Tuning Degradation to Achieve Specific and Efficient Protein Depletion
Summary
Here, we present a protocol to effectively and specifically deplete a protein of interest in the yeast Saccharomyces cerevisiae using the β-est AID system.
Abstract
The plant auxin binding receptor, TIR1, recognizes proteins containing a specific auxin-inducible degron (AID) motif in the presence of auxin, targeting them for degradation. This system is exploited in many non-plant eukaryotes, such that a target protein, tagged with the AID motif, is degraded upon auxin addition. The level of TIR1 expression is critical; excessive expression leads to degradation of the AID-tagged protein even in the absence of auxin, whereas low expression leads to slow depletion. A β-estradiol-inducible AID system was created, with expression of TIR1 under the control of a β-estradiol inducible promoter. The level of TIR1 is tunable by changing the time of incubation with β-estradiol before auxin addition. This protocol describes how to rapidly deplete a target protein using the AID system. The appropriate β-estradiol incubation time depends on the abundance of the target protein. Therefore, efficient depletion depends on optimal timing that also minimizes auxin-independent depletion.
Introduction
Conditional mutations, such as temperature-sensitive mutants, are a powerful tool for the study of essential proteins, allowing cell growth under the permissive condition but causing loss of function under non-permissive conditions. However, cell metabolism can be seriously perturbed by the change in growth conditions required to induce the defect and may also create off-target effects. Several methods have been developed, in which the protein of interest is conditionally sequestered1 or its expression is controlled2,3 by addition of a small molecule. This protocol uses auxin and the auxin-inducible degron (AID) system to efficiently deplete a target protein.
The AID system has its origin in plants, where an auxin (in this protocol indole-3-acetic acid (IAA) is used), stimulates interaction of the Aux/IAA protein with TIR1, a member of the SCF U3 ubiquitin ligase complex4. SCF complex interaction causes polyubiquitination of Aux/IAA family proteins, which results in their degradation by the proteasome5,6.
This system was previously adapted for use in the yeast Saccharomyces cerevisiae7,8 by expressing the TIR1 protein from Oriza sativa (osTIR) in yeast cells, where it is able to interact with the endogenous yeast SCF complex. The protein of interest was tagged with a motif from the Aux/IAA protein IAA17 to target it for degradation. Functional truncations of IAA17 were developed later, such as AID*8,9,10, containing the 43 amino acid auxin-sensitive motif from Arabidopsis thaliana IAA17, along with an epitope tag to enable detection.
The system initially adapted for use in budding yeast7,8 expressed the osTIR1 protein from a yeast GAL promoter. Expression requires shifting to growth medium with galactose as the sole carbon source, which, unfortunately, results in a diauxic shift with wide-ranging changes to cell metabolism11. On the other hand, it has been reported that constitutive expression of TIR1 can lead to degradation of the target protein in the absence of auxin/IAA12 if the expression level is high, whereas low TIR1 expression causes inefficient depletion. An improved AID system named β-est AID was developed in which the osTIR is under the control of an inducible promoter that is tunable to suit the target protein, with minimal effect on cell metabolism. To achieve this, an artificial transcription factor (ATF) was constructed in which the VP16 viral transcription activator is fused to an oestrogen receptor and a four Zn fingers DNA binding domain (DBD). When β-estradiol (an oestrogen) is present, the ATF can enter the nucleus and induce osTIR transcription by binding to its promoter (Z4EVpr)13,12.
