Synthesis of a Deuterated Standard for the Quantification of 2-Arachidonoylglycerol in Caenorhabditis elegans
Summary
This work describes a robust and straightforward method to detect and quantify the endocannabinoid 2-arachidonoylglycerol (2-AG) in C. elegans. An analytical deuterated standard us prepared and used for the quantification of 2-AG by isotopic dilution and liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS).
Abstract
This work presents a method to prepare an analytical standard to analyze 2-arachidonoyl glycerol (2-AG) qualitatively and quantitatively by liquid chromatography-electrospray Ionization-tandem mass spectrometry (LC-ESI-MS/MS). Endocannabinoids are conserved lipid mediators that regulate multiple biological processes in a variety of organisms. In C. elegans, 2-AG has been found to possess different roles, including modulation of dauer formation and cholesterol metabolism. This report describes a method to overcome the difficulties associated with the costs and stability of deuterated standards required for 2-AG quantification. The procedure for the synthesis of the standard is simple and can be performed in any laboratory, without the need for organic synthesis expertise or special equipment. In addition, a modification of Folch's method to extract the deuterated standard from C. elegans culture is described. Finally, a quantitative and analytic method to detect 2-AG using the stable isotopically labeled analog 1-AG-d5 is described, which provides reliable results in a fast-chromatographic run. The procedure is useful for studying the multiple roles of 2-AG in C. elegans while also being applicable to other studies of metabolites in different organisms.
Introduction
Endocannabinoids regulate multiple biological processes in a variety of organisms and are conserved lipid mediators1. The first discovered and most well-characterized endocannabinoids are anandamide (arachidonoylethanolamide, AEA) and 2-arachidonoyl glycerol (2-AG). Endocannabinoids play many critical roles, including those involved in brain reward systems as well as drug addiction, memory, mood, and metabolic processes2. AEA and 2-AG are only synthesized when needed and have short life spans, and they are degraded through transport protein reuptake and hydrolysis3.
The use of animal models like Caenorhabditis elegans (C. elegans) has become important to study the large variety of biological processes including apoptosis, cell signaling, cell cycle, cell polarity, gene regulation, metabolism, ageing, and sex determination4,5. Additionally, C. elegans is an excellent model for studying the physiological roles of polyunsaturated fatty acids (PUFAs). AEA has been identified in C. elegans and is reduced under dietary restriction6. This deficiency extends the lifespan of the nematode through a dietary restriction mechanism that can be suppressed by supplementation with the endocannabinoid. Recently, it was discovered that 2-AG and AEA play fundamental roles in the regulation of cholesterol trafficking in C. elegans7. More importantly, it was determined that supplementation with exogenous 2-AG can rescue dauer arrest, which is caused by the impaired cholesterol trafficking in Niemann-Pick type C1 C. elegans mutants.
To gain a better understanding of 2-AG's relationship with cholesterol trafficking and other biological processes in the nematode (i.e., monoaminergic signaling, nociception and locomotion), it is crucial to study this endogenous metabolite and how it is affected under certain environmental and dietary conditions8,9,10,11,12,13. Therefore, it is imperative to design and optimize a method to detect and quantify endogenous 2-AG in C. elegans that is simple to use for scientists of different fields, especially those who study the nematode's behavior in relation to this endocannabinoid.
In 2008, Lethonen and coworkers succeeded in identifying 2-AG and AEA in C. elegans using LC-MS analytical methods14. In 2011, they managed to expand this technique to other endocannabinoids15. More recent work has shown other analytical methods that have been successful in detecting and quantifying endocannabinoids in C. elegans, including mass spectrometry and GC-MS16,17,18, and it has also been reported that similar analytical methods can be expanded to other models19.
Previously reported analytical methods used for quantifying 2-AG in biological samples usually involve the use of deuterated standards that are commercially acquired and require availability for the purchase20,21. Many analytical standards for LC-MS/MS quantification of endocannabinoids are commercially available from different providers. Nevertheless, they are expensive, are sensitive, and become oxidized over time, due to the presence of multiple double bonds. The most common versions of these standards are based on the octa-deuterated arachidonic acid and are suitable for quantification by isotope dilution LC-MS/MS14,22. Also, most of these standards are substituted in position 2 of the glycerol, making them unstable under most conditions since they are prone to acyl migration19,23.
