Whole Mount Immunofluorescence and Follicle Quantification of Cultured Mouse Ovaries
Summary
Here, we present a protocol to quantify follicles in cultured ovaries of young mice without serial sectioning. Using whole organ immunofluorescence and tissue clearing, physical sectioning is replaced with optical sectioning. This method of sample preparation and visualization maintains organ integrity and facilitates automated quantification of specific cells.
Abstract
Research in the field of mammalian reproductive biology often involves evaluating the overall health of ovaries and testes. Specifically, in females, ovarian fitness is often assessed by visualizing and quantifying follicles and oocytes. Because the ovary is an opaque three-dimensional tissue, traditional approaches require laboriously slicing the tissue into numerous serial sections in order to visualize cells throughout the entire organ. Furthermore, because quantification by this method typically entails scoring only a subset of the sections separated by the approximate diameter of an oocyte, it is prone to inaccuracy. Here, a protocol is described that instead utilizes whole organ tissue clearing and immunofluorescence staining of mouse ovaries to visualize follicles and oocytes. Compared to more traditional approaches, this protocol is advantageous for visualizing cells within the ovary for numerous reasons: 1) the ovary remains intact throughout sample preparation and processing; 2) small ovaries, which are difficult to section, can be examined with ease; 3) cellular quantification is more readily and accurately achieved; and 4) the whole organ imaged.
Introduction
In order to study the cellular composition and morphological features of mammalian ovaries, scientists often rely on in vivo experiments followed by immunohistological staining of paraffin embedded ovaries. More recently though, whole ovary organ culture has proven to be an effective alternative to study ovarian function1,2,3,4 because the technique can be coupled with better visualization and quantification tools. Traditionally, analysis of ovarian morphology depends on reconstructing three-dimensional ovarian architecture from paraffin embedded serial sections, but, in addition to being laborious and time consuming, serially sectioning paraffin embedded tissue does not guarantee proper reconstruction of the organ, and sections are often lost or mis-ordered in the process.
In addition to the technical challenges associated with serial sectioning, there are also variations in the methods routinely used to quantify follicle numbers per ovary5,6. The methodological variability currently used impairs meta-analysis of ovarian reserve across studies5,7. For example, follicle numbers from different research articles can vary by 10-fold or more between similar developmental ages within a specific strain6. These large differences in reported follicle quantification can lead to confusion and have hindered cross-study comparisons. Experimentally, traditional approaches to follicle quantification from serial sections are performed by counting follicles of a pre-defined number of sections (e.g., every fifth, tenth, or other section). Variability in follicle counts using this approach arises not only from the periodicity in which sections are counted but also from variations in section thickness, and technical experience in generating serial sections5,6. In addition to its variability, another disadvantage of traditional tissue sectioning is that the sectioning of small ovaries from young animals is especially challenging and highly dependent on tissue orientation8.
The protocol below describes a routinely used ovary culture technique1 but greatly improves upon traditional follicle quantification by substituting physical sectioning with tissue clearing and optical sectioning using confocal microscopy8,9. Clearing using tissue immersion (without the need for transcranial perfusion or electrophoresis) in a urea- and sorbitol-based solution (e.g., ScaleS(0)10) proved compatible with the immunostaining and allowed for the reduction of clearing time without compromising depth of imaging. Other reported methods (e.g., ScaleA28,10, SeeDB11, ClearT12, and ClearT212) are either more time consuming or do not allow in-depth optical resolution of the sample. Optical sectioning is advantageous because it is less labor intensive and maintains the organ's three-dimensional architecture7,8. Another benefit of this approach is that preparation of the samples does not require costly reagents to clear the tissue and can be conducted with relative ease.
Specifically, the protocol described has been optimized for cultured mouse ovaries at postnatal day five but has been conducted on ovaries ranging from postnatal day 0 – 10. The method makes use of an ovary culture system in which the tissue naturally attaches to the membrane on which it is cultured, facilitating organ handling and manipulation. The culture system described can be used to maintain explanted ovaries for up to 10 days and to assess how different experimental conditions may interfere with oocyte survival13. The quantification procedure described is performed using the non-commercial image processing package FIJI-ImageJ14 and can be conducted on most personal computers. Furthermore, images used for quantification can be made widely available for the scientific community, thus allowing for future meta-analysis.
Protocol
Cornell University's Institutional Animal Care and Use Committee (IACUC) has approved all the methods described here, under protocol 2004-0038 to JCS.
