymers (~2% w/v) in tetrahydrofuran (THF), or other appropriate solvent.
- Spin coat circular glass coverslips of suitable size with the polymer solutions using a spin coater at 2,000 rpm for 10 sec.
NOTE: In absence of a spin coater, coverslips or other materials may be dip coated, although spin coating produces a more uniform surface. - Dry the coated coverslips in a convection oven at 40 °C overnight and sterilize using UV light for 30 min prior to inoculating bacteria.
- To Coat Coverslips with Agarose for Negative Controls:
- Clean coverslips either by plasma treatment for 10 min or immersing in 1 M NaOH for 4 hr.
- Immerse the coverslips in acetonitrile containing 1% (3-aminopropyl) triethoxysilane overnight. Clean the coverslips with acetone 3 times and put into an oven (100 °C) for 1 hr.
- Dip coat the dried coverslips with 1% agarose aqueous solution maintained in a water bath at 60 °C. Place the agarose coated coverslips on flat surface in dust-free ambient conditions for 24 hr and sterilize using UV light for 30 min prior to inoculating bacteria.
7. Attachment and Analysis of Cover Slip Using Scanning Electron Microscope (SEM)
- After sterilization with UV light for 30 min, place the cover slips (polymer/agarose coated or uncoated glass) in a standard 12-well plate or 24-well plate (depending on the size of the coverslips) and incubate with bacteria as described in section 4.
- After incubation with bacteria, wash the uncoated and coated coverslips twice with 0.1 M cacodylate buffer (pH 7.4) and then fix with 2.5% (w/v) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 hr.
- Post-fix samples with 1% (w/v) osmium tetroxide for 1 hr at room temperature and dehydrate with sequential ethanol washes at 50, 70, 90 and 100% (v/v) for 30 min each.
- Dry samples in CO2 according to standard protocols19. Drying time will depend on the nature of the sample.
- Coat samples in a gold/palladium alloy (60/40%) using a sputter coater with current at 30 mA and vacuum at 0.75 Torr. Examine under an electron microscope as per standard protocols20.
NOTE: Ensure adequate safety precautions to manage risks resulting from storage, handling and disposal of Osmium tetroxide, as it is highly toxic. - Visually compare images of the uncoated coverslips and those coated with agarose or the 'hit' polymers to confirm bacterial binding/repelling abilities of the polymers.
NOTE: Resistance of attachment should be easily visible as a reduction of cells present. - Choose the most promising 'hit' non-binding polymers for the coating studies with medical devices (e.g., central venous catheters)6.
8. Selection of a Solvent for Coating Catheters
- Evaluate various solvents for their compatibility with the indwelling part of the catheter, as well as their ability to dissolve the 'hit' polymer. Cut parts of cath-1 and into cylindrical pieces 5 mm in length, and measure with a digital Vernier calliper.
- Evaluate various solvents such as acetic acid, ammonia, acetone, acetonitrile, diethyl ether, tetrahydrofuran (THF), dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), ethanol, methanol, ethylene glycol, toluene and xylene. Immerse catheter pieces in the solvents for 12 hours and evaluate visually for integrity of catheter and clarity of solvent.
NOTE: Choose a solvent that does not cause the catheters to swell or disintegrate or result in a turbid solvent. The solvent must dissolve the 'hit' polymers.
9. Analysis of Bacterial Attachment on Catheters by Confocal Microscopy
- Prepare 2.5% (w/v) polymer solutions in acetone, or other suitable solvent as determined in section 8. Place 5 mm catheter pieces into a small glass vials (5 ml) and immerse them in 1 ml of the polymer solution for 2 min. Remove the excess polymer solution and dry the catheter pieces overnight in a vacuum oven at 40 °C.
- Prepare inoculum by mixing thawed bacterial stocks to give approximately 106 cells of each species in 50 ml LB6.
- Sterilize coated and uncoated catheter pieces with UV light for 30 min and incubate with 1 ml inoculated LB in a 24-well plate, at 37 °C for 3 days, with gentle agitation6.
- After incubation, wash the catheters with PBS (2x, 2 ml), fix the bacteria with 10% paraformaldehyde in PBS for 30 min, and wash with PBS (1 ml). Stain the bacteria on catheter pieces with DAPI (1 µg ml−1) for 20 min and wash with PBS (1 ml).