To overcome the difficulties associated with the costs and stability of these deuterated standards, a convenient and simple method is presented to prepare an analytical standard based on glycerol-d5. The sequence to prepare the penta-deuterated standard requires a three-step procedure that results in the standard 1-AG-d5, which is stable and does not undergo acyl migration (the main issue when aiming to synthesize 2-monoacylglycerols).
The main objective here is to show a simple and reproducible method to study 2-AG in C. elegans, including the synthesis of the analytical deuterated standard, preparation and extraction of the nematode samples, and analysis by LC-MS/MS (Figure 1). This synthetic procedure is achievable without the sophisticated organic synthesis knowledge or special equipment, making it suitable for scientists from different fields who are studying C. elegans behavior under endocannabinoid influence. The method is also expandable to other study models, making it useful for different targets. The standard, prepared as reported here, has been applied to successfully develop a fast and reliable chromatographic method that allows for effective detection and quantification of 2-AG in a reproducible manner.
Protocol
1. 1-AG-d5 preparation
NOTE: For obtaining 1-AG-d5 as a deuterated internal standard for quantification assays, follow the protocol as detailed below.
- Differential protection
- To only protect primary alcohols, first add 38 mg of glycerol-d8 to a 10 mL reaction tube using a Pasteur pipette and add a magnetic stirrer.
- Add 5 mL of anhydrous dichloromethane (DCM) using a 5 mL Hamilton syringe, and fill the tube with dry N2 to yield an inert atmosphere.
- Prepare a bath using a shallow Dewar flask filled with distilled ethyl acetate.
- Fit the hermetically closed reaction tube inside the bath and cool it by slowly adding liquid N2 to the ethyl acetate until the solvent is frozen.
CAUTION: Liquid violently boils at room temperature (RT) and can cause severe burns when contacting eyes and skin. - Add 54 mg of anhydrous collidine using a Hamilton syringe.
CAUTION: Collidine is volatile and has a very strong and unpleasant scent. - Add 70 mg of tert-butyldimethylsilyl chloride and stir the entire solution for 3 h at -78 °C on a magnetic stirrer.
- After 3 h, leave the reaction to warm at RT and keep stirring for an additional 12 h.
- Add 2 mL of brine to quench the reaction.
- Extract the solution 3x with 2 mL of distilled dichloromethane using a separating funnel, saving the organic extract each time.
- Combine the three organic extracts and dry them over sodium sulfate.
- Evaporate the dichloromethane under reduced pressure in a vacuum rotary evaporator carefully to avoid solvent projections.
- Purify the crude mixture by column chromatography using silica gel as the stationary phase and a 10% increasing hexane/ethyl acetate gradient, starting from 100% hexane and finishing with 100% ethyl acetate.
- Combine the product-containing fractions and remove the solvent under reduced pressure in a vacuum rotary evaporator to obtain the pure 1-O,3-O-bis-(TBDMS) glycerol-d5 as a colorless liquid.
- Esterification
- Add 10 mg of the 1-O,3-O-bis(TBDMS)-glycerol-d5 (previously synthesized) to a 10 mL reaction tube using a Pasteur pipette and add a magnetic stirrer.
- Add 2 mL of anhydrous dichloromethane using a 5 mL Hamilton syringe, and fill the tube with dry N2 to yield an inert atmosphere.
- Cool the solution to 0 °C using an ice bath.
- Add 36 mg of arachidonic acid using a multi-volume adjustable micropipette and stir.
- Add 15 mg of 4-dimethylaminopyridine and stir.
- Add 15 mg of N,N'-diisopropylcarbodiimide using a multi-volume adjustable micropipette and stir.
- Let the mixture react at 0 °C for 3 h.
- After 3 h, leave the reaction to warm at RT and keep stirring for an additional 12 h.
- Add 2 mL of water to quench the reaction.
- Extract the organic solution 3x with 2 mL of distilled dichloromethane (DCM) using a separating funnel.