1. Preparation of Instruments and Culture Media
- Wipe the working area clean with 10% bleach and allow the bleach to remain on the work area surface for at least 5 min. After 5 min, remove the excess bleach with clean paper towels and 70% ethanol (EtOH). Clean the dissecting microscope thoroughly with 70% EtOH.
NOTE: It is important that the work area be at least semi-sterile to avoid contamination of organ cultures. - Wrap clean forceps and scissors with a paper towel dampened with 70% EtOH until the time of use.
NOTE: The ideal dissection tools may vary according to the age/size of the ovary. Best results are obtained using one sharp micro dissecting scissor, two fine tip forceps (either number 5 or 55) and one micro dissecting iris scissor. - In a conical tube, make 50 mL of ovary culture media and warm in a 37 °C water bath.
- To make ovary culture medium4, supplement 1x Minimal Essential Media (MEM) with 10% Fetal Bovine Serum (FBS), 25 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 100 units/mL penicillin, 100 µg/mL streptomycin, 0.25 µg/mL Amphotericin B (e.g., 45 mL of MEM, 5 mL of heat inactivated FBS, 1.25 mL of HEPES, and 0.5 mL of 100x stock of penicillin, streptomycin, and Amphotericin B).
- Prepare plates and inserts prior to the ovary culture.
- In a tissue culture hood, add 1 mL of ovary culture medium to a 35 mm tissue culture plate.
- Pre-soak insert membrane with the culture medium. Add sufficient media, usually about 0.55 mL per well, to the 24-well carrier tissue culture plate, and place a cell culture insert in the well. Make sure the insert's membrane touches the media.
- Prepare the number of 35 mm plates and wells with cell culture inserts according to the expected number of ovaries in the experiment. Place the 35 mm plates containing 1 mL of culture media and the 24-well carrier plate containing culture media and inserts in a 37 °C, 5% CO2, and atmospheric O2 incubator.
- Arrange a hot plate next to the dissection scope and set the temperature to 37 °C. Keep a 37 °C, 5% CO2 and the atmospheric O2 incubator is close to the dissection room.
2. Ovary Dissection and Organ Culture
NOTE: Ovaries can be dissected at room temperature as long as the exposure to the non-ideal temperature is minimal.
- Euthanize the female mice at post-natal day 5, by decapitation, one at a time.
NOTE: Euthanasia must be conducted according to IACUC guidelines present at the facility where the research is being performed. - Spray the animal with 70% EtOH. Place the animal on the top of the dry paper towel under the dissection microscope. Place a 35 mm tissue culture dish containing ovary culture media close to the dissection microscope.
- With forceps or a micro dissection iris scissor, make an incision through the skin of the animal and into the abdominal cavity, using caution not to cut into the intestines. Push the intestines out of the abdominal cavity and locate the ovaries. Extract the ovaries and place them into the 35-mm tissue culture dish containing culture media.
- Once the ovaries have been dissected from the animal, increase the magnification of the dissection microscope in order to better visualize ovaries and any attached tissue. With clean fine tip forceps, remove all non-ovarian tissue, such as bursa and fatty tissue. Be careful to keep the ovaries intact when removing the attached non-ovarian tissue.
- Once the ovaries are isolated, place the pair into the pre-soaked cell culture inserts of the carrier 24-well plate (refer to steps 1.4.2 and 1.4.3) and adjust the volume of the medium to ensure that it is sufficient to keep the organ moist (without completely submerging it).
Caution: Be careful not to poke a hole in the membrane of the cell culture insert when transferring the ovaries. - Place explanted organs in a 37 °C, 5% CO2, and atmospheric O2 incubator.
- Repeat steps 2.1 – 2.6 until the ovaries of each animal are dissected and placed on the cell culture insert of the 24-well plate containing the ovary culture medium.
- Change the ovary culture medium every 2 days, using warmed ovary media aliquots from a 37 °C water bath and proceed to Part 3 of the protocol at the desired experimental time point.
3. Tissue Fixation
- In a 15 mL conical tube, prepare 4% paraformaldehyde (PFA) in 1x phosphate-buffered saline (PBS) (e.g., add 2 mL of 16% PFA electron microscopy grade to 6 mL of 1x PBS).
Caution: PFA is a hazardous substance and should be handled under a chemical hood. - Fix the cultured ovaries, without removing the tissue from the culture insert, by covering the tissue with the freshly prepared 4% PFA solution. Seal the edges of the plate with plastic adhesive (to avoid evaporation) and place the samples at 4 °C overnight.
