A Preclinical Model to Assess Brain Recovery After Acute Stroke in Rats
Summary
The purpose of this study is to establish and validate an animal model for research in the recovery and sequela stages of brain ischemia by testing brain infarction and sensorimotor function after middle cerebral artery occlusion/reperfusion (MCAO/R) after 1-90 days in rats.
Abstract
The purpose of this study was to establish and validate an animal brain ischemia model in the recovery and sequela stages. A middle cerebral artery occlusion/reperfusion (MCAO/R) model in male Sprague-Dawley rats was chosen. By changing the rat's weight (260−330 g), the thread bolt type (2636/2838/3040/3043) and the brain infarct time (2-3 h), a higher Longa's score, a larger infarct volume and a greater model success ratio were screened using the Longa's score and TTC staining. The optimum model condition (300 g, 3040 thread bolt, 3 h brain infarct time) was acquired and used in a 1-90 day observation period after reperfusion via assessment of sensorimotor functions and infarct volume. At these conditions, the bilateral asymmetry test had a significant difference from 1 to 90 days, and the grid-walking test had a significant difference from 1 to 60 days; both differences could be a suitable sensorimotor functional test. Thus, the most appropriate condition of a novel rat model in the recovery and sequela stages of brain ischemia was found: 300 g rats that underwent MCAO with a 3040 thread bolt for a 3 h brain infarct and then reperfused. The appropriate sensorimotor functional tests were a bilateral asymmetry test and a grid-walking test.
Introduction
Brain ischemia is divided into three stages with different post-stroke indicators: the acute stage (within 1 week), the recovery stage (1 week to 6 months), and the sequelae stage (more than 6 months). Presently, most studies focus on the acute stage of brain ischemia because of its significant effect and multi-relative research models1,2,3. However, the recovery and sequelae stages of brain ischemia cannot be ignored due to their long-term complication of disabilities. Therefore, the purpose of this study is to explore a stable, reliable and relatively simple animal model to research the recovery and sequela stages of brain ischemia.
Among the many experimental brain ischemia models, we use middle cerebral artery occlusion (MCAO) via thread bolt insertion into the right middle cerebral artery (MCA). This model is similar to human stroke, which can produce larger infarct volumes, result in many behavioral disorders related to stroke, and can allow blood reperfusion (R) by removing the thread bolt4,5,6. MCAO/R is also considered the gold standard animal model of brain ischemia7. Furthermore, the severity of the brain injury depends on the diameter and the insertion length of the thread bolt, the duration of brain ischemia, and the animal weight (larger rats have bigger brains and thicker cerebral vessels)8. Therefore, by changing the thread bolt type, the infarct time, and the rat weight, a suitable model can be found for the recovery and sequela stages of brain ischemia in MCAO/R rats. To validate the rat model, we performed a 1-day, 35-day, 60-day, and 90-day study of the MCAO/R model using TTC staining and sensorimotor function experiments (a bilateral asymmetry test, a grid-walking test, a rotarod test and a lifting rope test).
Protocol
The procedure and use of animal subjects have been approved by the National Institute of Health for the care and use of laboratory animals. This protocol is specifically adjusted for the tests of middle cerebral artery occlusion/reperfusion (MCAO/R) and sensorimotor function.
1. Experimental design and grouping
- Use a rat MCAO/R model to screen a rat brain ischemic model method with more severe brain injury and greater model success ratio using Longa's score and TTC staining.
- Perform the experiment on male Sprague-Dawley rats weighing 260−330 g that are 7−9 weeks of age. The real rat weight is 275 ± 15 g for 275 g, 300 ± 10 g for 300 g, and 320 ± 10 g for 320 g.
- Use the following seven groupings (weight, thread bolt type, infarct time): group 1 with 15 rats (275 g, 2636, 2 h); group 2 with 15 rats (275 g, 2636, 3 h); group 3 with 15 rats (275 g, 2838, 2 h); group 4 with 15 rats (275 g, 2838, 3 h); group 5 with 13 rats (300 g, 3040, 3 h); group 6 with 10 rats (320 g, 3040, 3 h); group 7 with 13 rats (300 g, 3043, 3 h).