osTIR expression is usually detectable about 20 min after addition of β-estradiol12. However, the optimal duration of osTIR expression to achieve efficient depletion of the tagged protein with auxin, while avoiding depletion before auxin addition, needs to be empirically determined for each target protein. An approximate time for this pre-incubation can be estimated from abundance values in the Saccharomyces Genome Database (SGD https://www.yeastgenome.org/). As can be seen in Figure 1, the abundant protein, Dcp1 (2880 to 4189 molecules/cell), requires 40 min of pre-incubation with β-estradiol, with no auxin-independent depletion observed. The much less abundant protein, Prp2 (172 to 211 molecules/cell), is strongly depleted after only 20 min of pre-incubation. It is advisable to test two additional pre-incubation times, 10 to 20 min before or after this initial estimated time (20 min is the minimum time that is recommended). The optimum pre-incubation time is the time at which target protein has not depleted before adding auxin and once auxin is added the depletion is acceptable or protein levels approach the minimum possible. So, from Figure 1b, for Prp22 with 30 min of pre-incubation, the levels have not declined much 10 min after auxin addition. Comparing this with 40 min of pre-incubation and 15 min with IAA, where there is little additional depletion, there is no benefit in incubating with auxin longer than 10 min or pre-incubating for longer than 30 min, particularly as there is evidence of non-auxin dependent depletion at 40 min. For Dcp1 with 40 min of pre-incubation (the last point at which the protein level is approximately 100% before auxin addition), 15 to 20 min of depletion with auxin is acceptable. It is recommended to keep the depletion time as short as possible to reduce secondary effects on cell metabolism14.
This article demonstrates how to use the β-est AID system by optimizing the timing of β-estradiol incubation for osTIR expression to achieve rapid target protein depletion upon IAA addition without depletion before adding auxin.
Protocol
NOTE: See Figure 2 for a graphical summary.
1. Strain Preparation
- Using a ura3-strain, introduce the β-est AID system (i.e., genes encoding the β-estradiol responsive transcription factor (ATF) and the osTIR) and AID* tag the target protein (see Figure 3 and Table 1 for a summary of the procedure).
- Transform15 either pZTRK (G418 resistance marker) or pZTRL (LEU2 marker) plasmid (available from the Yeast Genetic Resource Centre) into the ura3- yeast strain or use the plasmid as a template to produce the PCR product for genomic integration.
- PCR amplify the ATF (marked Z4EVATF on the plasmid map) and osTIR using a high fidelity polymerase from either of the plasmids pZTRK or pZTRL. Use primers with 50 to 60 base 3’ extensions with homology to the genomic region, to direct integration by homologous recombination16. For genomic integration of the two components either separately or together, see Table 1 for primers and conditions.
NOTE: The strain pZ4EV-NTR1 has the components already integrated in the genome (available from the Yeast Genetic Resource Centre, Japan). - Ensure that the target protein is AID* tagged using the Longtine procedure17 (see Figure 3b and Table 1).
- Perform a growth analysis on the strain without β-estradiol and IAA present to determine if the AID* tag affects growth and to predict growth rate for use at step 1.5.
2. General Procedure for Depletion
- Calculate how much culture is required for all samples to be collected; for example, 10 mL of culture at OD600 of 0.8 is sufficient for protein, RNA and DNA extraction for a single sample, so for 6 samples, at least 60 mL of culture is needed.
- From an overnight culture, set up sufficient new culture at OD600 0.1 to 0.2 and leave to grow at 30 °C. A rich medium such as YPDA is recommended, although other growth conditions can be used:
Yeast Extract 10 g Peptone 20 g Glucose 20 g Adenine sulphate 40 mg H2O to 1 L
NOTE: Autoclave or filter sterilize; filter sterilization is preferred as peptide/sugar complexes produced by autoclaving precipitate in the methanol used in sample collection. - Prepare to receive the samples.
- Put 30 to 50% of the intended sample volume of methanol into a tube. For example, if a 10 mL sample is to be taken, put 5 mL of methanol into a 15 mL falcon tube and close the tube tightly. Once closed, label the tube and put on dry ice or at -80 °C to chill.
CAUTION: Dispense the methanol in a fume hood. - Label 1.5 mL tubes for long term storage of the samples and place in ice to cool.
- Cool enough H2O (at least 1 mL per sample) on ice.
- Put 30 to 50% of the intended sample volume of methanol into a tube. For example, if a 10 mL sample is to be taken, put 5 mL of methanol into a 15 mL falcon tube and close the tube tightly. Once closed, label the tube and put on dry ice or at -80 °C to chill.
- Anticipate the culture’s growth. The target OD for collecting the samples is approximately 0.7 to 0.8, but the pre-incubation step (the incubation with β-estradiol to induce the osTIR), needs to be started earlier so that the culture will reach approximately the right OD by the time the samples are collected.
