Induction of Eryptosis in Red Blood Cells Using a Calcium Ionophore
Summary
A protocol for the induction of eryptosis, programmed cell death in erythrocytes, using the calcium ionophore, ionomycin, is provided. Successful eryptosis is evaluated by monitoring the localization phosphatidylserine in the membrane outer leaflet. Factors affecting the success of the protocol have been examined and optimal conditions provided.
Abstract
Eryptosis, erythrocyte programmed cell death, occurs in a number of hematological diseases and during injury to erythrocytes. A hallmark of eryptotic cells is the loss of compositional asymmetry of the cell membrane, leading to the translocation of phosphatidylserine to the membrane outer leaflet. This process is triggered by increased intracellular concentration of Ca2+, which activates scramblase, an enzyme that facilitates bidirectional movement of phospholipids between membrane leaflets. Given the importance of eryptosis in various diseased conditions, there have been efforts to induce eryptosis in vitro. Such efforts have generally relied on the calcium ionophore, ionomycin, to enhance intracellular Ca2+ concentration and induce eryptosis. However, many discrepancies have been reported in the literature regarding the procedure for inducing eryptosis using ionomycin. Herein, we report a step-by-step protocol for ionomycin-induced eryptosis in human erythrocytes. We focus on important steps in the procedure including the ionophore concentration, incubation time, and glucose depletion, and provide representative result. This protocol can be used to reproducibly induce eryptosis in the laboratory.
Introduction
Programmed cell death in erythrocytes, also known as eryptosis, is common in many clinical conditions and hematological disorders. Eryptosis is associated with cell shrinkage and the loss of phospholipid asymmetry in the cell plasma membrane1,2. Loss of asymmetry results in the translocation of phosphatidylserine (PS), a lipid normally localized in the inner leaflet3,4, to the cell outer leaflet, which signals to macrophages to phagocytose and remove defective erythrocytes5,6,7,8. At the end of the normal life span of erythrocytes, removal of eryptotic cells by macrophages ensures the balance of erythrocytes in circulation. However, in diseased conditions, such as sickle cell disease and thalassemia9,10,11, enhanced eryptosis may result in severe anemia2. Due to its importance in hematological diseases, there is significant interest in examining the factors inducing or inhibiting eryptosis and the molecular mechanisms underlying this process.
The plasma membrane of healthy erythrocytes is asymmetric, with different phospholipids localizing at the outer and inner leaflets. Membrane asymmetry is primarily regulated by the action of membrane enzymes. Aminophospholipid translocase facilitates the transport of aminophospholipids, PS and phosphatidylethanolamine (PE), by directing these lipids to the cell inner leaflet. On the other hand, floppase transports the choline containing phospholipids, phosphatidylcholine (PC) and sphingomyelin (SM), from the inner to the outer leaflet of the cell membrane12. However, unlike healthy cells, the membrane of eryptotic erythrocytes is scrambled. This is due to the action of a third enzyme, scramblase, which disrupts phospholipid asymmetry by facilitating the bidirectional transport of aminophospholipids13,14,15,16. Scramblase is activated by elevated intracellular levels of Ca2+. Therefore, calcium ionophores, which facilitate the transport of Ca2+ across the cell membrane12, are efficient inducers of eryptosis.
Ionomycin, a calcium ionophore, has been widely used to induce eryptosis in erythrocytes12,17,18,19,20,21,22,23,24,25,26. Ionomycin has both hydrophilic and hydrophobic groups, which are necessary to bind and capture Ca2+ ion, and transport it to the cytosolic space27,28,29. This leads to the activation of scramblase and translocation of PS to the outer leaflet, which can be easily detected using annexin-V, a cellular protein with a high affinity to PS12. Although triggering eryptosis by ionomycin is commonly reported, there is considerable method discrepancy in the literature (Table 1). The population of erythrocytes undergoing eryptosis depends on different factors such as ionophore concentration, treatment time with ionophore, and the sugar content of extracellular environment (glucose depletion activates cation channels and facilitates the entry of Ca2+ into the cytosolic space)30,31. However, there is little consistency in these factors in the literature, making it difficult to perform eryptosis reproducibly in vitro.
In this protocol, we present a step-by-step procedure to induce eryptosis in human erythrocytes. Factors affecting successful eryptosis including Ca2+ concentration, ionophore concentration, treatment time, and pre-incubation in glucose-depleted buffer are examined and optimal values are reported. This procedure demonstrates that pre-incubation of erythrocytes in a glucose-free buffer significantly increases the percentage of eryptosis compared to glucose-containing buffer. This protocol can be used in the laboratory to produce eryptotic erythrocytes for various applications.