- Study the brain recovery status by TTC staining, and use suitable sensorimotor function tests to indicate the long-term functional deficits by the bilateral asymmetry test, grid-walking test, rotarod test and lifting rope test after 1, 35, 60, 90 days of MCAO/R.
- Use male Sprague-Dawley rats weighing 300 ± 10 g that are 8−9 weeks of age.
- Use the following five groupings: a control (normal) group with 20 rats; a 1 day group with 16 rats; a 35 day group with 16 rats; a 60 day group with 17 rats; and a 90 day group with 19 rats.
- After the Longa's score in step 1.1 or sensorimotor functional tests in step 1.2, anaesthetize and decapitate all rats for TTC staining.
2. Establishment of a unilateral MCAO/R model in rats9
NOTE: During the operation, use the microforceps gently to prevent breakage of the blood vessel. Avoid damage to the nerves and other blood vessels in the neck of the rat when the vessel is isolated. Care must be taken to present appropriate aseptic technique for all survival surgical procedures. The technique illustrated later in the video should be practiced through the entire procedure.
- Throughout the surgery, maintain the body temperature of the rats at 37.0 ± 0.5 °C in a small animal thermostat. Prepare four 6 cm 5-0 sutures.
- Set the oxygen flow rate of a small animal anesthesia machine (with a waste gas treatment device) at 0.4−0.6 L/min and the concentration of isoflurane to be 5%. Place the rat in the anesthesia machine.
- After the animal has fainted, place the rat on a surgical fixing table. Connect the mouth of the rat to the mask of the anesthesia machine (the oxygen flow rate remains unchanged; adjust the concentration of isoflurane to be 3%). Confirm that the animal has entered deep anesthesia by observing a lack of extremity tension, corneal reflexes, and pain.
- Fix its limbs to lie on the operating table with paper bandages (or other tools).
- Remove the neck coat with an electric shaver and sterilize with 75% alcohol (iodophor is better). Fix the mouth of the rat with a hook.
- Cut 2−3 cm along the central longitudinal shape of the neck with ophthalmic scissors.
- Separate the common carotid artery. Separate the subcutaneous muscle with ophthalmic forceps. Use homemade retractor to fully expose the field of vision. After separating the anterior muscle of the trachea with ophthalmic forceps, separate along the right sternocleidomastoid tendon until the common carotid artery is visible.
- Isolate the common carotid artery, the external carotid artery and the internal carotid artery with ophthalmic forceps. Ligate the common carotid artery (hard knot), external carotid artery far from the heart end (hard knot), and internal carotid artery (loose knot) with 5-0 sutures. Line the external carotid artery near the heart end with 5-0 sutures.
- Insert a thread bolt. Cut a small opening in the external carotid artery using ophthalmic scissors and gently insert a thread bolt. Ligate the suture of the external carotid artery that has been in loose knot and cut off the external carotid artery.
- Loosen the loose knot of the internal carotid artery and continue inserting the thread bolt to the beginning of the middle cerebral artery (suture marked). Then cut off the exposed thread bolt.
- After the ischemic time is reached (2-3 h), fix the fracture of the external carotid artery with one microforceps, and gently pull out the thread bolt with another microforceps. When the front end of the thread bolt is completely withdrawn from the internal carotid artery, ligate the external carotid artery that was lined with 5-0 sutures, and then pull out the thread bolt completely.
- Loosen the common carotid artery, and daub ~50,000 U of penicillin sodium powder on the surface of the wound to prevent infection. Suture subcutaneous muscles and skin with 4 sutures.
- Give ~0.2 mL of sterile saline to the rat orally using a 1 mL syringe (SQ-PEN injection is better) to prevent postoperative water shortage after placing the rat back to the cage.
- Choose the animals after 24 hours of reperfusion according to the Longa's score10. Select animals with a Longa's score of 1−3 for the next TTC staining in step 1.1, and animals with a Longa's score of 2−3 score for the 1, 35, 60, 90 days study in step 1.2.
NOTE: Longa's score10: 0 score, no neurological deficit; 1 score, failure to extend left foreclaw; 2 score, circling to the left; 3 score, falling to the left; 4 score, cannot walk spontaneously and has a depressed consciousness. - Analyze the Longa's score by one-way ANOVA. Values shown represent mean ± S.D. P < 0.05 indicate difference.