NOTE: It is advisable to perform a growth curve in the conditions to be used in the experiment so that this starting OD can be estimated. - Once the target OD for the start of the pre-incubation has been reached, take a sample (usually 10 mL), into the pre-prepared tube containing cold methanol. Invert briefly to mix and place back in dry ice.
NOTE: The sample can be moved to water ice after about 5 min, if convenient to do so. - Immediately add the β-estradiol, 1 µL/mL of culture (final concentration of 10 µM); have the β-estradiol pre-measured in a pipette ready for use in order to reduce the time taken between collecting the sample and adding the β-estradiol. Rapidly mix by swirling vigorously.
- Continue to grow the culture as before (step 2.2), incubate (this is the “pre-incubation” step) with β-estradiol for the optimal time (for determination of the optimal pre-incubation time see Figure 1).
- Prepare to add IAA (auxin). Take up the volume of IAA needed for step 2.10 (i.e., 0.5 µL of IAA per mL of culture). This makes step 2.20 faster.
- Collect a sample as step 2.5.
- Immediately add IAA 0.5 µL/mL of culture to a final concentration of 750 µM as prepared in step 2.8. Rapidly mix by swirling vigorously.
- Collect samples, as step 2.5, according to your experimental design. Either a single sample, at a time when it is expected that the protein will be reliably depleted, or multiple samples in a time course of depletion. For example, 5 min intervals are convenient for timing and provide a range of protein levels. The optimization strategy, as shown in Figure 1, will give an indication of suitable times.
- Process the samples.
- Place the samples on ice, if not done already. Ensure that none of the samples has frozen; if they have, gently warm in the hand, inverting constantly so the temperature does not rise locally.
NOTE: This is best done in the hand as the sample’s temperature can be assessed, it should always feel cold. Place on ice. This is not a pause point – once all the samples are fluid, proceed to the next step. - Once all samples have been collected and are no longer frozen, spin at 3,500 x g for 2 min (at 4 °C if possible).
- Pour off the methanol/medium mix and place back on ice; do not worry if not all the liquid has been removed.
- Resuspend the cell pellet in 1 mL of ice cold H2O (from step 2.3.3) and transfer to a labelled 1.5 mL tube (prepared in step 2.3.2) on ice.
- Spin briefly (e.g., 10 s total time) at >15,000 x g to re-pellet the cells, place back on ice and remove the liquid.
- Remove the H2O by aspiration. The cell pellets can be stored at -20 °C, or -80 °C for long term storage.
- Place the samples on ice, if not done already. Ensure that none of the samples has frozen; if they have, gently warm in the hand, inverting constantly so the temperature does not rise locally.
- Check the level to which the protein has been depleted by Western blot analysis18.
NOTE: Sufficient protein19 and/or nucleic acid can be extracted from a single cell pellet for most purposes, although rare RNA species might require more sample volume.
Representative Results
Representative examples of depletion are displayed in Figure 1. The three experiments presented in this figure were optimization experiments for depletion of the proteins Prp2, Prp22 and Dcp1. The low abundance, spliceosomal Prp2 and Prp22 proteins both depleted to less than 20% after 40 min pre-incubation with β-estradiol followed by 15 min with auxin. Longer pre-incubation times lead to faster depletion but also show undesirable protein depletion before auxin addition. In comparison, the more abundant Dcp1 was only depleted to approximately 30% with the same treatment, but 60 min of pre-incubation resulted in depletion to 13% with the same auxin treatment, at the cost of depletion before the auxin is added. It is possible that 50 min of pre-incubation with β-estradiol and 15 min with auxin would have achieved similar results at a shorter time point and so would have been more optimal.