NOTE: In order to facilitate handling and tissue integrity, avoid displacing ovaries from the cell culture insert throughout the entire procedure. Cultured ovaries attached to the surface of the insert are advantageous for handling without damaging or losing the organ. - Rinse the tissue 3x with 70% EtOH and either proceed to step 4.1 or store it in scintillation vials filled with 70% EtOH at 4 °C until further processing.
NOTE: If using tissue endogenously expressing fluorescent proteins, storing the samples with 70% EtOH may greatly reduce the signal. Alternatively, tissue can be rinsed and stored in the PBS with 0.2% sodium azide (NaN3). NaN3 is, however, highly toxic and poses a serious inhalation hazard. Make the stock solution in the fume hood and handle wearing appropriate personal protective equipment such as a laboratory coat and nitrile gloves.
4. Whole Mount Immunofluorescence
- Place the fixed tissue in 1x PBS at RT for a minimum of 4 h prior to the permeabilization step 4.3.
- Prepare permeabilization solution and blocking solution.
- Prepare permeabilization solution as described. To 1x PBS, add 0.2% polyvinyl alcohol (PVA), 0.1% sodium borohydride (NaBH4) solution (from 12 wt.% in 14 M sodium hydroxide (NaOH) solution), and 1.5% polyethylene glycol tert-octylphenyl ether (non-ionic surfactant detergent). In a 50 mL conical tube, add 5 mL of 10x PBS, 0.1 g of PVA, 50 µL of NaBH4, and 750 µL of non-ionic surfactant detergent, then add ultrapure water up to 50 mL and agitate well at room temperature until the mixture is entirely in solution.
- Prepare blocking solution as described. To 1x PBS, add 0.1% non-ionic surfactant detergent, 0.15% of 2.5M glycine pH 7.4, 10% normal goat serum, 3% bovine serum albumin (BSA), 0.2% NaN3, 100 units/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL Amphotericin B. Prepare a stock solution of 2.5 M glycine by dissolving 93.8 g of glycine in ultrapure water to a 500 mL final volume (pH 7.4) and a stock solution of 10% NaN3 by dissolving 5 g in 50 mL of ultrapure water; then, in a 50 mL conical tube prepare the blocking solution by adding 5 mL of 10x PBS, 50 µL of non-ionic surfactant detergent, 750 µL of glycine stock solution, 5 mL of goat serum, 1.5 g of BSA, 1 mL of NaN3 stock solution, and 0.5 mL of 100x stock of penicillin, streptomycin, and Amphotericin B . Add ultrapure water up to a final volume of 50 mL and syringe filter the final solution.
- Remove and discard the 1x PBS from the vial, and then add enough permeabilization solution to completely submerge the ovaries. Place the sample in permeabilization solution on an orbital shaker (e.g., nutator) at room temperature for 4 h.
NOTE: Incubation time may vary according to the organ thickness. - Replace the permeabilization solution with enough blocking solution to completely submerge the ovaries. Seal the vial with the plastic adhesive and leave the tissue incubating for a minimum of 12 h at room temperature on an orbital shaker.
- Prepare primary antibody dilution in blocking solution as per the manufacturer's datasheet.
NOTE: Note that not all antibodies are compatible with the clearing agent. The average volume needed per sample is approximately 750 µL.- For oocyte quantification, use TAp63 (nuclear oocyte marker) and MVH (cytoplasmic germ cell marker).
NOTE: The antibodies used in the immunofluorescence images are mouse anti-p63 and rabbit anti-MVH). The primary antibody solution can be reused 2x or more if stored at 4 °C between staining protocols and properly handled to avoid bacterial growth.
- For oocyte quantification, use TAp63 (nuclear oocyte marker) and MVH (cytoplasmic germ cell marker).
- Place the insert containing the tissue in a 24-well plate and add about 750 µL of primary antibody solution. Ensure that ovaries are completely submerged. Seal the plate with plastic adhesive to minimize evaporation and leave the tissue incubating for 4 days at room temperature on an orbital shaker.
- Prepare washing solution.
- Prepare washing solution as described. Make 1x PBS, add 0.2% PVA and 0.15% non-ionic surfactant detergent. In a 50 mL conical tube, add 5 mL of 10x PBS, 0.1 g of PVA, 75 µL of non-ionic surfactant detergent and ultrapure water up to a final volume of 50 mL.