3. TTC staining
NOTE: The rat brain slice mold and blade must be pre-cooled in a -20 °C refrigerator before use to prevent adhesion caused by a large temperature difference. During staining, prevent adhesion between the brain slices and the culture plate, which can result in insufficient staining.
- Anesthetize the rat by intraperitoneal injection of 400 mg/kg chloral hydrate after the Longa's score in step 1.1 or sensorimotor functional tests in step 1.2.
- Decapitate the rat with surgical scissors or with a rat decapitation apparatus. Remove the brain with surgical scissors and hemostatic forceps.
- Put the brain in the refrigerator at -20 °C for 30 min to facilitate slicing.
- Remove the brain from the refrigerator and place it in pre-cooled rat brain slice mold. Cut the brain into six 2-mm-thick consecutive sections with a pre-cooled blade.
- Stain the sections with 2% 5-triphenyl-2H-tetrazolium chloride (TTC) in a 6-well culture plate.
- Culture the sections for 30−60 min at 37 °C in a shaking bed. Flip the sections every 10 min until the brain ischemia area and the normal area are clearly white and red.
- Line the brain slices vertically in order from the back to the front of brain. Use a ruler to ensure that the total length of each line is same. Take pictures with digital camera.
- Analyze the infarct volume.
- Pre-treatment the photo with photoshop software
- Import the photo using photoshop CS6. 00:00-00:14
- Click Select to select the brain slices, click Select | Inverse. 00:15-00:36
- Click Foreground to select black and click OK. 00:37-00:42
- Press Alt +Delete to fill the background color, and CTRL +D to deselect. 00:43-00:46
- Click File | Save to desktop. 00:47-01:08
- Pre-treatment the photo with Image Pro Plus software.
- Open Image Pro Plus 6.0 software and import the photo. 01:09-01:24
- For defect modification, adjust the brightness with the Contrast Enhancement tool, so that the background is black. 01:25-01:37
- Use the Median tool in Filter to remove the highlights. 01:38-01:46
- Calculate the left (normal) brain area with Image Pro Plus software
- Select color using Segmentation and adjust the value of H/S/I, so that the brain slices are separated from the black background. 01:47-02:12
- Return to Count | Size. 02:13-02:16
- Click on Count | Split Objects in Edit to separate the brain from the midline. The software will automatically distinguish the left and right brain areas. 02:17-02:49
- Calculate the right infarct brain area with Image Pro Plus software
- Implement step 3.8.1-3.8.2. 02:50-03:14
- Select Count | Size. 03:15-03:21
- Click on Draw | Merge Objects tool in Edit. Manually select the ischemic area and click Count to calculate the ischemic area. 03:16-05:31
- Calculate the health brain area with Image Pro Plus software
- Implement step 3.8.1-3.8.2. 05:32-06:44
- Select color using Segmentation and adjust the value of H/S/I, so that the normal part of brain slices are separated from the black background. 06:45-07:10
- Return to Count | Size and click on Count to calculate this area. 07:11-07:21
- Click on Split Objects in Edit to separate the brain from the midline. The software will automatically distinguish the left and right brain areas. 07:22-08:08
- Pre-treatment the photo with photoshop software
- Calculate the infarct volume (%) and infarct and shrink volume (%):
Infarct volume (%) = [right infarct area/(2 x left brain area)] x 100.
Infarct and shrink volume (%) = [(left brain area – right health brain area) / (2 x left brain area)] x 100.
NOTE: The right brain is the injured part. The data were analyzed by one-way ANOVA. Values shown represent mean ± S.D. P < 0.05 indicate difference.
4. Assessment of sensorimotor function
NOTE: Rats (300 g, 3040 thread bolt, 3 h brain infarct time) with a Longa's score of 2−3 were selected to perform the sensorimotor function experiments from 1-90 days. Keep quiet and do not disturb the animals during this period of study. The data were analyzed by two-way ANOVA. Values shown represent mean ± S.D. P < 0.05 indicate difference.
- Bilateral asymmetry test11
- Wrap paper tape (5 cm long, 0.8 cm wide) on the saphenous part of each foreclaw of a rat three times with equal pressure.
- For each rat, record the number of times each foreclaw is contacted and tape removed in 5 min with camera, including unaffected paw times and affected paw times.
- After 30 min, repeat steps 4.1.1 and 4.1.2 again.