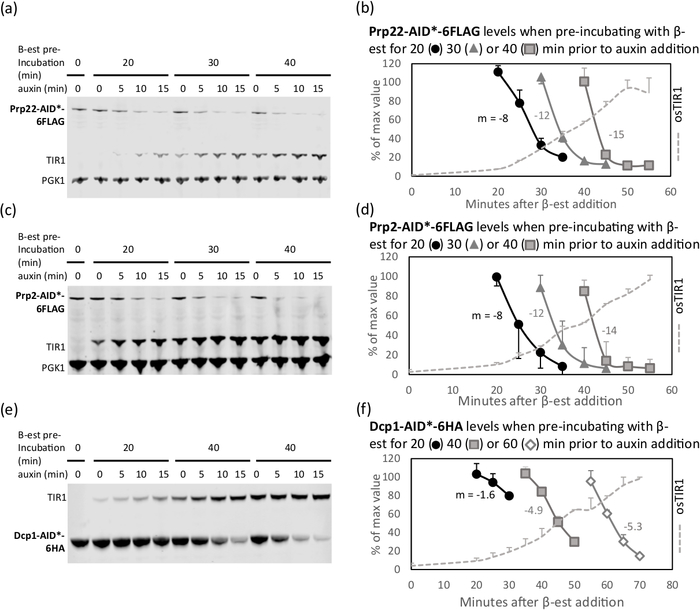
Figure 1: Depletion rate can be tuned by modulating the duration of β-estradiol pre-incubation. Western blot18 of target proteins: (a and b) Prp22-AID*-6FLAG, (c and d) Prp2-AID*-6FLAG, and (e and f) Dcp1-AID*-6HA, from cultures pre-incubated with β-estradiol (β-est) for 20, 30, 40, or 60 min prior to auxin addition12. Equal amounts of total protein were loaded in each lane. Pgk1 is detected as a visual loading control, except for panel e, where Pgk1 and Dcp1 co-migrate. Quantification of protein bands in panels a, c and e are shown in panels b, d, and f, respectively. As a measure of depletion rate, the slope (m) was calculated for the linear section (from 100% to 30% of initial values) of each curve. The optimal pre-incubation time is the time at which the protein levels are still close to the un-induced levels (100%) and the subsequent rate of depletion is fast. For Dcp1 (f), 60 min of pre-incubation is too long, as the protein has begun to degrade in the absence of auxin, whereas 20 min is too short, as the protein does not appreciably deplete in this time course. After 40 min pre-incubation, 15 min with auxin can be used as the protein is approximately 70% depleted and, although 20 min would result in further depletion, it could also result in secondary effects. Error bars represent standard deviation of two biological replicates. For each experiment, one representative blot is shown. This figure is derived from previous publication9. Please click here to view a larger version of this figure.
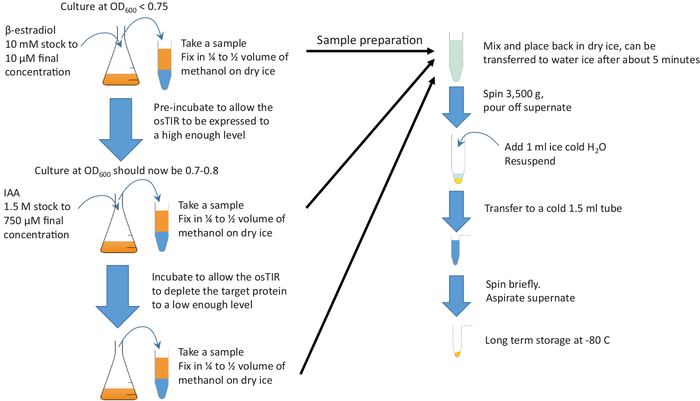
Figure 2: Graphical summary. Add β-estradiol to sufficient culture growing in rich medium and at the required temperature in order to start pre-incubation. Continue growth for the required pre-incubation time before adding IAA (auxin) to start depletion. The pre-incubation and depletion times depend on the protein to be depleted, but pre-incubation is often in the range of 20-60 min and the depletion time is typically in the order of 10 to 20 min. 10 mL samples should be taken at the start and end of pre-incubation and during the depletion. These samples are rapidly fixed in cold methanol before pelleting and storage. Please click here to view a larger version of this figure.