- Remove primary antibody dilution and add a generous amount of washing solution. Always make sure to submerge the ovaries. Allow the ovaries to soak in the solution overnight at room temperature on the orbital shaker.
- Replace the washing solution and incubate on the orbital shaker for 2 h or more.
- Repeat the step 4.9 one additional time.
- Prepare the secondary antibody dilution in blocking solution.
NOTE: The secondary antibodies used are anti-mouse 488 fluorescent dye raised in goat and anti-rabbit 594 fluorescent dye raised in goat. See step 4.5. for the average volume needed per sample. - Remove washing solution from the ovaries and add secondary antibody solution. Protect the samples from light (wrap plate with aluminum foil) and seal the plate with plastic adhesive to minimize evaporation. Incubate samples on an orbital shaker at room temperature for 2 days.
NOTE: Continue protecting the samples from light using aluminum foil during all subsequent incubation steps. - Remove secondary antibody solution from the samples and add washing solution. Incubate on an orbital shaker for 8 h.
NOTE: If 4,6-Diamidino-2-phenylindole (DAPI) staining is desired, add 50 ng/mL of DAPI to the first wash step (~8 h incubation). - Replace the washing solution for an overnight wash.
- While preparing the clearing solution (see section 5), replace the overnight washing solution with fresh solution and keep the samples on the orbital shaker at room temperature.
5. Ovary Clearing and Imaging
- Prepare ScaleS(0) clearing solution.
- Prepare ScaleS(0) clearing solution10 by adding reagents in the given proportion: 40% D-(-) sorbitol (w/v), 10% glycerol, 4.3 M urea, and 20% dimethyl sulfoxide (DMSO), pH 8.1 (e.g. in a 50 mL conical tube, add 2.5 mL of glycerol, 10 g of sorbitol, 5 mL of DMSO, and 12 mL of 9 M urea for a total volume of 25 mL. Mix solution by inversion at 50 °C for 30 min and degas prior to use.)
- Remove the washing solution and submerge the samples in clearing solution. For better results maintain samples on the orbital shaker.
- Replace the clearing solution 2x daily until the tissue becomes transparent (approximately 2 days).
- Once the tissue is cleared, prepare the sample for imaging by carefully removing the insert membrane containing the cultured ovaries with a fine tip scalpel and placing the sample on a glass slide.
- Proceed to imaging the samples on a microscope capable of optical sectioning.
6. Oocyte Quantification
NOTE: There are many different computer programs that are able to quantify cells. Described below is a protocol for oocyte quantification using the non-commercial image processing package, FIJI-ImageJ14.
- Using a flattened maximum intensity projection of the Z-stack image series for the specific cellular markers of interest (without the DAPI channel), convert the image into a black and white 8-bit image under Type in the Image drop-down menu.
- Under the Image drop-down menu, highlight the Adjust tab and then select Threshold.
- Manually adjust the threshold to a level that best identifies each individual oocyte. Once the level is determined, click Apply to generate a black and white image. Once completed, quantify the sample by delineating using the Oval or Freehand icon.
NOTE: FIJI-ImageJ has different options to fine tune the image that will be used for the particle quantification. For instance, in Process | Binary tab, there are different options that can be used to improve the detection of what will be counted. In Figure 4, the options used to better individualize follicles was Erode, followed by Fill holes, and lastly Watershed. - Under the Analyze drop-down menu, select Analyze particles. Set the parameters according to the desired resolution.
Representative Results
This protocol includes 6 major steps following dissection of the ovaries, as outlined in Figure 1. Figure 2, Figure 3, Figure 4 highlight the most novel features of this protocol, which include optimization of tissue clearing for ovaries and whole tissue oocyte quantification using FIJI-ImageJ. Figure 2A shows images of an uncultured 5-day postnatal fixed ovary before (left) and 1 h after (right) adding clearing solution to the ovary. The ovary will begin to become translucent within minutes of being submerged in clearing solution. Once cleared, small ovaries like the one imaged in Figure 2A become difficult to handle without damaging. Therefore, working with cultured explanted ovaries is advantageous because they become attached to the porous surface of the insert membrane on which they are cultured. Attachment of the ovary to the insert membrane allows the experimenter to handle the membrane insert rather than the ovary itself (Figure 2B and Figure 3). Also, ovaries cultured in close proximity will fuse and Figure 2B shows attached fused ovaries before (left) and after clearing (right) on hematoxylin stained samples.