- Calculate the average value of the sensorimotor bias (%):
Sensorimotor bias (%) = (unaffected paw times – affected paw times)/(unaffected paw times + affected paw times) x 100
- Grid-walking test
- Place the rat in the center of an elevated grid surface platform (area: 1 m2; height: 90 cm) with grid openings of 2.5 cm2.
- Push the rat's hips lightly to encourage the rat to traverse the grid surface.
- Record the number of foot faults made by the unaffected (right) and affected (left) limbs and the total step number in 1 min with camera.
- Calculate the error times:
Error times (%) = [unaffected (right) limb – affected (left) limb]/total step number x 100.
NOTE: Total number of steps below 20-step data were removed.
- Rotarod test12,13
- Set up the rat rotating bar fatigue apparatus (diameter 90 mm) of rats using the supporting software to a speed of 13 rpm over a 5 min period on the computer.
- Start the computer programs and place the rat on the rotarod rungs at the same time.
- End a trial if the rat falls off the rung or keeps walking for 5 min and record the rotating time.
- Have the rat rest for 30 min.
- Repeat steps 4.3.2−4.3.4 twice more and choose the maximum value to be the last rotating time.
- Lifting rope test14
- Place the lifting rope instrument (70 cm high; the rope is 0.2 cm in diameter and 40 cm long) on the desk.
- Have the rat grip the rope with its forelimbs and hang the rat.
- Record the time of hanging and calculate the scores.
NOTE: A score of 3: 0−2 s on the rope; A score of 2: 3−4 s on the rope; A score of 1: 5−6 s on the rope; A score of 0: more than 7 s on the rope.
Representative Results
Using the abovementioned procedure for a MCAO/R model with a Longa's score and TTC staining, different treatments of average weight (275/300/320 g), bolt types (2636/2838/3040/3043; Table 1) and ischemic times (2-3 h) and 1 day reperfusion were used to screen for the optimal brain ischemia model in rats. Model parameters of 300 g weight, 3040 thread bolt, and 3 h brain infarct time were the most suitable for the largest cerebral infarction, highest Longa's score and greatest model success ratio. This was significantly improved on the conventional treatment of a 275 g weight, 2636 thread bolt, and 2 h brain infarct time (Figure 1).
Furthermore, rats with 300 g weight, 3040 thread bolt, 3 h brain infarct time and a 2−3 Longa's score underwent sensorimotor function tests (a bilateral asymmetry test, a grid-walking test, a rotarod test, and a lifting rope test) and TTC staining to study the recovery status of brain ischemia from 1-90 days. The infarct and shrink volume were 23.4%, 19.6%, 16.1% (P < 0.01, compared with the first day) and 15.7% (P < 0.01, compared with the first day) after 1, 35, 60, and 90 days post MCAO/R, respectively (Figure 2). On the first day after MCAO/R, infarct volume was biggest. In time, the infarct volume became smaller and the shrink volume became larger. The infarct and shrink volume no longer changed after 60 days of MCAO/R.
The sensorimotor bias in the bilateral asymmetry test, the grid-walking error times in the grid-walking test and the lifting rope score in the lifting rope test all significantly increased, while the rotarod time in the rotarod test decreased significantly after 1 day of MCAO/R (Figure 3), which indicated that all four tests were meaningful in the stage of acute brain ischemia. However, only sensorimotor bias maintained large functional disorders with a time-dependent manner after 35, 60 and 90 days of MCAO/R. There were significant differences of grid-walking error times in the grid-walking test after 35 and 60 days of MCAO/R. These results indicated that the bilateral asymmetry test and the grid-walking test could be suitable sensorimotor function tests for the stage of recovery and sequela in rats.