Protocol
All human blood samples used in the protocol described below were purchased as de-identified samples. No human subjects were directly involved or recruited for this study. The guidelines of the Declaration of Helsinki should be used when research involves human subjects.
1. Erythrocyte isolation from whole blood
- Add 500 µL of whole blood in acid citrate dextrose (ACD) (stored at 4 °C) to a microcentrifuge tube.
NOTE: Whole blood was purchased in ACD. According to the company, 1.5 mL of ACD is added to 7 mL of whole blood (8.5 mL total volume). - Centrifuge the whole blood at 700 x g for 5 min at room temperature (RT) and remove the clear plasma and the thin buffy coat using a pipette to leave the red erythrocyte layer.
- Prepare 1 L of Ringer solution containing 125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 32 mM HEPES, 5 mM glucose, and 1 mM CaCl2. Adjust the pH to 7.4 by adding 2 µL drops of 1.0 M NaOH. To prepare glucose-free Ringer solution, follow the same protocol, but do not include glucose in the solution.
- Wash the erythrocytes 2x in Ringer solution by suspending the cell pellet in 1.5 mL of Ringer solution, centrifuging at 700 x g for 5 min at RT, and removing the supernatant.
- Make a 0.4% hematocrit by resuspending 40 µL of the erythrocyte pellet in 9,960 µL of glucose-free Ringer solution to reach a final volume of 10 mL.
NOTE: Hematocrit is a term used to refer to the volume fraction of erythrocytes in suspension. A 0.4% hematocrit is a suspension containing 0.4% erythrocytes. - Incubate the cell suspension at 37 °C for 7 days.
2. Treatment of erythrocytes with ionomycin and measurement of hemolysis
- Dissolve 1 mg of ionomycin calcium salt in 630 µL of dimethyl sulfoxide (DMSO) to reach a final concentration of 2 mM. Aliquot and store at -20 °C.
- Take 1 mL of the 0.4% hematocrit from step 1.5 and add 0.5 µL of 2 mM ionomycin to reach a final concentration of 1 µM. Incubate for 2 h at 37 °C.
- Use 1 mL of the hematocrit with no ionomycin treatment as a negative control.
- Centrifuge the ionomycin-treated and untreated hematocrits at 700 x g for 5 min at RT, and remove their supernatants to leave the cell pellets at the bottom of the tubes.
- Wash the cells 3x with Ringer solution by suspending the cell pellets in 1.5 mL of Ringer solution, centrifuging at 700 x g for 5 min at RT and discarding the supernatants.
- To measure hemolysis, add 1 mL of the untreated 0.4% hematocrit from step 1.5 to a microcentrifuge tube and incubate for 2 h at 37 °C as the negative control for hemolysis (0%).
- Add 1 mL of the untreated 0.4% hematocrit from step 1.5 to a microcentrifuge tube and centrifuge at 700 x g for 5 min at RT. Remove the supernatant and add 1 mL of distilled water to the cell pellet and incubate for 2 h at 37 °C as the positive control for hemolysis (100%).
- Add 1 mL of the ionomycin-treated 0.4% hematocrit from step 2.2 to a microcentrifuge tube.
- Centrifuge the untreated cells, treated cells, and the cells in distilled water at 700 x g for 5 min at RT.
- Take 200 µL of the supernatants and add to a 96-well plate.
- Measure the absorbance at 541 nm using a microplate reader.
- Calculate the hemolysis using Equation 132:
%Hemolysis = (AT – A0)/(A100 – A0)*100
where A0 is the absorbance of erythrocytes in Ringer solution, A100 is the absorbance of erythrocytes in water, and AT is the absorbance of treated erythrocytes by ionomycin.
3. Annexin-V binding assay
- Dilute 2 mL of the 5x annexin V binding buffer in 8 mL of phosphate-buffered saline (PBS) to obtain 1x binding buffer.
- Resuspend the ionomycin-treated and untreated cell pellets from step 2.4 in 1 mL of 1x binding buffer.
- Take 235 µL of the cell suspensions in the binding buffer and add 15 µL of Annexin V-Alexa Flour 488 conjugate.
- Incubate the cells at RT for 20 min in a dark place. Centrifuge at 700 x g for 5 min at RT and remove the supernatant.
- Wash the cells 2x with 1x binding buffer, by suspending the cell pellet in 1.5 mL of the binding buffer, centrifuging at 700 x g for 5 min at RT and removing the supernatant.
- Resuspend the cell pellets in 250 µL of 1x binding buffer for flow cytometry measurements.
4. Flow cytometry
- Transfer 200 µL of the annexin-V stained erythrocytes to 1 mL round bottom polystyrene tubes compatible with flow cytometry.