- Obtain confocal images of the catheter pieces using the following settings: 405nm blue diode laser, detector range 414 to 502 nm, pin hole – Airy1, image Size – 1,024 × 1,024 pixels, voxel width – 105.2 nm, Z-stacks spacing 0.5µ m, magnification – 40X 1.25.
- Complete the confocal imaging by Z-stacking 50 images across 100 µm length of the catheter.
- Analyze the images using suitable analysis software: flatten the Z-stacked images in the Z plane, using the function to extend depth of field, to create a single image of a catheter piece.
NOTE: This image should then be analyzed to obtain the area of the catheter covered by bacteria, after background-correction using the 'flatten background' function.
10. Analysis of Bacterial Attachment on Catheters by SEM
- Prepare 10% polymer solution (w/v) in acetone.
- Press a pipette tip (for 200 µl micropipette) into the mid portion of the cut piece of the catheter to hold it and dip the piece in the polymer solution for approximately 30 sec. Dry the coated piece in ambient conditions for 30 min. Apply a second coating by immersing again into the polymer solution and dry overnight in ambient conditions.
- After UV treatment and incubation with bacteria wash the pieces (n = 3) with PBS (2x, 1 ml) and transfer them into 48-well plates containing 10% formaldehyde in PBS for 30 min. After fixing, wash the pieces with PBS (1 ml), dry overnight at room temperature, mount on stubs with conductive carbon disks, and gold coat using a sputter coater (see step 7.5).
- Obtain images using a scanning electron microscope6.
Representative Results
Figure 2 shows bacterial attachment (normalized fluorescence intensity) to a number of polymers as determined by microarray analysis. Spots printed without polymer (NMP only) are the negative control, as agarose strongly resists bacterial binding6 — recorded fluorescence is very low. The polymers displayed are all low-binding although in most cases, the repellent properties of a polymer varies significantly between bacterial species tested. This reflects the wide differences between attachment mechanisms across different species. The selection of an appropriate polymer is therefore dependent on the application, but the low-binding polymers are easily identified by comparison with agarose. High-binding polymers are likely to be identified in an array, and can be used as positive controls for subsequent experiments. For polymers that do not show auto-fluorescence in the DAPI channel, qualitative comparison of the spot images can be made visually (as shown in Figure 3, highlighting one representative high-binding polymer and three example low-binding polymers). Such comparison, while providing less information than the statistical analysis, can be a useful visual validation of the intensity values calculated.
With 22 appropriate polymers identified using the microarray, scale-up experiments are carried out to confirm their bacteria-repellent property when used for coating larger surfaces. Shown are the best-performing examples, used to coat glass cover slips (analyzed by SEM (Figures 4 and 5)) and catheter slices (analyzed by confocal microscopy (Figure 6) and SEM (Figure 7)). Both microscopic methods have the benefit of allowing direct cell counts on the surface, providing unambiguous data; reduction in cell attachment is easily visible. Those coatings which best retained their repellent properties on scale-up, as well as being amenable to large-scale coating techniques, were investigated further. The results shown illustrate the best-performing polymers from each stage.
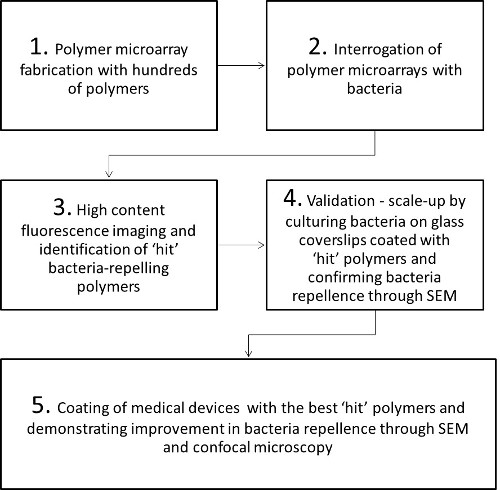
Figure 1: Steps involved in the identification of bacteria repellent polymers through polymer microarrays for biomedical applications. SEM = scanning electron microscopy.