- Place the three organic extracts in the same tube and dry them over sodium sulfate.
- Evaporate the dichloromethane under reduced pressure in a vacuum rotary evaporator carefully to avoid solvent projections.
- Purify the crude mixture by column chromatography using silica gel as the stationary phase and a 10% increasing hexane/ ethyl acetate gradient, starting from 100% hexane and finishing with 50% hexane/50% ethyl acetate.
- Combine the product-containing fractions and remove the solvent under reduced pressure in a vacuum rotary evaporator to obtain the pure 1-O, 3-O-bis(TBDMS)-2-AG-d5 as a yellowish liquid.
- Deprotection
- Add 15 mg of the 1-O,3-O-bis(TBDMS)-2-AG-d5 (previously synthesized) to a 10 mL reaction tube using a Pasteur pipette and add a magnetic stirrer.
- Add 2 mL of anhydrous THF using a 5 mL Hamilton syringe, and fill the tube with dry N2 to yield an inert atmosphere.
- Cool the solution to 0 °C using an ice bath.
- Add 150 µL dropwise of 1 M tetrabutylammonium fluoride solution in THF using a Hamilton syringe.
- Let the reaction warm to RT and stir for 1 h.
- After 1 h, add 2 mL of water to quench the reaction.
- Extract the solution 3x with 2 mL of distilled dichloromethane using a separating funnel.
- Combine the three organic extracts and dry them over sodium sulfate.
- Evaporate the dichloromethane under reduced pressure in a vacuum rotary evaporator to obtain the pure 1-AG-d5 as a yellowish liquid.
- Monitor all reactions by thin layer chromatography performed on silica gel 60 F254 pre-coated aluminum sheets. Visualize the bands under a 254 nm UV lamp after staining with an ethanolic solution of 4-anisaldehyde.
2. Preparation of standard stock and measuring solutions
- Dissolve 1 mg of the internal standard 1-AG-d5 in 1 mL of ACN and sonicate for 1 min to obtain the 1,000 ppm standard stock solution.
- To prepare the 1,000 ppb solution used for quantification in worms, first prepare a 10 ppm solution: take 10 µL of the stock solution using a Hamilton syringe and dilute it to a final volume of 1 mL by adding 990 µL of ACN.
- Take 100 µL from the solution produced in step 2.2 using a Hamilton syringe, and dilute it to a final volume of 1 mL by adding 900 µL of ACN to obtain the 1,000 ppb solution used for the quantification.
- Sonicate for 1 min between each step to ensure complete solubilization. Store the solutions at -78 °C to maintain the concentrations and integrity of the standards. After the standard solution is used, flow some nitrogen before closing the vial to prevent oxidation.
3. Growth and maintenance of C. elegans
NOTE: Seed the nematode growth medium (NGM) agar plates with E. coli OP50 and propagate the worms on these plates.
- Mix 3 g of NaCl with 17 g of agar.
- Add 2.5 g of peptone, then add 975 mL of H2O.
- Autoclave for 50 min, then cool the flask to 55 °C.
- Mix the following: 1 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, 25 mL of 1 M KH2PO4 buffer (all of which have been previously autoclaved), and 1 mL of 5 mg/mL cholesterol in ethanol.
- While maintaining a sterile environment, dispense the NGM solution into 60 mm Petri plates, filling the plates to two-thirds of their volume. Store the plates at 4 °C.
- Streak the E. coli bacterial culture from a -80 °C glycerol stock onto the LB agar plate. Let it grow on the plate overnight at 37 °C.
- Pick up a single colony to inoculate 100 mL of liquid LB overnight at 37 °C with agitation.
NOTE: It is not necessary to check the O.D. because this strain can reach stationary phase over this time. - Remove the stored NGM plates, remove the lids in the laminar flow hood, and leave open to allow evaporation of excess moisture from the plates.
- Once the plates are dried, use a Pasteur pipette to add 100 µL of OP50 E. coli to the center of the plate without spreading.
- Leave the OP50 E. coli lawn to grow overnight at RT or at 37 °C for 8 h.
- Add the desired number worm embryos obtained by hypochlorite treatment or "bleaching" (Section 4).