- Login to the flow cytometry software and click on the new experiment button. Click on the new tube button. Select the global sheet and choose the apply analysis to measure the fluorescence intensity with an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
- Set number of cells to 20,000 to be collected for fluorescence-activated cell sorting (FACS) analysis.
- Select the desired tube and click on load button. Click on record button for forward scatter and side scatter measurements. Repeat for all samples.
- Right click on specimen button and click on apply batch analysis to generate the result file.
- Right click on specimen button and click on generate FSC files.
- Add the flow cytometry data (FSC files) into the workplace of flow cytometry software.
- Analyze the control data by selecting the cell population of interest and adding statistics for eryptosis value.
- Double click on control and select histogram versus fluorescence intensity.
- Click on gate button to draw a gate on the histogram which indicates the percentage of eryptosis.
- Apply the same statistics for all other experimental tubes to obtain the eryptosis values. Right click on control and select copy analysis to group.
- After properly gating all samples, transfer the analyzed data by dragging and dropping them into the layout editor.
- Overlay the analyzed data with control in layout editor.
- Set the desired histograms and intensities by changing the x and y axis of the overlaid graphs.
- Export image files by clicking on export button and save the graphs in desired location.
5. Confocal microscopy
- Transfer 5 µL of annexin-V-stained cells on a microscope slide and cover it with a cover slip. Keep in a dark place to prevent photobleaching.
- Use Argon laser of the confocal fluorescence microscope to observe the cells excited at 488 nm with desired magnifications.
NOTE: A confocal microscope is not necessarily needed and any microscope with fluorescence capabilities can be used to obtain fluorescence images that demonstrate annexin-V binding. - Obtain fluorescence images of the control (non-treated cells) and treated cells.
NOTE: Non-treated cells are expected to show very weak fluorescence signals, whereas treated cells are expected to show bright green fluorescence on their membranes.
Representative Results
Optimization of ionomycin concentration
While ionomycin is required to induce eryptosis, increased ionomycin concentrations can lead to hemolysis (i.e. lysis of erythrocytes and release of hemoglobin), which needs to be avoided. Treatment of erythrocytes with 1 µM ionomycin in Ringer solution for 2 h is enough to induce eryptosis, as evidenced by successful labeling with annexin-V Alexa Flour 488 conjugate and quantification by FACS analysis (Figure 1A). Higher concentrations of ionomycin (5 and 10 µM) result in a slight increase in eryptosis (Figure 1A-D). However, such concentrations also enhance hemolysis (Figure 1E), which is not desired. In order to stay below 5% hemolysis, 1 µM ionomycin should be used.
Treatment time with ionomycin
Incubation of erythrocytes with ionomycin in Ringer solution for as little as 30 min is enough to induce eryptosis (Figure 2A). Increased incubation time increases the level of eryptosis, as measured by the annexin V-binding assay, for up to 2 h (Figure 2B,C). However, further incubation time results in a slight decrease in the level of eryptosis (Figure 2D). Maximum eryptosis was obtained after 2 h of treatment with 1 µM ionomycin, and for all other treatment times, lower eryptosis was obtained (Figure 2E). Representative flow cytometry histograms are presented in Figure 2A-D. In addition, average percentage eryptosis and hemolysis, for various treatment times with 1 µM ionomycin, are presented in Figure 2E and Figure 2F, respectively. The higher value of hemolysis after 180 min explains the reduction in eryptosis after the same amount of incubation (Figure 2E) as less viable cells exist upon 180 min of treatment with ionomycin.
Moreover, cells were treated with low concentrations of ionomycin including 0, 0.25, 0.5, and 1 µM for longer treatment times including 6 and 12 h, and eryptosis was measured (Figure 3). Cells treated with ionomycin concentrations of lower that 1 µM for 6 and 12 h show lower eryptosis compared to the cells treated with 1 µM ionomycin (Figure 3). Since decreasing the concentration and increasing the exposure time did not enhance eryptosis, 1 µM was used to trigger eryptosis.
Eryptosis is dependent on incubation time and extracellular glucose concentration
Extracellular glucose concentration affects the outcome of the process. Higher eryptosis values are observed when erythrocytes are pre-incubated in glucose-free Ringer solution compared to glucose-containing Ringer solution prior to incubation with 1 µM ionomycin for 2 h. The highest eryptosis values are obtained after 7 days of pre-incubation in both solutions. However, eryptosis is higher after pre-incubation in glucose-free Ringer solution compared to normal Ringer solution, which contains 5 mM glucose (see Figure 4A for representative plots and Figure 4B for comparison of global means). In addition, forward scatter histograms indicate the effect of glucose depletion on erythrocyte shrinkage (Figure 5A-D). Forward scatter is a measure for cell size based on the light refraction, and the level of light scattered is directly proportional to the size of cells33. The cells incubated in glucose-free Ringer solution show less forward scatter compared to the cells incubated in glucose-containing buffer (Figure 5E), indicating cell shrinkage in the glucose-free environment.