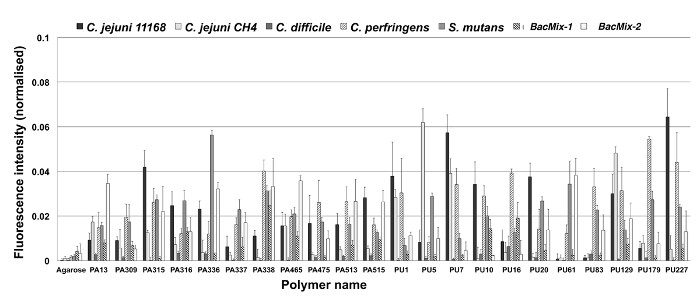
Figure 2: Bacterial binding on a series of polymers, determined by microarray analysis. Low bacterial binding is seen on a number of polyacrylates/acrylamides (PA) and polyurethanes (PU). Results from polymer microarrays probed with several bacterial species (C. jejuni, C. difficile, C. perfringens, S. mutans, and two consortia; BacMix-1 and BacMix-2) are shown. Bacterial binding expressed as background corrected mean DAPI fluorescence intensity (normalized). Error bars represent standard deviation. Adapted from reference6. Please click here to view a larger version of this figure.
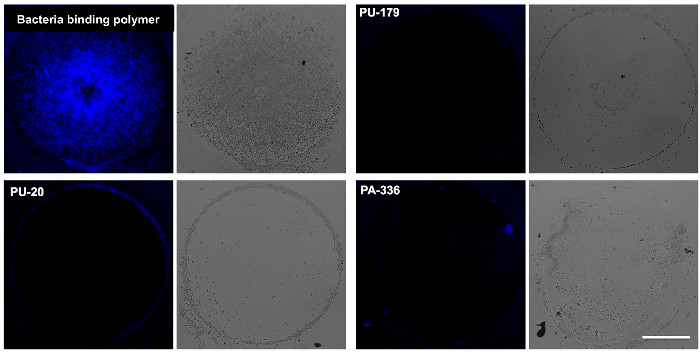
Figure 3: Example images of polymer spots. Fluorescence (DAPI) and brightfield microscopy images of BacMix-2 showing bacterial attachment on representative polymers. A strongly-binding polymer is included for comparison to several non-binding polymers (PU-20, PA-336 and PU-179). Scale bar = 100 µm. Adapted from reference6. Please click here to view a larger version of this figure.
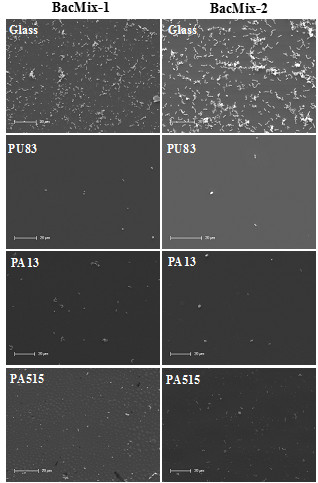
Figure 4: Scale-up experiments with glass cover slips. SEM images showing bacterial attachment on uncoated glass and glass coated with 'hit' polymers (examples chosen from Table 1). Scale bars = 20 µm.
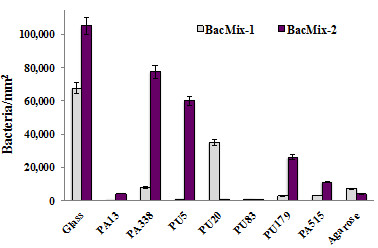
Figure 5: Bacterial binding quantified by cell counts from SEM images. Comparison of cells attached per unit area of surfaces coated with the best 'hit' polymers. Agarose was used as a control 'non-binding' surface, with glass as a control binding surface. Error bars represent standard deviation.
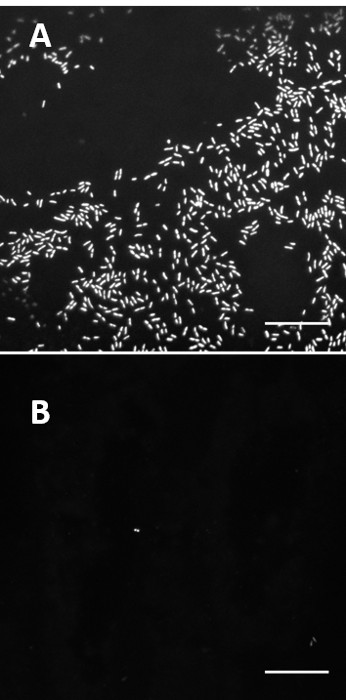
Figure 6: Comparison of bacterial binding on coated and uncoated catheter slices. Confocal images comparing untreated catheter (cath-1) (A) and catheter coated with PA13 (B) after incubation with BacMix-2. Images taken with a 40X objective (Scale bar = 20 µm). Adapted from reference6.