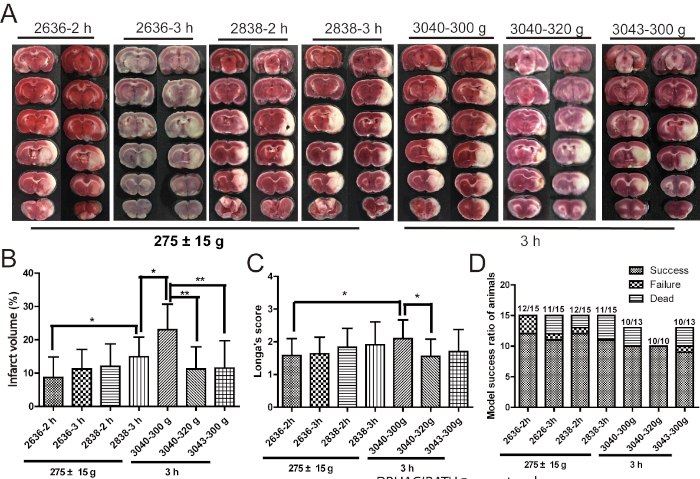
Figure 1: 300 g weight, 3040 thread bolt, 3 h brain infarct time may be the optimum condition of the brain ischemic injury induced by MCAO/R. (A,B) Pictures and cartogram of infarct volume of brain tissue (n = 9−12). (C) Longa's score (n = 9−12). (D) The statistics of model success ratio of rats (n = 10−15). Model success ratio = (total number of rats – death rats after MCAO/R – failure rats after MCAO/R)/total number of rats. Failure rats are the model rats that do not have a suitable Longa's score. Error bars represent S.D., *P < 0.05, **P < 0.01. This figure has been modified from Liu et al.15. Please click here to view a larger version of this figure.
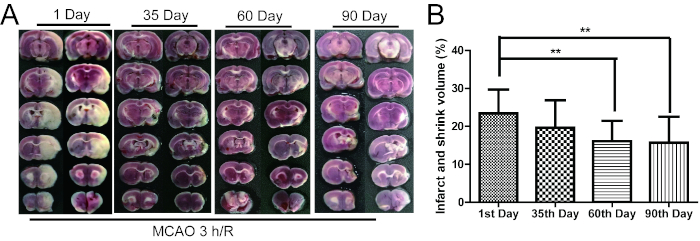
Figure 2: The infarct and shrink volume gradually decreased from 1 to 90 days after MCAO/R. (A) The TTC staining of rat brain tissue. (B) The cartogram of infarct and shrink volume (n = 16−19). Error bars represent S.D., **P < 0.01 vs. the first day after MCAO/R. This figure has been modified from Liu et al.15. Please click here to view a larger version of this figure.
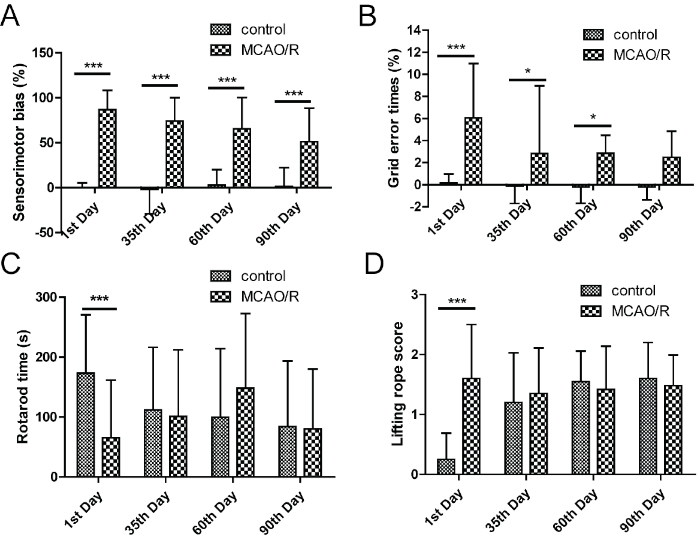
Figure 3: Bilateral asymmetry test and grid-walking test were the suitable sensorimotor function tests in the recovery and sequela stage of brain ischemia. (A) The right limb tearing favorability in debonding experiment. (B) The grid-walking error times in grid-walking test. (C) The length of time in rotarod test. (D) The score in lifting rope test. Error bars represent S.D., n = 15−19, *P < 0.05, ***P < 0.001. This figure has been modified from Liu et al.15. Please click here to view a larger version of this figure.
| Type | The diameter of thread bolt | The diameter of thread bolt head | Recommended weight of rat | Level |
| 2636 | 0.26 mm | 0.36 mm | 250-280 g | A4 |
| 2838 | 0.28 mm | 0.38 mm | 280-350 g | A4 |
| 3040 | 0.30 mm | 0.40 mm | 360-400 g | A4 |
| 3043 | 0.30 mm | 0.43 mm | >400 g | A4 |
| Note: A4 level thread bolt is the standard that the head end is hemispherical, the front end is covered with poly-lysine, marked, sterilized, and buy-on-use without any treatment (This Table has been modified from Liu et al., 2018). | ||||
Table 1: Thread blot information. This table has been modified from Liu et al.15.
