Rapid Collection of Floral Fragrance Volatiles using a Headspace Volatile Collection Technique for GC-MS Thermal Desorption Sampling
Summary
Here, we present a protocol for collecting the floral fragrance volatiles from blooming flowers, using a non-destructive sampling procedure.
Abstract
Fragrances of many flower families have been sampled and the volatiles analyzed. Knowing the compounds that make up the fragrances can be an important step to conservation of flowers that are threatened or endangered. Because floral fragrance is critical for attracting pollinators, this method could be used to better understand or even enhance pollination. We present a protocol using a portable charcoal air filter and vacuum to collect floral fragrance volatiles, which are then analyzed by a GC-MS. By using this method, fragrance volatiles can be sampled using a non-destructive method with a machine that is easily transported. This methodology uses a rapid sampling procedure, cutting sampling time down from 2-3 hours to approximately 10 minutes. Using GC-MS, the fragrance compounds can be identified individually, based on authentic standards. The steps used for collecting fragrance and control data are presented, from material setup to collecting the data output.
Introduction
Flowers typically produce a fragrance used to attract pollinators. These fragrances are made up of many chemical compounds all acting together as a floral blend1,2,3. Without these fragrances, flowers would be less likely to pass on their genetic information using pollinators. Floral fragrance has been documented in many flowering plant families, with Orchidaceae being one of the more common families studied4. To understand the role of floral fragrance in pollination, it is important to nondestructively collect and analyze the chemical compounds being emitted from the flowers at different times of the day and during the several days to weeks the flower blooms are open, as fragrance can vary over time5.
An early protocol for this kind of sampling was developed by Heath and Manukian6. The goal of their sampling methods was to reduce stress on the specimen (e.g., plants, insects) being studied. Earlier papers documented that destructive procedures to the plant were required, such as removing blooming flowers in order to collect the fragrance. More recent floral fragrance publications by Cancino and Damon7,8 used similar methods. This study put the flowers in glass chambers and passed purified air over them; then fragrance compounds from the chamber were absorbed onto porous polymer adsorbents in clear Pasteur pipettes. The fragrances were collected for at least two hours during this study. Sadler et al.9 carried out floral fragrance studies on an epiphytic orchid in south Florida, much like the original study10. Again, this study required the flowers to be sampled for over two hours to collect the fragrance volatiles, with fragrance collected onto the porous polymer adsorbent. The paper here presents a non-destructive method that allows for much quicker sampling, lasting only 10 minutes. Also, instead of using a glass chamber oven baking bags are used, which allow for more flexible movement of the chamber and reduce the chances of damage to the flowers. These bags come in several sizes allowing the option to select the size of bag that will easily fit individual samples without damaging the sample or the surrounding material. The adsorbent used in this study was Tenax Porous Polymer Adsorbent. This differs from Porapak, because the sample can be thermally desorbed onto the GC-MS column for analysis, eliminating the use of a chemical solvent.
The methods in this study provide a way to quickly sample fragrance volatiles produced by flowers and could be used to sample volatiles from other specimens as well, such as insect pheromones, or mushroom volatiles. The reduced time for sampling means there is less stress on the sample and the ability to collect many samples in a short period of time. For example, in Sadler et al.9, the flower was only fragrant at night, so only two or three samples could be collected each night. With the method here, samples could be taken all night at 15-20-minute intervals from the same flower. Additionally, by using bags instead of glass chambers, the headspace can be suspended easier for sampling in the field for in situ collection on endangered or threatened plant species. Using the method presented here, we were able to sample flowers 1.5 to 2 meters above ground. These methods are incredibly useful for fragrance collection in the laboratory and the field, and provides researchers with a sampling technique that is fast and non-destructive to the sample.
Protocol
NOTE: Perfumes or scented lotions and products must not be worn during any of these procedures.
1. Flower selection
NOTE: Flowers used can be either naturally growing in the environment or kept under artificial environmental conditions. Temperature, humidity, and light level during collection can vary based on the specific flower species used, and what type of data is being collected. For example, data has been collected during the day and at night for the same flower to determine if fragrance varies over time of day, and collected from both in situ and greenhouse flowers.