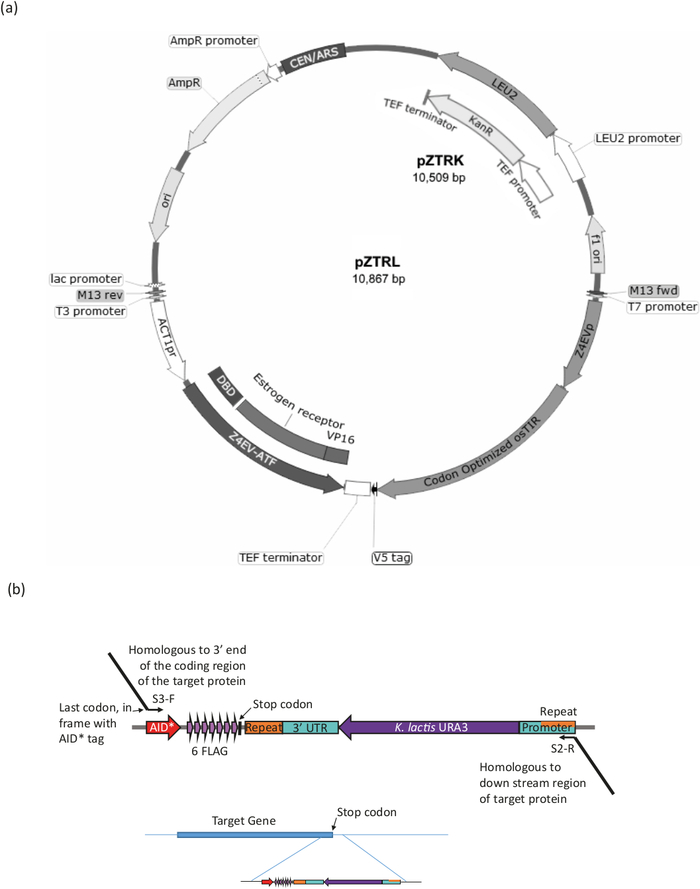
Figure 3: Strain generation for the B-est system. (a) To generate a yeast strain with the AID* system, either the pZTRL (LEU2) or the pZTRK (kanamycin (G418) resistance), plasmid should be introduced into the strain or, alternatively, the ATF and osTIR can be inserted into the genome by homologous recombination of a fragment generated by PCR from 3’end primers (see Figure 3b and Table 1). (b) C-terminal tagging of the target protein is achieved by PCR amplification of the appropriate region of the plasmid pURA3-AID*-6FLAG (pURA3_AID*-6HA differs only in the tag and can be treated in exactly the same way), using Longtine primers S3-F and S2-R with 3’ extensions homologous to the 3’ end of the target protein. The forward primer extension should include the last amino acid codon in frame with the start of the AID* tag and must not include the stop codon. The reverse primer extension should be to a region downstream of the coding region. Once inserted into the genome, cells that have lost the URA3 marker (by homologous recombination between the identical regions found at both ends of the marker) can be selected by growth with 5-FOA, that counter-selects URA3 cells. Please click here to view a larger version of this figure.
| a. Primer Sequences | |||||||
| Target | Location | Primer | Name | Sequence | Tm (°C) | ||
| pZTRL | 516 | F | pZTRL_F | <-region of homology->GCGACAGCATCACCGACTTCG | 61.23 | ||
| 7897 | R | pZTR_R | CGCCGCCTCTACCTTGCAGA<-region of homology (RC)-> | 61.30 | |||
| pZTRK | 9154 | F | pZTRK_F | <-region of homology->ACGTTGAGCCATTAGTATCAATTTGCTTACC | 59.40 | ||
| 5897 | R | pZTR_R | CGCCGCCTCTACCTTGCAGA<-region of homology (RC)-> | 61.30 | |||
| pURA3-AID*-6FLAG or pURA3_AID*-6HA | F | S3-F | <-region of homology->CGTACGCTGCAGGTCGAC | 59.21 | |||
| R | S2-R | ATCGATGAATTCGAGCTCG<-region of homology (RC)-> | 52.76 | ||||
| pZRTL/K is to amplify the β-est AID system | |||||||
| pURA3-AID*-6FLAG/6HA to amplify the AID* and epitope tag to tag the target protein (Lontine procedure) | |||||||
| <-region of homology-> | Region homologous to the flanking regions where the system is to be inserted. The longer this region is the more likely the modification is to be successful; 50 – 100 bases is recommended. | ||||||
| <-region of homology (RC)-> | Region homologous to the flanking regions where the system is to be inserted, remember to use the reverse complement. As above, the longer this region is the better. | ||||||
| Tm (°C) | Tm using the %GC method with 50 mM NaCl | ||||||
| b. PCR Mix | |||||||
| Component | Volume (µL) | ||||||
| Template | <10 | ||||||
| NEB Phusion HF Buffer (5x)* | 100 | ||||||
| Forward Primer 100 µM | 2.5 | ||||||
| Reverse Primer 100 µM | 2.5 | ||||||
| dNTPs 10 mM each | 10 | ||||||
| H2O | to 500 | ||||||
| * The NEB Phusion GC Buffer (5x) can also be used but is not preferred | |||||||
| Make this mix, split into 10 tubes of 50 µl mix each and perform the PCR as Table 1 c. | |||||||
| Check the PCR has worked by running on an agarose gel | |||||||
| Combine all successful reactions into one tube and ethanol precipitate | |||||||
| Transform the yeast with all the material produced by the PCR | |||||||
| c. PCR Conditions | |||||||
| Step | Temp (°C) | Time | |||||