NOTE: Cool the plates to RT before the addition of worms.
4. Bleaching technique for synchronizing C elegans cultures
- Seed and chunk worms onto 6 cm NGM plates.
- Leave the worms growing for 2-3 days to obtain sufficient numbers of eggs and gravid adults on the plate.
- Once there are enough eggs/adults, pour 5 mL of M9 onto the plate.
- Transfer the worms to a 15 mL centrifuge tube using a glass pipette.
- Centrifuge the tube for 2 min at 2,000 x g and pellet the worms.
- Suction out most of the M9, avoiding disturbance of the worm pellet.
- Add 3 mL of bleaching solution (2:1:1 ratio of NaOH:NaOCl:H2O).
- Invert gently to mix the solution for 5 min or until the number of intact adult worms decreases.
CAUTION: Do not bleach for more than 5 min. - Centrifuge for 1 min at 2,000 x g and suction most of the bleaching solution without disturbing the worm pellet.
- Add 15 mL of M9 and mix well.
- Centrifuge again at 2000 x g for 1 min.
- Suction out most of the M9 without disturbing the worm pellet.
- Repeat steps 4.10-4.12 one or two more times.
- Add 5 mL of fresh M9 and agitate.
- Let the eggs hatch overnight with gentle rocking.
5. Worm sample preparation
- Let the N2 embryos obtained by the bleaching procedure hatch overnight in M9 buffer (5 mL in a 15 mL centrifuge tube) at 20 °C.
- Harvest the synchronized L1s by centrifuging the tube for 2 min at 2,000 x g.
- Wash the worms with M9 buffer 1x, then quantify the number of live L1 worms.
- Seed approximately 10,000 worms into NGM plates (10 cm diameter) with 1 mL of OP50 E. coli (previously dried).
- Incubate the plates for 48 h at 20 °C until worms reach the L4 stage.
- Harvest the worms using cold M9 buffer in a 15 mL centrifuge tube, wash them 1x, then and transfer them to a 1.5 mL tube.
- Pellet the worms by centrifugation at 2,000 x g for 1 min, eliminate most of the supernatant, immerse the tubes in liquid nitrogen, and store at -80 °C.
6. Lipid extraction
- Thaw approximately 100 µL of frozen worm pellets belonging to N2 on ice, add 1.3 mL of methanol, and sonicate the sample for 4 min.
- Add 2.6 mL of chloroform, and 1.3 mL of 0.5 M KCl/0.08 M H3PO4 to a final ratio of 1:2:1, 1,000 ppb of the internal standard 1-AG-d5, and butylated hydroxytoluene as an antioxidant agent at a final concentration of 50 µg/mL.
- Vortex the samples for 1 min and sonicate in an ultrasonic water bath for 15 min on ice.
- Vortex the samples 2x for 1 min and centrifuge for 10 min at 2,000 x g to induce the phase separation.
- Collect the lower phase and collect it in a clean tube, dry it under nitrogen, and resuspend the solid residue in 100 µL of ACN.
7. Endocannabinoid analysis by HPLC-MS/MS
- Use liquid chromatography coupled with an ESI triple quadrupole mass spectrometer to detect and quantify 2-AG from nematode samples.
- Use the following ratio for reversed-phase HPLC: from 0.0-0.5 min H2O:ACN (40:60), 0.5-6.5 min H2O:ACN (40:60) to (25:75), 6.5-7.5 min H2O:ACN (25:75), 7.5-8.0 min H2O:ACN (25:75) to (40:60), 8.0-12.0 min H2O:ACN (40:60).
- Maintain the column temperature at 40 °C and set the autosampler tray temperature to 10 °C.
- Set the following ionization conditions: positive-ion mode; drying gas (N2) temperature = 300 °C; drying gas flow rate = 10 L/min; nebulizer pressure = 10 UA; and cap. voltage = 4 kV.
- For the analyte detection, use MRM with the following transitions: 379.2 m/z to 289.2 m/z for 2-AG; and 384.2 m/z to 289.2 m/z for 1-AG-d5.
8. Endocannabinoid quantification in worms
- Use deuterated internal standard 1-AG-d5 and calculate the peak area ratios of the analyte to the internal standard.