- Select a flower that is initially unopened, to standardize sample collection time. This controls for a flower changing fragrance over time.
- Depending on the duration of the blooming time, if possible, wait at least 24 hours after blooming to collect the sample, setting a standard time for all samples.
- If there are multiple blooming flowers on a plant, mark the one that will be used with flagging tape or something similar to ensure repeated sampling of the same flower.
2. Material preparation
- Use oven bags (approximately 40.5 cm × 44.5 cm) and corrugated PTFE tubing.
- Initially, boil oven bags in water for ~30 min to remove residual plastic compounds. To dry, bake in an oven at 175 °C.
- Once the bags have dried, add a polypropylene bulkhead union to each corner of the closed end of the oven bags. These attachments allow connection of tubes to push charcoal filtered air in and pull fragrance out of the headspace.
- Rinse all bags and tubes with 75% ethanol. Let both air dry after rinsing.
- After the oven bags have dried, bake bags and tubes in an oven at low heat, approximately 74-85 °C for 30 min.
3. Volatile collection
NOTE: Sterile neoprene gloves need to be worn throughout this process, as contacting the bag or filter cartridges can contaminate the samples.
- Cover the selected flower with a baked oven bag. Cinch the bag together tightly with a plastic zip tie below the flower to prevent unwanted airflow into the bag.
- Attach a tube from the air outlet of the collection equipment and connect it to one of the bulkhead unions on the oven bag.
- On the other bulkhead union, attach a glass filter cartridge containing porous polymer adsorbent.
- Attach a second tube to the collection equipment on the vacuum input. Connect end of the second tube onto the glass volatile collection filter cartridge.
- Turn on both the air pump and vacuum at the same time set at ~0.05 L/min. The headspace around the flower will fill with air, but not become overinflated. The system will pull air from the bag through the filter, trapping the floral volatiles.
- Allow the machine to run for 10 min and then turn off both the air pump and vacuum.
NOTE: Flower species that produce/emit a smaller amount of fragrance may need to be sampled for a longer period of time. - Disassemble the tubes and the glass filter cartridge. Place the filter into a glass vial with a screw-on cap. Once the cap is on, seal the vial with PTFE pipe thread tape.
- Store samples in a freezer until analyzed using GC-MS.
- Repeat this process with a clean oven bag and glass filter, this time with an empty oven bag, to collect a blank air sample as a control. This allows any background volatiles collected to be identified.
NOTE: Repeating sample collection needs to be done at approximately the same time each day, as some flowers produce varying fragrance levels over the course of a day.
4. GC-MS
- Remove the glass filter cartridge from the freezer and place into a GC-MS in the injector port.
- Release headspace volatiles collected on porous polymer adsorbent from the adsorbent by heating in the thermal collection trap (TCT) to 220 °C for 8 min within a flow of helium gas (rate: 1.2 mL/min).
- Collect desorbed compounds in the TCT cold trap unit at -130 °C. The cold trap temperature is regulated by the GC-MS program.
- Flash heat the TCT cold trap unit to inject the compounds into the capillary column of the gas chromatograph to which the TCT cold trap unit was connected. The method for the TCT begins at -20 °C and ends at 150 °C.
- Program the GC-MS to rise from 40 °C to 280 °C at 15 °C/min, with a 5 min hold at 40 °C.
5. Data analysis
- For identification, compare the mass spectra of the sample to those from mass spectra libraries (NIST and Department of Chemical Ecology, Goteborg University, Sweden11), as well as retention times of the volatiles to times of authentic compound standards12.
- Compare chromatograms of collected volatiles to identify common reoccurring peaks.
- After identifying the peak volatiles, use Pherobase (online database of semiochemicals and pheromones) to determine if they have been previously described in floral fragrances10.