| Initial Denaturation | 98 | 30 s | |||||
| 25-35 Cycles | Denature | 98 | 10 s | ||||
| Anneal | 45–60 | 20 s | |||||
| Extension | 72 | 30 s/kb | |||||
| Final Extension | 72 | 10 min | |||||
| Hold | 8 | ||||||
| Anneal at 45 °C for the Lontine primer set (S3-F and S2-R) and 60 °C for the pZTRL/K primers | |||||||
| Extend for 3 minutes for the Lontine PCR and 3 minutes for pZTRL/K | |||||||
Table 1: Primer sequences, PCR mix and PCR conditions.
Discussion
A well optimized protocol can produce rapid and efficient depletion of the target protein. Determining the approximate pre-incubation time with β-estradiol is important, as this increases reproducibility of the depletion, but small variations in pre-incubation time can be tolerated. On the other hand, care must be taken with timing after auxin addition, as the protein level declines very rapidly.
An advantage of this approach is that tuned depletion can be achieved by varying combinations of pre-incubation time with β-estradiol and IAA incubation time. For example, if desired, the target protein can be more slowly depleted by reducing the pre-incubation time.
The β-est AID system offers certain advantages over systems where OsTIR is constitutively expressed. For example, if the target protein is essential for viability, regulated expression of osTIR can avoid premature depletion of the target protein. Moreover, expression of osTIR can be tuned to suit the abundance of the target protein and its susceptibility to degradation, and the depletion can be either fast or slow. The two small molecule effectors, β-estradiol and auxin, do not perturb the yeast metabolism under the conditions used here, unlike rapamycin, used in the anchor-away system1.
It should be noted that tagging some proteins disrupts their function, which is a problem with any targeted depletion system. In this case, an N-terminal tag may work when a C-terminal tag does not. Also, not all proteins will be depleted efficiently; for example, the AID-tag on the target protein may be inaccessible to the osTIR protein. Therefore, after AID-tagging, each target protein should be tested for any effect of the tag on growth, and to determine whether depletion is effective, before the timings of β-estradiol pre-incubation and auxin treatment are optimized.
This AID* system is very simple and is compatible with any subsequent experimental procedure that does not involve further growth, such as protein, DNA or RNA analysis or microscopy. In addition, the system works well when combined with thiolabelling to purify nascent RNA20.
This system provides a rapid, specific, and reproducible means of depleting a protein without otherwise affecting the metabolism of the yeast cell.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Thanks to Jane Reid for initiating this programme, Barbara Terlouw for development, Vahid Aslanzadeh for the “ura looper” constructs and Susana de Lucas for many helpful discussions. This work was supported by a scholarship to GIMO from the Consejo Nacional de Ciencia y Tecnología, Mexico (CONACYT) and the University of Edinburgh School of Biological Sciences, a Wellcome PhD studentship to IEM [105256] and by Wellcome funding [104648] to JD Beggs. Work in the Wellcome Centre for Cell Biology is supported by Wellcome core funding [092076].