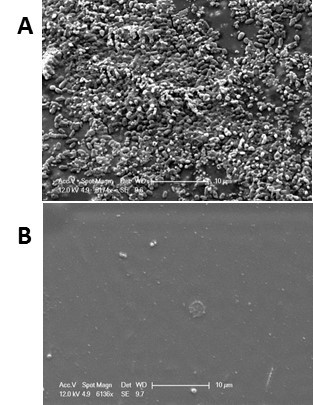
Figure 7: Comparison of bacterial binding on coated and uncoated catheter slices. SEM images show comparison of untreated catheter (cath-1) (A) and catheter coated with PA13 (B) after incubation with a bacterial cocktail consisting of BacMix-2. Scale bars = 10 µm. Adapted from reference6.
| Polymer | Monomer 1 | Monomer 2 | Monomer 3 | Ratio of Monomers | ||
| PA465 | MEMA | DEAEMA | HEA | 8 | 1 | 1 |
| PA475 | MEMA | DEAEA | HEMA | 6 | 1 | 3 |
| PA513 | MEMA | DEAEMA | MMA | 8 | 1 | 1 |
| PA515 | MEMA | DEAEA | MMA | 6 | 1 | 3 |
| PA13 | MMA | DMAA | – | 9 | 1 | – |
| PU1 | PEG2000 | HDI | – | 4.9 | 5.2 | – |
| PU16 | PEG2000 | MDI | – | 4.9 | 5.2 | – |
| PU161 | PEG2000 | MDI | BD | 2.5 | 5.2 | 2.3 |
| PU7 | PEG900 | BICH | – | 4.9 | 5.2 | – |
| PU83 | PEG900 | HMDI | BD | 2.5 | 5.2 | 2.3 |
| PU227 | PPG-PEG-1900 | HDI | – | 4.9 | 5.2 | – |
| PU129 | PPG425 | BICH | DMAPD | 2.5 | 5.2 | 2.3 |
| PU10 | PTMG2000 | BICH | – | 4.9 | 5.2 | – |
| PU179 | PTMG2000 | HDI | NMAPD | 2.5 | 5.2 | 2.3 |
| PU20 | PTMG2000 | MDI | – | 4.9 | 5.2 | – |
| PU5 | PTMG2000 | HDI | – | 4.9 | 5.2 | – |
Table 1: Composition of the bacteria non-binding 'hit' polymers in Figure 2.
Discussion
Attachment of bacteria to a surface is a complex process determined by a wide range of factors dependent on the bacterial species, the properties of the surface, the surrounding medium and the physical environment. Although certain chemical groups are known to affect bacterial binding (polyglycols, for instance, typically resist attachment11), correlating the biological impact of polymers with their chemical structures is difficult, making rational design of polymers for specific functions challenging. In the absence of detailed attachment mechanisms, other studies have attempted to mimic naturally-occurring repellent surfaces, with lengthy and extensive optimization processes21. The miniaturized high-throughput method presented here overcomes these challenges by facilitating parallel screening of hundreds of polymers to identify leads for further study.
Results from the microarray method principally serve to identify likely lead candidates. Figure 2 illustrates 22 candidates with low binding of at least one species, while Figure 3 demonstrates the clear reduction in binding capacity. All 22 low-binding polymers shown in in Figure 2 were taken forward into scale-up experiments, during which the best (in terms of repellence and coating properties) were determined to be PU83, PA13, and PA515 (Figures 4 and 5). Polyacrylates offer greater flexibility in terms of polymerization methods and so the lowest-binding polyacrylate, PA13, was chosen for catheter coating studies (Figures 6 and 7). More detailed further work on other candidates was carried out and has been reported elsewhere6.