- Use the following transitions: 384.2 m/z to 287.2 m/z for 2-AG; and 379.2 m/z to 287.2 m/z for 1-AG-d5.
- Calculate the concentration of the endogenous 2-AG by comparing to the peak area ratios of the deuterated standard using the concentration value of the standard.
Representative Results
An isotopically labeled analog was successfully synthesized from commercially available d8-glycerol and arachidonic acid using a 3-step synthetic method (Figure 2, Figure 3). These steps are straightforward and do not require sophisticated equipment, specially controlled conditions, or expensive reagents. Thus, this method is robust and may be successfully extended to synthesize monoacylglycerides containing different fatty acids.
1-AG-d5 was structurally characterized using nuclear magnetic spectroscopy. 1H NMR showed the characteristic multiplet at 5.44 ppm to 4.93 ppm, which integrates for the eight vinyl protons of the arachidonoyl chain and triplet at 2.40 ppm, corresponding to the two protons of the alpha position to the carbonyl group. In 2D NMR, it is also possible to see a 2.9 ppm to 2.7 ppm multiplet assignable to the five deuterium of the glycerol portion.
The chemically synthesized 1-AG-d5 was used as an internal standard in C. elegans samples. The standard was added to the samples before extraction then extracted with the endogenous lipids, using a straightforward method adapted from Folch24. This modified method provides a high recovery value of the standard, as shown by HPLC quantification.
The method was optimized using the transitions 1) 384.2 m/z to 287.2 m/z for 2-AG and 2) 379.2 m/z to 287.2 m/z for 1-AG-d5, in which the glycerol molecules are lost (Figure 4). The limits of detection (LOD) and quantification (LOQ) were calculated for the standard using a calibration curve, resulting in values of 5 ppb and 16.6 ppb, respectively. The retention time for the standard was 6.8 min.
2-AG endogenous from the C. elegans samples was successfully detected and quantified by isotopic dilution with the chemically synthesized 1-AG-d5 using HPLC-MS/MS (Figure 5).
Since the original concentrations of the deuterated standards in samples 1 and 3 were each 1,000 ppb, from the peak area ratio it was possible to calculate the endogenous concentration of 2-AG at 340 ppb for sample 1 and 360 ppm for sample 3, yielding an average of 350 ppm (Table 1).
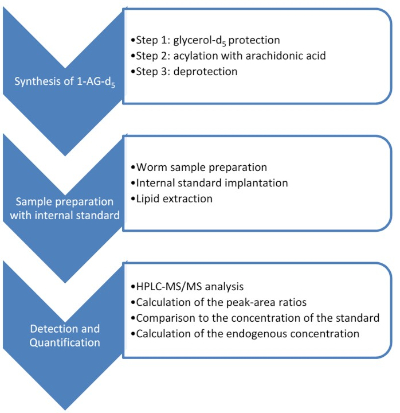
Figure 1: Summary of synthesis, worm sampling, and quantification. To achieve successful quantification of the endogenous 2-AG, it was necessary to synthesize its deuterated analog using a three-step sequence. Afterwards, it was added to worm samples, extracted, and analyzed by HPLC-MS/MS. Used as an internal standard, the synthetic of 1-AG-d5 was the tool used to quantify the endogenous metabolite. Please click here to view a larger version of this figure.
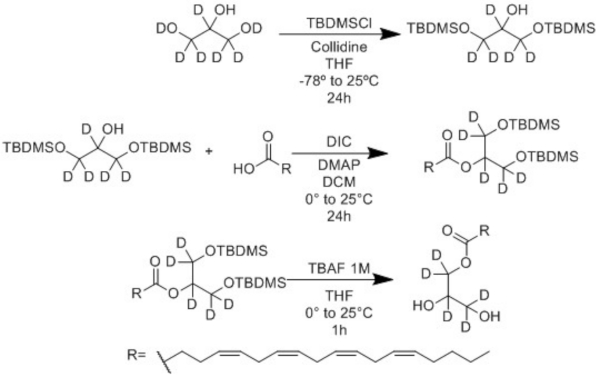
Figure 2: Synthetic scheme for obtaining 1-AG-d5. A mass of 10 mg of the deuterated analog was obtained using the three-step method involving 1) protection of the glycerol-d8, 2) acylation with arachidonic acid, and 3) deprotection. Please click here to view a larger version of this figure.