Materials
| Adenine sulphate | Formedium | DOC0230 | |
| Agar | Formedium | AGA03 | |
| Β-estradiol | Sigma Aldrich | E2758-1G | 10mM solution in ethanol. Store at -20 oC |
| DMSO | Alfa Aesar | 42780 | DMSO should be solid at 4 oC |
| Glucose | Fisher Scientific | G/0500/60 | |
| IAA 1H-Indole-3-acetic acid | Across Orgainics | 122150100 | Auxin analogue. 1.5 M in DMSO. The solution will be a russet colour and darken as time goes on; a deep red solution should be discarded and a new one made. Store at -20 oC. |
| Methanol | Fisher Scientific | M/4000/PC17 | CAUTION Toxic and flammable |
| Phusion High-Fidelity DNA Polymerase | NEB | M0530 | |
| Peptone | Formedium | PEP03 | |
| SCSM single drop-out –ura | Formedium | DSCS101 | |
| Yeast Extract | Formedium | YEA03 | |
| Yeast nitrogen base without amino acids with amonium sulphate | Formedium | CYN0410 |
References
- Haruki, H., Nishikawa, J., Laemmli, U. K. The Anchor-Away Technique: Rapid, Conditional Establishment of Yeast Mutant Phenotypes. Molecular Cell. 31, 925-932 (2008).
- Bellí, G., Garí, E., Piedrafita, L., Aldea, M., Herrero, E. An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucleic Acids Research. 26, 942-947 (1998).
- Alexander, R. D., et al. RiboSys, a high-resolution, quantitative approach to measure the in vivo kinetics of pre-mRNA splicing and 3′-end processing in Saccharomyces cerevisiae. RNA. 16, 2570-2580 (2010).
- Deshaies, R. J., Joazeiro, C. A. P. RING Domain E3 Ubiquitin Ligases. Annual Review of Biochemistry. 78, 399-434 (2009).
- Tan, X., et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 446, 640-645 (2007).
- Teale, W. D., Paponov, I. A., Palme, K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology. 7, 847-859 (2006).
- Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T., Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nature Methods. 6, 917-922 (2009).
- Morawska, M., Ulrich, H. D. An expanded tool kit for the auxin-inducible degron system in budding yeast. Yeast. 30, 341-351 (2013).
- Kubota, T., Nishimura, K., Kanemaki, M. T., Donaldson, A. D. The Elg1 Replication Factor C-like Complex Functions in PCNA Unloading during DNA Replication. Molecular Cell. 50, 273-280 (2013).
- Brosh, R., et al. A dual molecular analogue tuner for dissecting protein function in mammalian cells. Nature Communications. 7, 11742 (2016).
- DeRisi, J. L., Iyer, V. R., Brown, P. O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 278, 680-686 (1997).
- Mendoza-Ochoa, G. I., et al. A fast and tuneable auxin-inducible degron for depletion of target proteins in budding. Yeast. , (2018).
- McIsaac, R. S., et al. Synthetic gene expression perturbation systems with rapid, tunable, single-gene specificity in yeast. Nucleic Acids Res. 41, e57 (2013).
- Prusty, R., Grisafi, P., Fink, G. R. The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. PNAS. 101, 4153-4157 (2004).
- Geitz, D., St Jean, A., Woods, R. A., Schiest, R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Research. 20, 1425 (1992).
- Widlund, P. O., Davis, T. N. A high-efficiency method to replace essential genes with mutant alleles in yeast. Yeast. 22, 769-774 (2005).
- Longtine, M. S., et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14, 953-961 (1998).
- Eaton, S. L., et al. A Guide to Modern Quantitative Fluorescent Western Blotting with Troubleshooting Strategies. Journal of Visualized Experiments. , e52099 (2014).
- Volland, C., Urban-Grimal, D., Géraud, G., Haguenauer-Tsapis, R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. Journal of Biological Chemistry. 269, 9833-9841 (1994).
- Barrass, J. D., et al. Transcriptome-wide RNA processing kinetics revealed using extremely short 4tU labeling. Genome Biology. 16, 282 (2015).