Performing the optical sectioning of cultured ovaries without clearing is possible; however, cells deep within the tissue have a signal that is difficult to differentiate from the background and this lack of clear signal impedes proper oocyte quantification (Figure 3A). In contrast to Figure 3A, Figure 3B is a representative image of a cleared sample in which oocyte quantification throughout the entire organ is possible. Figure 3B demonstrates that whole organ imaging of cleared cultured ovaries can be conducted without significant loss of signal deep within the tissue and images such as the one shown (Figure 3B) can be readily quantified. One approach for quantifying oocytes in samples such as these is by converting the Z-stack series of optical sections into a Maximum Intensity Projection and then further processing the file with an image processing package. Figure 4 and Supplemental Figure 1 highlight the steps used to quantify the number of oocytes in Figure 3B using FIJI-ImageJ. Supplemental Table 1 includes FIJI-ImageJ's particle (oocyte) quantification. Quantification of particles using this method also allows for the analysis of different follicles based on oocyte size, because information on both the number of particles and the corresponding area of each particle is calculated by the software and provided to the user.
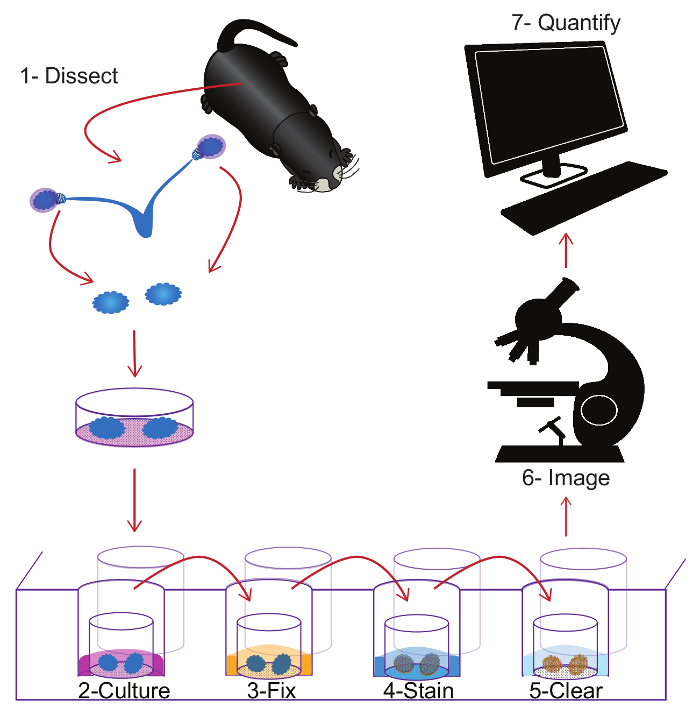
Figure 1: Schematic representation of the entire protocol. Visual summary of the protocol beginning with dissection of the ovary (1), followed by ovary culture (2), tissue fixation with 4%PFA (3), immunostaining using specific antibodies (4), clearing tissue for deep imaging (5), obtaining the optical section using a confocal microscope (6) and ending with oocyte quantification (7). Please click here to view a larger version of this figure.
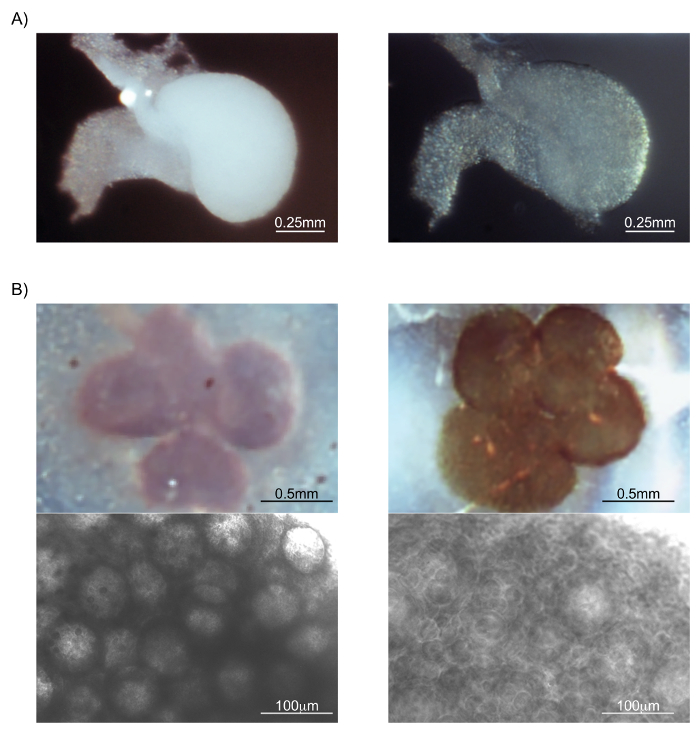
Figure 2: Difference in tissue opacity between un-cleared and cleared ovaries. (A) Ovary from a postnatal day five pup without clearing (left) and after mild clearing of about 1 h (right). (B) Multiple ovaries cultured in close proximity to each other for seven days. Two different magnifications of each sample are shown. Leftmost images are without clearing and rightmost images are with clearing. Ovaries will attach to the membrane of the culture insert and can be better handled if kept attached throughout the protocol. In order to improve the contrast of tissue for imaging using white light, ovaries were submerged in hematoxylin for 5 min after fixation. Please click here to view a larger version of this figure.