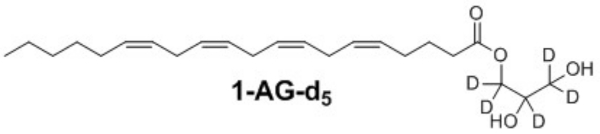
Figure 3: Chemical structure of the isotopically labeled 2-AG analog. Please click here to view a larger version of this figure.
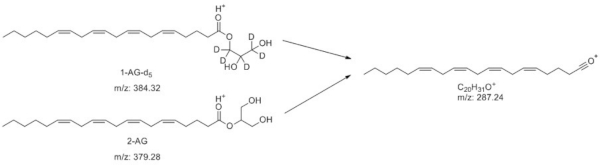
Figure 4: Selected fragmentations for quantification of 1-AG-d5 and 2-AG. Please click here to view a larger version of this figure.
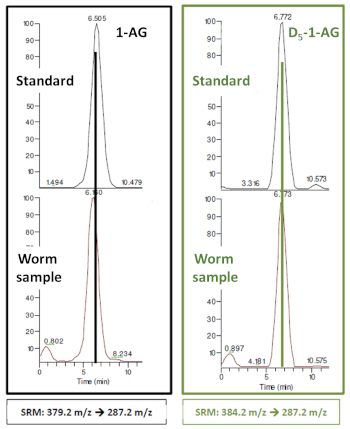
Figure 5: HPLC chromatograms for 1-AG-d5 and 1-AG as pure standards and internal standards in a worm sample. It was possible to analyze retention times and see that 1) the worm appears not to have endogenous 1-AG and 2) it would only have 2-AG, but the standard 1-AG-d5 will still work as a good analytical standard for quantification by isotopic dilution. The transitions used were: 384.2 m/z to 287.2 m/z for 2-AG, and 379.2 m/z to 287.2 m/z for 1-AG-d5. Please click here to view a larger version of this figure.
| 1-AG-d5 | 2-AG | Ratio (2-AG/1-AG-d5) | |
| Sample 1 | 71964.74 | 210616.08 | 0.34 |
| Sample 3 | 74311.36 | 205648.43 | 0.36 |
Table 1: Peak area ratios for the deuterated standard and endogenous 2-AG. The ratios were calculated as a quotient between the peak areas of 2-AG and 1-AG-d5, respectively, for two isolated samples, both with deuterated standard added prior to extraction.
Discussion
Endocannabinoids are a class of lipids that have been implicated in the regulation of dauer formation in C. elegans7. More specifically, the synthesis of polyunsaturated fatty acids (PUFAs) is important for cholesterol trafficking and the reproductive development of worms. It is revealed here that 2-AG, an arachidonic acid containing endocannabinoid, is responsible for restituting the dauer larva to its normal cycle in worms that have impaired cholesterol metabolism7.
Given the recently discovered importance of 2-AG in the enhancement of cholesterol trafficking and other biological processes and how little is known about how lipids influence this process, a reliable detection method for this endocannabinoid is necessary. The successful development of this simple and robust synthetic method to obtain the deuterated analog 1-AG-d5 is a key step in this protocol.
Most of the reported methods to quantify monoacylglycerols involve the use of commercially available analytical standards, which are usually expensive and unstable under regular storage conditions. This makes them inconvenient for researchers who require larger quantities of standards and fresh stocks. They are also unreachable for lower budget laboratories. However, this method overcomes this obstacle by proposing synthesis of the standard using more accessible starting materials.
It is also remarkable that contrary to other reported methods (which use deuterated analytical standards of 2-substituted monoacylglycerols that suffer acyl-migration under many conditions, so that two chromatographic peaks are seen and affect the relative quantification by isotopic dilution25), this method efficiently uses a 1-substituted deuterated analytical standard, which is a single isomer and does not undergo acyl-migration.