Discussion
Many models establishing methods and behavioral indicators that are well used in acute cerebral ischemia may not have significant changes in the recovery and sequela stages of brain ischemia16,17. However, the number of patients with brain ischemic in the recovery and sequela stages is the greatest. It is essential to select a suitable animal model for the recovery and sequela stages of ischemia stroke.
We use the MCAO/R model in rats to screen the suitable weight of rats (260−330 g), the type of thread bolt (2636/2838/3040/3043), and the time of brain infarct (2-3 h) for the most severe infarct injury, a high model success ratio, and visible behavioral indicators, which will be suitable for the recovery and sequelae stages of brain ischemia.
Rats that weigh 300 g with a 3040 thread bolt and 3 h brain infarct time have larger infarct volumes, more severe behavioral defects, and a greater model success ratio (Figure 1). Furthermore, we provided validation methods of this rat model by TTC staining and sensorimotor function tests (bilateral asymmetry test, grid-walking test, rotarod test and lifting rope test) 1-90 days after reperfusion. We found that the bilateral asymmetry test and grid-walking test could be used to research the recovery and sequela stages of ischemia because the significant differences of these indicators last 90 days and 60 days, respectively. The larger the infarct and shrink volume is, the more severe the sensorimotor deficits, which can be seen in Figure 2 and Figure 3.
This method is mainly suitable for brain ischemia caused by MCAO. However, the model has differences in brain anatomies between humans and rats, such as the grade of collateral circulation. Another limitation is that white matter recovery cannot be seen by TTC staining. Further studies of collateral circulation and white matter recovery with MR imaging or other methods can confirm the predictive value of this model.
The most critical matter is that the skill of creating a MCAO/R model in rats is not easy and requires practice. Before the experiment, confirm an acceptable and parallel model success ratio. More instruments and methods are needed to test the sensorimotor function in the recovery and sequela stages of stroke. If a more difficult task, such as increasing the speed from 10 to 30 rpm was used, a longer period of deficit may appear in the rotarod test. Other behavioral tests may be also suitable for this model, such as gait detection. More precise detection methods should be used for patients in the recovery and sequela stages of brain ischemia, which can identify the effect of drug or other therapeutic tools.
As a new animal model to study brain ischemia in the recovery and sequela stages, the method presented here is meaningful and deserves popularization.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81603315, 81603316), Key R & D plan of Jiangxi province in China (20171ACH80001), Industrial and academic cooperation projects in colleges and universities of Fujian province in China (2018Y41010011).
Materials
| Anatomical Microscope | Leica (Germany) | S8 | Microscopic operating instrument |
| Blade | Gellette | / | Cutting brain sections |
| Constant Temperature Shaking Bed | Taicang Experimental Equipment Factory | THZ-C | To keep the brain sections stained evenly and at a constant temperature |
| Digital Camera | Canon | 700D | For taking pictures of TTC staining |
| Electric Shaver | Shanghai Yuyan Scientific Instruments Co., Ltd. | 3000# | Removal of hair from the neck of rats |
| Forceps Hamostatic | Shanghai Medical device Co., Ltd. | 14 cm | Using for brain removing |
| Image Pro Plus Software | Media Cybernetics Inc. | 6.0 | Analyze the infarct volume |
| Isoflurane | RWD Life Science | 217170702 | Anesthetic gas |
| Microforceps | Shanghai Jinzhong Medical Devices Co., Ltd. | 10 cm | Vascular micromanipulation |
| Microshear | Shanghai Jinzhong Medical Devices Co., Ltd. | 10 cm | Vascular micromanipulation |