Through a number of experimental iterations we found a number of minor steps were key to success and reproducibility. As well as facilitating the adhesion of the polymers to the glass slides, using an agarose under-coating provides a clean background, as agarose is highly resistant to bacterial colonization. Likewise consistency in the polymer spots themselves, both within the same array and between arrays, is vital and therefore the printing of the arrays must be carefully controlled. Careful adjustment of the pins in the print-head and also uniform filling of the 384-well plate are required to ensure uniform spotting. As some of the polymers we used exhibited a degree of autofluorescence, taking background fluorescence data for each slide before incubation with bacteria was vital. To account for variations and to obtain robust data replicates of microarrays are advised.
The stain employed here (DAPI) has no selectivity for bacterial species, binding non-specifically to DNA. Therefore, good aseptic technique is essential once bacterial cultures are introduced as contaminants may go unnoticed, confusing the interpretation of the results. The same is true of later experiments using scanning electron microscopy, where it is only possible to distinguish rods and cocci but not genus or species.
After microarray screening, promising polymers should be chosen for further validation. In the example presented here, seven polymers of interest were identified by their clear reduction in fluorescence on the microarray and their inhibition of attachment was confirmed by coating them on larger surfaces. Figures 4 and 5 show the reduction in binding achieved on glass coverslips, a practical means to test the behavior of the polymers as bulk coatings rather than as microarray spots. Subsequently, these polymers were coated on medical devices to fully quantify reduction in bacterial attachment. It is important that the solvent chosen (see protocol section 8) for these coating studies is benign to the desired substrate (here, the catheter) while retaining ability to dissolve the polymer of interest, in order to allow coating. Here, we used acetone which, as well as the properties mentioned, has a low boiling point and evaporates quickly to leave a uniform coating.
The means of validation chosen will depend on the specific application being studied. As observation of cells by electron and fluorescence microscopy allows direct quantification of individual cell attachment, we chose these techniques as a complement to the bulk staining microarray assay. Results are shown in Figures 6 and 7, which demonstrate the importance of complimentary validation methods. The confocal images in Figure 6 provide very clear images of individual cells, while the SEM has the added benefit of allowing an assessment of the surface of the polymer, which is here smooth and uniform. These methods are limited by the field of view of the microscopes used, and therefore it is important to take a series of snapshots to have confidence in the results. The method described above cannot quantitate bacterial adherence over the entire surface, only infer coverage from a number of small regions. We believe this is sufficient for the application described. Reduction in bacterial binding could be assessed by enumerating surface adhered bacteria on the entire coated and uncoated catheter pieces using methods as described elsewhere22. However such methods require the biomaterial surfaces screened to have a uniform surface area, which is difficult to maintain when assays are performed with medical devices, which often have complex geometry.
Clearly, any device intended for clinical use must go through substantial further testing to ensure safety and efficacy in humans. The method presented here represents the beginning of this process and further work must include confirmation of in vivo activity. In this case, studying venous catheters, initial work could investigate the binding of blood components and whole cells to the polymer. The effect of blood components on bacterial binding should also be considered, possibly by repeating the binding assays in the presence of inactivated serum or de-fibrinated blood23. The definitive test of the technology will be in an in vivo model such as a subcutaneous implant infection model24.
We demonstrate the potential of the polymer microarray method for screening of surface-altering polymers. Such polymers (both resisting and promoting bacterial binding) have a great number of applications in medicine, the food industry and biotechnology, meaning this method may be useful in many areas of research. Although the work here uses bacteria, the method could be adapted to other cell types and likewise other chemical microarrays.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank EASTBIO (the East of Scotland BioScience Doctoral Training Partnership funded by the BBSRC) (S. V.) and the Medical Research Council (P.J.G) for funding.