Representative Results
Representative data from the GC-MS are shown as a chromatogram in Figure 1. In addition to the chromatogram, a data file of results is also provided (Supplementary File 1). This data file provides the retention time for each peak (RT), and an identification of what compound that peak is (Library/ID). Peaks between 10:00 and 15:00 minutes are floral volatiles, due to the molecular weight of the compounds10. The numbers above the peaks signify the retention times of the identified compounds which are referenced the data file of the results (Supplementary File 1). By obtaining the chromatogram and data file for each fragrance sample, the compounds can be compared and those that are reoccurring for each flower sample can be identified. Collections can be identified from this document under the category "Sample", named to represent the flower sampled, and the time and date of the collection (example: UF1 8AM 03/16/15). Page 1 of this document also shows the identification of specific compounds identified from the sample (LibraryID), which peak retention time from Figure 1 the compound corresponds to (Pk#), and the percentage of the total fragrance that each volatile comprises (Area %). All the collected volatiles listed under "Library/ID" can be referenced in Pherobase to determine if they have previously described in a floral fragrance. For example, in the Supplementary File 1, compound #21, with a retention time (RT) of 10.311 has been identified as benzaldehyde. In future samples, if benzaldehyde is present, it can be referenced on Pherobase to determine if it is a likely floral compound for the flower. In Figure 2, benzaldehyde was searched on Pherobase. Once a compound has been selected, the page shows a list of all flower species, organized by plant family, from which that fragrance compound has been identified. Highlighted in the bottom right corner of Figure 2 is a small subset of the orchid species (Orchidaceae) from which benzaldehyde has been determined to be present in the floral fragrance.
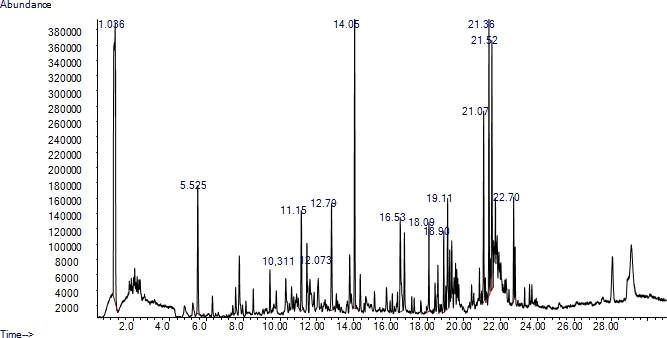
Figure 1: GC-MS volatile peak results. Graphical results showing the peak volatiles of the floral fragrance sample. Numbers above the peaks correspond to a list of all collected volatile compounds, identifying the peak to the specific volatile. Peaks between 10:00 and 15:00 minutes are most likely to be volatiles from a floral fragrance. Please click here to view a larger version of this figure.
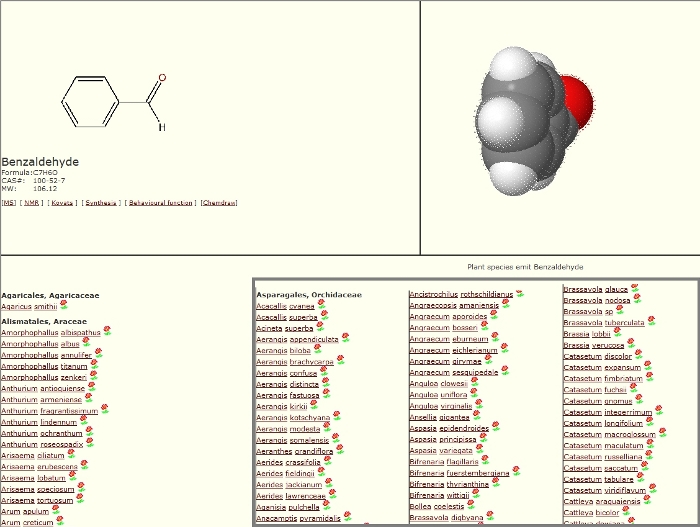
Figure 2: Pherobase example results. An example of results from a Pherobase search for a fragrance compound. In this figure Benzaldehyde was searched, and the results show a list of all flower species from which this fragrance has been identified. Please click here to view a larger version of this figure.
Supplementary File 1: Results Data. Please click here to view this file (Right click to download).