| Ophthalmic Forceps | Shanghai Jinzhong Medical Devices Co., Ltd. | 10 cm | Auxiliary skin and muscle anatomy |
| Pphthalmic Scissors | Shanghai Jinzhong Medical Devices Co., Ltd. | 10 cm | Using for cutting the skin of neck |
| Rat Brain Slice Mold | Shanghai Yuyan Scientific Instruments Co., Ltd. | 400 g | For standard, uniform cutting of brain tissue |
| Rat Rotating Bar Fatigue Apparatus | Anhui Zhenghua Biological Instrument and Equipment Co., Ltd. | ZH-300B | To test the sensorimotor function |
| Small Animal Anaesthesia Machine | Shanghai Yuyan Scientific Instruments Co., Ltd. | ABM3000 | A gas anesthetic machine |
| Small Animal Thermostat | Beijing Damida Technology Co., Ltd. | DM.7-YLS-20A | To maintain animal body temperature constant during operation |
| Surgical Scissors | Shanghai Medical device Co., Ltd. | 16 cm | Using for decapitate and brain removing |
| Suture | Shanghai Jinhuan Medical Devices Co., Ltd. | 4-0 / 5-0 | Using for skin and muscle sutures / Using for vascular ligations |
| Thread Bolt | Beijing Cinontech Co. Ltd. | 2636/2838/3040/3043-A4 | Blockage of the middle cerebral artery in rats |
| 5-triphenyl-2H-tetrazolium chloride (TTC) | Sigma | LOT#BCBP3272V | Brain section staining reagent |
References
- Kong, L. L., et al. Neutralization of chemokine-like factor 1, a novel C-C chemokine, protects against focal cerebral ischemia by inhibiting neutrophil infiltration via MAPK pathways in rats. Journal of Neuroinflammation. 11, 112 (2014).
- Jiang, M., et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. Journal of Neuroinflammation. 11, 167 (2014).
- Thomas, A., Detilleux, J., Flecknell, P., Sandersen, C. Impact of Stroke Therapy Academic Industry Roundtable (STAIR) Guidelines on Peri-Anesthesia Care for Rat Models of Stroke: A Meta-Analysis Comparing the Years 2005 and 2015. PLoS One. 12, e0170243 (2017).
- Kumar, A., Aakriti, V., Gupta, A review on animal models of stroke: An update. Brain Research Bulletin. 122, 35-44 (2016).
- Tong, F. C., et al. An enhanced model of middle cerebral artery occlusion in nonhuman primates using an endovascular trapping technique. AJNR Am. Journal of Neuroradiology. 36, 2354-2359 (2015).
- Li, F., Omae, T., Fisher, M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke. 30, 2464-2470 (1999).
- Herson, P. S., Traystman, R. J. Animal models of stroke: translational potential at present and in 2050. Future Neurology. 9, 541-551 (2014).
- Abrahám, H., Somogyvári-Vigh, A., Maderdrut, J. L., Vigh, S., Arimura, A. Filament size influences temperature changes and brain damage following middle cerebral artery occlusion in rats. Exp. Brain Res. 142, 131-138 (2002).
- Sun, M. N., et al. Coumarin derivatives protect against ischemic brain injury in rats. European Journal of Medicinal Chemistry. 67, 39-53 (2013).
- Longa, E. Z., Weinstein, P. R., Carlson, S., Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20, 84-91 (1989).
- Smith, E. J., et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cell. 30, 785-796 (2012).
- Zhang, S., et al. Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology. 52, 72-83 (2016).
- Milani, D., et al. Poly-arginine peptides reduce infarct volume in a permanent middle cerebral artery rat stroke model. BMC Neuroscience. 17, 19 (2016).
- DeGraba, T. J., Ostrow, P., Hanson, S., Grotta, J. C. Motor performance, histologic damage, and calcium influx in rats treated with NBQX after focal ischemia. Journal of Cerebral Blood Flow and Metabolism. 14, 262-268 (1994).
- Liu, P., et al. Validation of a preclinical animal model to assess brain recovery after acute stroke. European Journal of Pharmacology. 835, 75-81 (2018).
- Zuo, W., et al. IMM-H004 prevents toxicity induced by delayed treatment of tPA in a rat model of focal cerebral ischemia involving PKA-and PI3K-dependent Akt activation. European Journal of Neuroscience. 39, 2107-2118 (2014).
- Yang, L., et al. L-3-n-butylphthalide Promotes Neurogenesis and Neuroplasticity in Cerebral Ischemic Rats. CNS Neuroscience & Therapeutics. 21, 733-741 (2015).