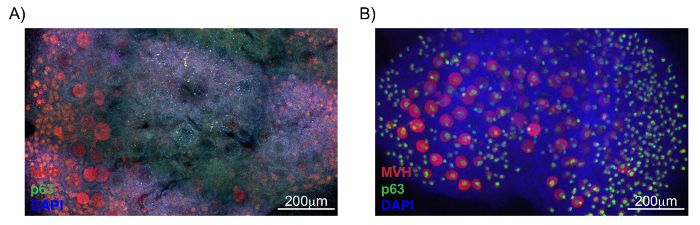
Figure 3: Immunofluorescence imaging of un-cleared and cleared ovaries. Ovaries from postnatal day 5 pups were cultured for 7 days and stained according to the whole mount immunofluorescence staining protocol described. Shown in red are cells labeled with mouse vasa homolog (MVH) to identify germ cells and in green are cells labeled with the nuclear oocyte-specific marker, p63. DAPI, in blue, was used to label all cell nuclei. (A) Immunofluorescence image of ovaries without clearing. (B) Immunofluorescence image of ovaries with clearing. Please click here to view a larger version of this figure.
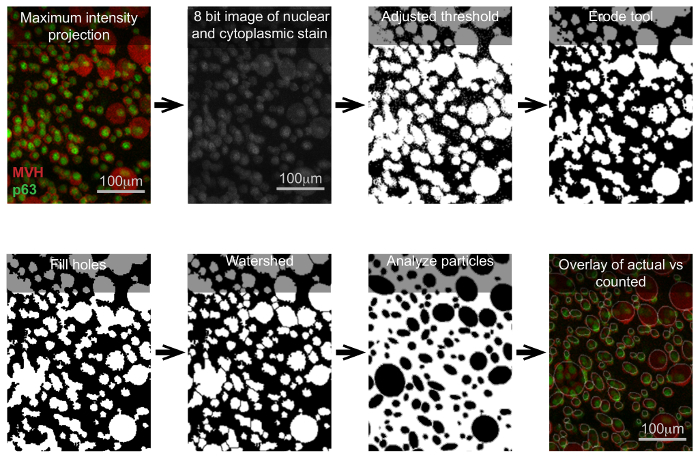
Figure 4: Visual workflow for how to quantify oocytes using FIJI-ImageJ software. In order to facilitate data analysis, images derived from the confocal planes can be reduced to a maximum intensity projection instead of a 3D image. With the maximum intensity projection, the user can define image threshold parameters in such a way that the target particles become evident. Once the simplified image is obtained, the software is used to count the oocytes. Critically examining images while setting the parameters is crucial. This figure shows an example of how the particle/oocyte threshold can be set. The text above each image highlights the parameter used to obtain that image in FIJI-ImageJ. The software generates a table with the area measurement of the particles (see Supplemental Table 1). Please click here to view a larger version of this figure.
Supplemental Figure 1: Visual workflow for how to quantify oocytes using FIJI-ImageJ software (lower magnification). This figure shows oocyte quantification for two ovaries in close proximity. The steps performed are the same as in Figure 4. 2,436 particles/follicles were counted by the software. Quantification data obtained from the software can be found in Supplemental Table 1. Please click here to download this file.
Supplemental Table 1: Follicle quantification computed by FIJI-ImageJ. This table contains the list of counted follicles and the corresponding area generated from the image in Supplemental Figure 1. The particle size used for quantification was set from 10 µm2-infinity. Please click here to download this file.