Discussion
Though this technique is incredibly valuable for its sampling speed and portability, one limitation is using it for epiphytic species, or those growing on trees and not from the ground. In the original study10, one of the flowers sampled was epiphytic. Because the machine is too heavy to hang freely, a stable, elevated base must be made for sampling. Additionally, the machine can either be plugged in to an electrical outlet or battery powered, so if there is prolonged field sampling, there must be a power source to charge the batteries when the machine is not in use.
The methods here allow for an in situ non-destructive sampling, with rapid repeated sampling and a much quicker sampling time. While some floral fragrance studies require the fragrance to be collected for 2-3 hours for one sample, the presented method can accurately collect the volatiles in approximately 10 min due to the collection material (porous polymer adsorbent) used in the glass filter.
These collection methods provide a way to quickly and safely sample the fragrance produced by flowers, without destroying or harming the flower. With so many flowers, especially those in the family Orchidaceae, being categorized as threatened or endangered, analyzing the fragrances they are producing in a non-destructive manner is critical as work is conducted to understanding their pollination biology. The information gained from these studies could potentially be used to boost pollination using synthetic blends based on peak chemicals found to attract more pollinators to areas with blooming orchids.
Disclosures
The authors have nothing to disclose.
Acknowledgements
USDA-ARS Research Project number 6036-22000-028-00D. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agriculture Research Service of any product or service to the exclusion of others that may be suitable. Additionally, the University of Florida Biology Department-Lewis and Varina Vaughn Fellowship in Orchid Biology (2017), and a University of Florida Graduate Research Fellowship (2014-2018) provided funding as well. We also thank Cindy Bennington from Stetson University for the orchid plant used during filming of this video.
Materials
| Bulkhead Union | Cole-Palmer | UX-06390-10 | |
| FEP tubing | Cole-Palmer | UX-06407-60 | |
| Gas Chromatography | Hewlett Packard | 6890 | |
| Glass Wool, Silanized | Sigma-Aldrich | 20411 | |
| Inlet liner | Agilent | 5062-3587 | |
| Mass Spectrometer | Hewlett Packard | 5973 | |
| Reynolds oven bag | Reynolds Consumer Products | Turkey size | |
| Tenax Porous Polymer Adsorbent | Sigma-Aldrich | 11982 |
References
- Knudsen, J. T., Tollsten, L., Bergstrom, L. G. Floral scents- A checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 33, 253-280 (1993).
- Dudareva, N. A., Pichersky, E. . Biology of floral scent. , (2006).
- Altenburger, R., Matile, P. Rhythms of fragrance emission in flowers. Planta. 174, 242-247 (1988).
- Dodson, C. H., Dressler, R. L., Hills, H. G., Adams, R. M., Williams, N. H. Biologically active compounds in orchid fragrances. Science. 164, 1243-1249 (1969).
- Theis, N., Raguso, R. A. The effect of pollination on floral fragrance in thistles. Journal of Chemical Ecology. 31 (11), 2581-2600 (2005).
- Heath, R. R., Manukian, A. Development and evaluation of systems to collect volatile semiochemicals from insects and plants using a charcoal-infused medium for air purification. Journal of Chemical Ecology. 18, 1209-1226 (1992).
- Cancino, A., Damon, A. Comparison of floral fragrance components of species of Encyclia and Prosthechea (Orchidaceae) from Soconusco, southeast Mexico. Lankesteriana. 6, 83-139 (2006).
- Cancino, A., Damon, A. Fragrance analysis of euglossine bee pollinated orchids from Soconusco, south-east Mexico. Plant Species Biology. 22, 129-134 (2007).
- Sadler, J. J., Smith, J. M., Zettler, L. W., Alborn, H. T., Richardson, L. W. Fragrance composition of Dendrophylax lindenii (Orchidaceae) using a novel technique applied in situ. European Journal of Environmental Science. 1, 137-141 (2011).
- Ray, H. A., Stuhl, C. J., Gillett-Kaufman, J. L. Floral fragrance analysis of Prosthechea cochleata (Orchidaceae), an endangered native, epiphytic orchid, in Florida. Plant Signaling and Behavior. , (2018).
- . National Institute of Standards and Technology. U.S. Department of Commerce Available from: https://www.nist.gov/ (2019)