Materials
| Agarose | Sigma | 05066 | |
| Silane-prep slides | Sigma | S4651 | |
| Polymers | Synthesised in-house | Not applicable | |
| NMP | Sigma | 494496 | |
| LB Broth | Oxoid | CM1018 | |
| DAPI | Thermo Fisher | D1306 | |
| Tetrahydrofuran | Sigma | 401757 | |
| (3-aminopropyl) triethoxysilane coated glass slides | Sigma | Silane-prep | |
| Cacodylate buffer | Sigma | 97068 | |
| Catheter 1 | Arrow International | CS12123E | |
| Catheter 2 | Baxter Healthcare | ECS1320 | |
| Osmium tetroxide | Sigma | 201030 | |
| Equipment | |||
| Contact printer | Genetix | Qarraymini | |
| Microarray microscope | IMSTAR | Pathfinder | |
| Spin Coater | Speedline Technologies | 6708D | |
| Confocal microscope | Leica | SP5 | |
| Image analysis software | Media Cybernetics | Image-Pro Plus | |
| Scanning electron microscope | Philips | XL30CP | |
| Sputter coater | Bal-Tec | SCD050 |
References
- Hansen, A., Mjoseng, H. K., et al. High-Density Polymer Microarrays: Identifying Synthetic Polymers that Control Human Embryonic Stem Cell Growth. Adv. Healthc. Mat. 3 (6), 848-853 (2014).
- Tourniaire, G., Collins, J., et al. Polymer microarrays for cellular adhesion. Chem. Commun. (20), 2118-2120 (2006).
- Pernagallo, S., Wu, M., Gallagher, M. P., Bradley, M. Colonising new frontiers-microarrays reveal biofilm modulating polymers. J. Mat. Chem. 21 (1), 96-101 (2010).
- Hay, D. C., Pernagallo, S., et al. Unbiased screening of polymer libraries to define novel substrates for functionala hepatocytes with inducible drug metabolism. Stem Cell Res. 6 (2), 92-102 (2011).
- Anderson, D. G., Levenberg, S., Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 22 (7), 863-866 (2004).
- Venkateswaran, S., Wu, M., et al. Bacteria repelling poly(methylmethacrylate-co-dimethylacrylamide) coatings for biomedical devices. J. Mat. Chem. B. 2 (39), 6723-6729 (2014).
- Pickering, H., Wu, M., Bradley, M., Bridle, H. Analysis of Giardia lamblia Interactions with Polymer Surfaces Using a Microarray Approach. Environ.l Sci. Technol. 46 (4), 2179-2186 (2012).
- Mizomoto, H. The Synthesis and Screening of Polymer Libraries using a High Throughput Approach. , (2004).
- How, S. E., Yingyongnarongkul, B., Fara, M. A., Díaz-Mochón, J. J., Mittoo, S., Bradley, M. Polyplexes and lipoplexes for mammalian gene delivery: from traditional to microarray screening. Comb. Chem. High Throughput Screen. 7 (5), 423-430 (2004).
- Thaburet, J. -. F., Mizomoto, H., Bradley, M. High-Throughput Evaluation of the Wettability of Polymer Libraries. Macromol. Rapid Commun. 25 (1), 366-370 (2004).
- Hook, A. L., Chang, C. -. Y., et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol. 30 (9), 868-875 (2012).
- Hall-Stoodley, L., Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 11 (7), 1034-1043 (2009).
- Lewis, K. Persister Cells. Ann. Rev. Microbiol. 64 (1), 357-372 (2010).
- Sauer, K., Camper, A. K., Ehrlich, G. D., Costerton, J. W., Davies, D. G. Pseudomonas aeruginosa Displays Multiple Phenotypes during Development as a Biofilm. J. Bacteriol. 184 (4), 1140-1154 (2002).
- Reisner, A., Haagensen, J. A. J., Schembri, M. A., Zechner, E. L., Molin, S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48 (4), 933-946 (2003).
- Watnick, P. I., Kolter, R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34 (3), 586-595 (1999).
- Campoccia, D., Montanaro, L., Arciola, C. R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 34 (34), 8533-8554 (2013).
- Imstar. . Pathfinder Technology for High Content Cellular Screening in Cell Biology and Genetoxicity Software version: 6.04I. , (2012).
- Williams, J. R., Clifford, A. A. . Supercritical Fluid Methods and Protocols. 13, (2000).
- Kuo, J. . Electron microscopy methods and protocols. , (2007).
- Leslie, D. C., Waterhouse, A., et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 32 (11), 1134-1140 (2014).
- Bechert, T., Steinrücke, P., Guggenbichler, J. P. A new method for screening anti-infective biomaterials. Nat. Med. 6 (9), 1053-1056 (2000).
- May, R. M., Magin, C. M., et al. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin. Transl. Med. 4, (2015).
- Chen, R., Willcox, M. D. P., Ho, K. K. K., Smyth, D., Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials. 85, 142-151 (2016).