Discussion
The study of mammalian reproduction requires using and quantifying specialized cells that are not routinely amenable to cell culture. However, ex vivo culture systems are effective at maintaining ovary and follicle viability1,15. During ovary culture, the tissue requires a larger surface area for exchange of nutrients through diffusion. Therefore, 5-day old mouse ovaries are ideal in size and shape for organ culture. This protocol was optimized for ovaries maintained for seven days in culture, but ovary culture length can be adjusted according to the specific scientific question1,4. Comparable results have been achieved in ovaries cultured for as little as three days and as long as ten days (data not shown), but ovaries from animals older than ten days are large and culture may not be as effective, thus highlighting a limitation of the protocol.
If ex vivo culturing of the ovary is not needed, it is possible to fix and stain older ovaries with the protocol described, although modifications may be required to adjust to the larger tissue size9,16. The staining protocol described depends on passive diffusion of the antibodies into the organ, which indicates that it may be possible to stain larger ovaries with either longer antibody incubation steps or by partitioning the tissue into few smaller pieces that can be reconstructed after imaging. The use of urea and sorbitol in the ScaleS(0) clearing agent proved effective for neonatal cultured ovaries because it required a shorter incubation period than those reported for clearing agents with urea and glycerol (ScaleA2)8,10. Furthermore, the use of a low concentration of sodium borohydride (NaBH4) in the permeabilization solution decreased the background noise, and, together with longer incubations of the samples with primary and secondary antibodies, facilitated staining deep within the tissue. Additionally, the use of PVA in the permeabilization and washing solutions prevents spurious particles from sticking to the tissue17,18.
In this protocol, cultured healthy ovaries will attach to the insert membrane, while unhealthy ovaries may detach from it upon fixation and handling. Handling loose tissue with forceps will damage the ovary and likely compromise imaging. Alternatively, loose tissue can be embedded in 5% agarose plugs or handled with transfer pipettes as long as the tip opening is wide enough not to damage it. With regard to immunostaining, primary antibodies other than the ones used in this protocol may perform differently and may require optimization in the permeabilization and incubation steps.
Lastly, the protocol describes an alternative approach towards quantifying follicles from young ovaries as compared to traditional paraffin embedded serial sections or other previously described volumetric quantification of follicles from whole mount images5,9,16. Depending on the researcher's requirements, the procedure for oocyte quantification described in the protocol can also be used the characterize different stages of follicular development19. The particle area generated by the software can be used to determine the cell diameter and thus infer follicular stage.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Rebecca Williams and Johanna Dela Cruz from the Cornell BRC Imaging and Andrew Recknagel for helpful suggestions and technical assistance. This work was supported to National Institutes of Health grant S10-OD018516 (to Cornell's Imaging Facility), T32HD057854 to J.C.B. and R01-GM45415 to J.C.S.
Materials
| Micro dissecting scissor 4.5", straight, sharp points | Roboz | RS-5912 | Micro dissecting scissor |
| Jewelers style forceps 4-3/8", style 5 | Integra Miltex | 17-305X | Fine tip forceps |
| Micro Iris Scissors 4", straight, sharp points | Integra Miltex | 18-1618 | Micro dissecting iris scissor or micro dissecting spring scissor |
| 1X Minimal Essential Media (MEM) | ThermoFisher Scientific | 11090-081 | |
| Fetal Bovine Serium | Corning | 35011CV | |
| HEPES (1M) | Gibco | 15630080 | |
| Antibiotic-Antimycotic (100X) | Gibco | 15240062 | Used in Step 1.3.1 |
| 35mm Tissue Culture Treated Dish | Corning | 430165 | |
| Nunc™ 24-Well Carrier Plate for Cell Culture Inserts | ThermoFisher Scientific | 141006 | Pore size: 8μm |