In addition to flow cytometry measurements, cells were observed under a confocal fluorescence microscope to confirm eryptosis. Erythrocytes with no treatment (Figure 6A) and with ionomycin treatment (Figure 6B) were labeled with annexin-V Alexa Flour 488 conjugate and observed under microscope. Treated cells showed a bright fluorescence signal (Figure 6B) due to the binding of annexin-V to PS in the outer leaflet. In contrast, cells with no treatment showed a very weak fluorescence signal (Figure 6A) indicating very low eryptosis. Further example images of eryptotic erythrocytes labeled with annexin-V with high fluorescence signal are shown in Figure 6C.
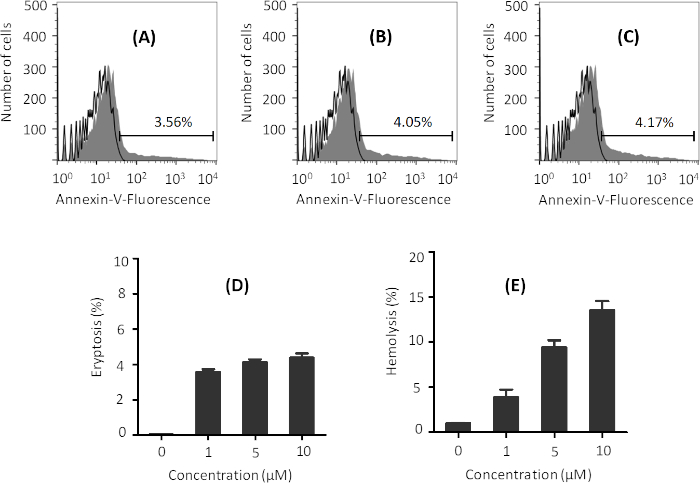
Figure 1: Representative graphs of the effect of various ionomycin concentrations on eryptosis and hemolysis. Flow cytometry histograms of erythrocytes treated with (A) 1 µM, (B) 5 µM, and (C) 10 µM ionomycin (gray) at 37 °C at 0.4% hematocrit in Ringer solution for 2 h. Black line indicates non-treated cells. Percentage of eryptosis is indicated in each figure. Phosphatidylserine exposure was measured using annexin-V binding. (D) Arithmetic means ± SD (n = 3) of the percentage eryptosis of cells treated with different concentrations of ionomycin after 2 h treatment, and (E) arithmetic means ± SD (n = 3) of hemolysis of erythrocytes by different concentrations of ionomycin under same conditions. Please click here to view a larger version of this figure.
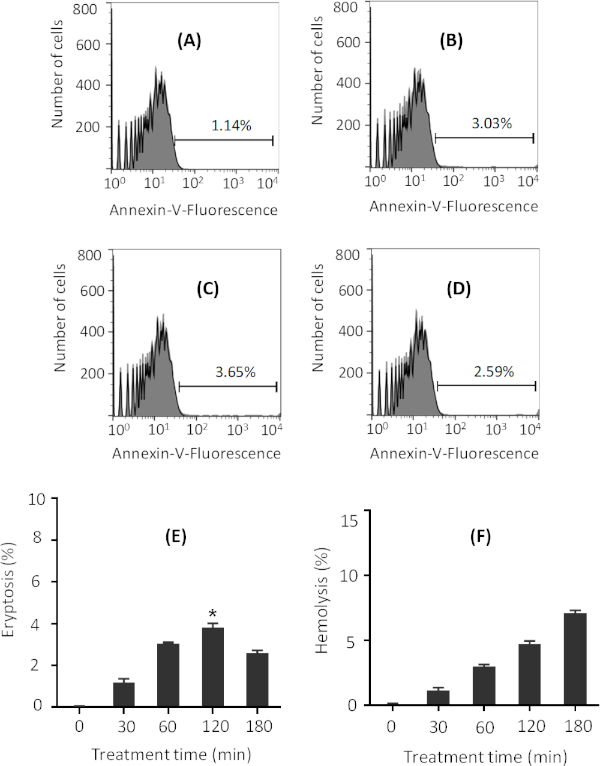
Figure 2: Representative figures on the effect of various ionomycin treatment times on eryptosis. Flow cytometry histograms of erythrocytes treated with 1 µM ionomycin (gray) at 37 °C for (A) 30 min, (B) 60 min, (C) 120 min, and (D) 180 min at 0.4% hematocrit in Ringer solution. Black line indicates non-treated cells. Percentage of eryptosis is indicated in each figure. Phosphatidylserine exposure was measured through annexin-V binding. (E) Arithmetic means ± SD (n = 3) of percentage eryptosis of cells treated with 1 µM ionomycin for different times. The highest eryptosis was obtained after 120 min treatment. (F) Arithmetic means ± SD (n = 3) of percentage hemolysis of cells treated with 1 µM ionomycin for different times. For statistical analysis, one-way non-parametric ANOVA with Kruskal-Wallis test was performed, and eryptosis after 120 min treatment was significantly higher than control as indicated in panel E. * is for p < 0.05. Please click here to view a larger version of this figure.