The synthetic method is straightforward and requires no sophisticated conditions, making it ideal for any laboratory having minimal equipment, budget, and access to reactants. It is also a simple technique that can be used by any scientist working in the field, without the need for special training in organic synthesis. The worm sample preparation is the conventional method, without further complications. Finally, the lipid extraction method to obtain the final samples is a modification from Folch's protocol24 that allows for better recovery values, since it does not require chromatographic column purification.
The critical step is to ensure that the sample preparation and lipid extraction are performed adequately to achieve good and detectable recovery of the standard. It is also important to 1) produce fresh stock solutions monthly to maintain conditions of the standard and 2) check by NMR-spectroscopy or LC-MS that the standard is still pure and has not undergone oxidation or degradation. The only limitation of this technique relies in its expansion to other studies that may have endogenous 2-AG concentrations lower than the presented LOQ. In this case, the method should be modified to ensure that the concentration falls between the limits.
In the case of failure during the protocol in which 1) there is no visible chromatographic signal of the standard or 2) the recovery value of the standard after extraction is lower than expected, it is recommended to repeat sample preparation and lipid extraction. Since the synthetic route involves synthesis of a protected deuterated glycerol building block that is finally acylated with arachidonic acid in the last step, this method can be expanded to the synthesis of deuterated standards of other monoacylglycerols, diacylglicerols, phospholipids, and structurally related metabolites.
In summary, this new procedure describes a straightforward and reproducible method for detecting and quantifying 2-AG, which will help address some of the unanswered questions regarding the role of this endocannabinoid in C. elegans.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by a research grant from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT 2014-3693). J.F.d.L., G.P., and B.H.C. are fellows from CONICET. D.d.M. and G.R.L. are members of the Research Career of CONICET. We are thankful to Gonzalo Lamberto (INMET) for LC-MS/MS analysis and helpful discussion. Video shooting and editing has been done by Ramiro Ortega and María Soledad Casasola from Dirección de Comunicación de la Ciencia, Facultad de Ciencia Política y Relaciones Internacionales, Universidad Nacional de Rosario in Rosario, Argentina.
Materials
| 4-dimethylaminopyridine | Sigma-Aldrich | 107700 | reagent grade, 99% |
| antioxidant BHT | Sigma-Aldrich | W21805 | |
| Arachidonic acid | Sigma-Aldrich | 10931 | |
| Glycerol-d8 | Sigma-Aldrich | 447498 | |
| Mass detector Triple Quadrupole | Thermo Scientific | TSQ Quantum Access Max | |
| N,N’-diisopropylcarbodiimide | Sigma-Aldrich | D125407 | |
| NMR spectrometer | Bruker | Avance II 300 MHz | |
| reversed-phase HPLC column | Thermo Fisher | 25003-052130 | C18 Hypersil-GOLD (50 x 2.1 mm) |
| tert-Butyldimethylsilyl chloride | Sigma-Aldrich | 190500 | reagent grade, 97% |
| tetrabutylammonium fluoride | Sigma-Aldrich | 216143 | 1.0M in THF |
| UHPLC System | Thermo Scientific | Ultimate 3000 RSLC Dionex | |
| worm strain N2 Bristol | Caenorhabditis Genetics Center (CGC) |
References
- McPartland, J. M., Matias, I., Di Marzo, V., Glass, M. Evolutionary origins of the endocannabinoid system. Gene. 370, 64-74 (2006).
- Le Foll, B., Goldberg, S. R. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. Journal of Pharmacology and Experimental Therapeutics. 312 (3), 875-883 (2005).
- Pesce, M., Esposito, G., Sarnelli, G. Endocannabinoids in the treatment of gastrointestinal inflammation and symptoms. Current Opinion in Pharmacology. 43, 81-86 (2018).
- Hulme, S. E., Whitesides, G. M. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angewandte Chemie International Edition. 50 (21), 4774-4807 (2011).
- Aitlhadj, L., Stürzenbaum, S. R. Caenorhabditis elegans in regenerative medicine: a simple model for a complex discipline. Drug Discovery Today. 19 (6), 730-734 (2014).