| Paraformaldehyde (PFA) | Electron Microscopy Sciences | 15710 | 16% solution |
| 1X Phosphate-Buffered Saline (PBS) | Gibco | 10010023 | |
| Nutator mixer GyroTwister™ | Labnet | S1000-A-B | three dimensional shaker |
| Normal Goat Serum | VWR | 103219-578 | |
| Bovine Serum Albumen (BSA) | VWR | 97061-416 | |
| Sodium Azide (NaN3) | Sigma-Aldrich | S2002-25G | |
| Sodium borohydride solution (NaBH4) | Sigma-Aldrich | 452904-25ML | Use the solution, rather than the tablet or powder form |
| Polyvinyl Alcohol (PVA) | Sigma-Aldrich | P8136-250G | Cold water soluble |
| Triton™ X-100 | Sigma-Aldrich | 93443-100ML | polyethylene glycol tert-octylphenyl ether |
| Syringe filters | ThermoFisher Scientific | 725-2520 | 25mm |
| 10mL Syringes | BD | 309695 | |
| Mouse anti-p63 antibody (4A4) | Biocare Medical | CM 163A | Dilution 1:500 |
| Rabbit anti-MVH antibody | Abcam | ab13840 | Dilution 1:600 |
| Alexa Fluor® goat anti-mouse 594 | ThermoFisher Scientific | A-11032 | Dilution 1:1000 |
| Alexa Fluor® goat anti-rabbit 488 | ThermoFisher Scientific | A-11034 | Dilution 1:1000 |
| 4,6-Diamidino-2-phenylindole (DAPI) | ThermoFisher Scientific | 62248 | |
| D-(-)sorbitol | Sigma-Aldrich | 240850-100G | |
| Glycerol | Sigma-Aldrich | G9012-100ML | |
| Urea | Sigma-Aldrich | U5378-500G | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2650-5X10ML | |
| FIJI-ImageJ | Image processing software | ||
| Disposable plastic transfer pipettes | VWR | 414044-034 |
References
- O’Brien, M. J., Pendola, J. K., Eppig, J. J. A Revised Protocol for In Vitro Development of Mouse Oocytes from Primordial Follicles Dramatically Improves Their Developmental Competence. Biology of Reproduction. 68 (5), 1682-1686 (2003).
- Morgan, S., Campbell, L., Allison, V., Murray, A., Spears, N. Culture and Co-Culture of Mouse Ovaries and Ovarian Follicles. Journal of Visualized Experiments. (97), (2015).
- Livera, G., Rouiller-Fabre, V., Valla, J., Habert, R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Molecular and Cellular Endocrinology. 165 (1), 225-231 (2000).
- Livera, G., Petre-Lazar, B., Guerquin, M. -. J., Trautmann, E., Coffigny, H., Habert, R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 135 (1), 3-12 (2008).
- Tilly, J. L. Ovarian follicle counts – not as simple as 1, 2, 3. Reproductive Biology and Endocrinology. 1, 11 (2003).
- Bucci, T. J., Bolon, B., Warbritton, A. R., Chen, J. J., Heindel, J. J. Influence of sampling on the reproducibility of ovarian follicle counts in mouse toxicity studies. Reproductive Toxicology. 11 (5), 689-696 (1997).
- Skodras, A., Marcelli, G. Computer-Generated Ovaries to Assist Follicle Counting Experiments. PLOS ONE. 10 (3), e0120242 (2015).
- Malki, S., Tharp, M. E., Bortvin, A. A Whole-Mount Approach for Accurate Quantitative and Spatial Assessment of Fetal Oocyte Dynamics in Mice. Biology of Reproduction. 93 (5), (2015).
- Feng, Y., et al. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Scientific Reports. 7, (2017).
- Hama, H., et al. ScaleS: an optical clearing palette for biological imaging. Nature Neuroscience. 18 (10), 1518-1529 (2015).
- Ke, M. -. T., Fujimoto, S., Imai, T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nature Neuroscience. 16 (8), 1154-1161 (2013).
- Kuwajima, T., Sitko, A. A., Bhansali, P., Jurgens, C., Guido, W., Mason, C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development. 140 (6), 1364-1368 (2013).
- Rinaldi, V. D., Hsieh, K., Munroe, R., Bolcun-Filas, E. M., Schimenti, J. C. Pharmacological Inhibition of the DNA Damage Checkpoint Prevents Radiation-Induced Oocyte Death. 遗传学. , (2017).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Obata, Y., Kono, T., Hatada, I. Oogenesis: Maturation of mouse fetal germ cells in vitro. Nature. 418 (6897), 497 (2002).
- Faire, M., et al. Follicle dynamics and global organization in the intact mouse ovary. 发育生物学. 403 (1), 69-79 (2015).
- Byrne, C., Hardman, M. J. Whole-Mount Assays for Gene Induction and Barrier Formation in the Developing Epidermis. Epidermal Cells. , 127-136 (2005).
- Lauter, G., Söll, I., Hauptmann, G. Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural Development. 6, 10 (2011).
- Laronda, M. M., et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nature Communications. 8, (2017).