- Lucanic, M., et al. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature. 473 (7346), 226-229 (2011).
- Galles, C., et al. Endocannabinoids in Caenorhabditis elegans are essential for the mobilization of cholesterol from internal reserves. Scientific Reports. 8 (1), 6398 (2018).
- Kurihara, J., et al. 2-Arachidonoylglycerol and Anandamide Oppositely Modulate Norepinephrine Release from the Rat Heart Sympathetic Nerves. The Japanese Journal of Pharmacology. 87 (1), 93-96 (2001).
- Harris, G., et al. Dissecting the Serotonergic Food Signal Stimulating Sensory-Mediated Aversive Behavior in C. elegans. PLoS ONE. 6 (7), e21897 (2011).
- Pastuhov, S. I., Matsumoto, K., Hisamoto, N. Endocannabinoid signaling regulates regenerative axon navigation in Caenorhabditis elegans via the GPCRs NPR-19 and NPR-32. Genes to Cells. 21 (7), 696-705 (2016).
- Oakes, M. D., Law, W. J., Clark, T. Cannabinoids Activate Monoaminergic Signaling to Modulate Key C. elegans Behaviors. Journal of Neuroscience. 37 (11), 2859-2869 (2017).
- Sofia, R. D., Nalepa, S. D., Harakal, J. J., Vassar, H. B. Anti-edema and analgesic properties of Δ9-tetrahydrocannabinol (THC). Journal of Pharmacology and Experimental Therapeutics. 186 (3), 646-655 (1973).
- Mills, H., et al. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. The EMBO Journal. 31 (3), 667-678 (2012).
- Lehtonen, M., Reisner, K., Auriola, S., Wong, G., Callaway, J. C. Mass-spectrometric identification of anandamide and 2-arachidonoylglycerol in nematodes. Chemistry & Biodiversity. 5 (11), 2431-2441 (2008).
- Lehtonen, M., et al. Determination of endocannabinoids in nematodes and human brain tissue by liquid chromatography electrospray ionization tandem mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 879 (11-12), 677-694 (2011).
- Aarnio, V., et al. Caenorhabditis Elegans Mutants Predict Regulation of Fatty Acids and Endocannabinoids by the CYP-35A Gene Family. Frontiers in Pharmacology. 2 (12), 12 (2011).
- Annibal, A., Karalay, &. #. 2. 1. 4. ;., Latza, C., Antebi, A. A novel EI-GC/MS method for the accurate quantification of anti-aging compound oleoylethanolamine in C. elegans. Analytical Methods. 10 (22), 2551-2559 (2018).
- Oakes, M., Law, W. J., Komuniecki, R. Cannabinoids Stimulate the TRP Channel-Dependent Release of Both Serotonin and Dopamine to Modulate Behavior in C. elegans. The Journal of Neuroscience. 39 (21), 4142-4152 (2019).
- Batugedara, H. M., et al. Helminth-Derived Endocannabinoids That Have Effects on Host Immunity Are Generated during Infection. Infection and Immunity. 86 (11), e00441 (2018).
- Zhang, M. Y., et al. Simultaneous determination of 2-arachidonoylglycerol, 1-arachidonoylglycerol and arachidonic acid in mouse brain tissue using liquid chromatography/tandem mass spectrometry. Journal of Mass Spectrometry. 45 (2), 167-177 (2010).
- Zoerner, A. A., et al. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: Minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. Journal of Chromatography B. 883-884, 161-171 (2012).
- Ivanov, I., Borchert, P., Hinz, B. A simple method for simultaneous determination of N-arachidonoylethanolamine, N-oleoylethanolamine, N-palmitoylethanolamine and 2-arachidonoylglycerol in human cells. Analytical and Bioanalytical Chemistry. 407 (6), 1781-1787 (2015).
- Keereetaweep, J., Chapman, K. D. Lipidomic Analysis of Endocannabinoid Signaling: Targeted Metabolite Identification and Quantification. Neural Plasticity. , (2016).
- Folch, J., Lees, M., Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 226 (1), 497-509 (1957).
- Zoerner, A. A., et al. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochimica et Biophysica Acta. 1811 (11), 706-723 (2011).