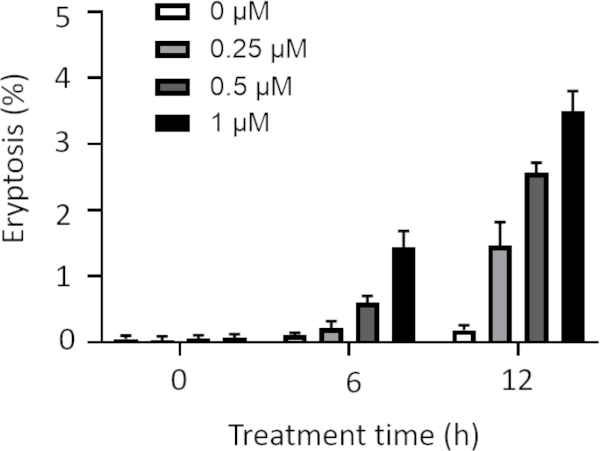
Figure 3: Effect of various ionomycin concentrations and treatment times on eryptosis. Arithmetic means ± SD (n = 3) of the percentage eryptosis of cells treated with different concentrations of ionomycin is shown after various treatment times. The cells were treated with low concentrations of ionomycin including 0, 0.25, 0.5, and 1 µM for longer exposure (6 h and 12 h). Higher concentrations and longer treatments resulted in higher eryptosis values. Please click here to view a larger version of this figure.
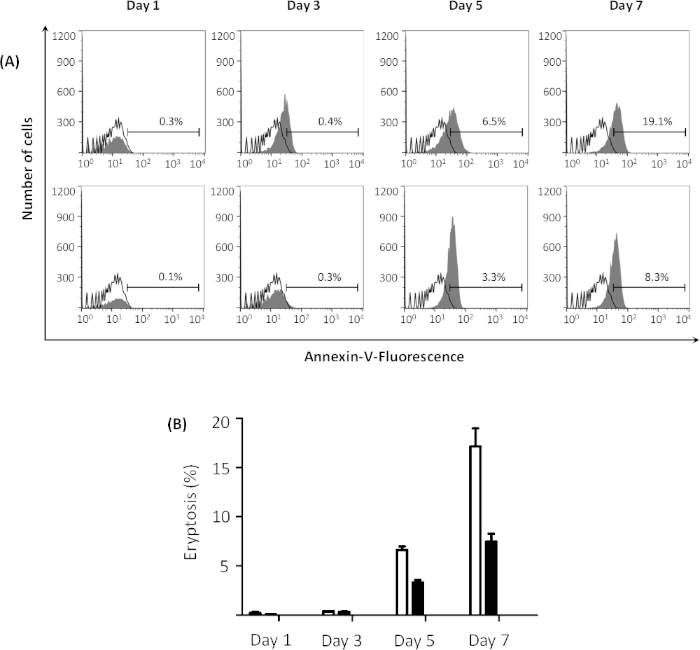
Figure 4: Effect of energy depletion on eryptosis. (A) Flow cytometry histogram for erythrocytes treated with 1 µM ionomycin (gray) at 37 °C for 2 h at 0.4% hematocrit, after pre-incubation in glucose-free Ringer solution (top figures) and Ringer solution (bottom figures) from 1 to 7 days, reveals that energy depletion facilitates eryptosis. Black line indicates non-treated cells. Percentages of eryptosis are indicated in the graphs for each day. (B) Arithmetic means ± SD (n = 3) of the percentage eryptosis of erythrocytes treated with 1 µM ionomycin at 37 °C for 2 h at 0.4% hematocrit, after pre-incubation in Ringer solution (black bars) and glucose-free Ringer solution (white bars) from 1 to 7 days. Please click here to view a larger version of this figure.
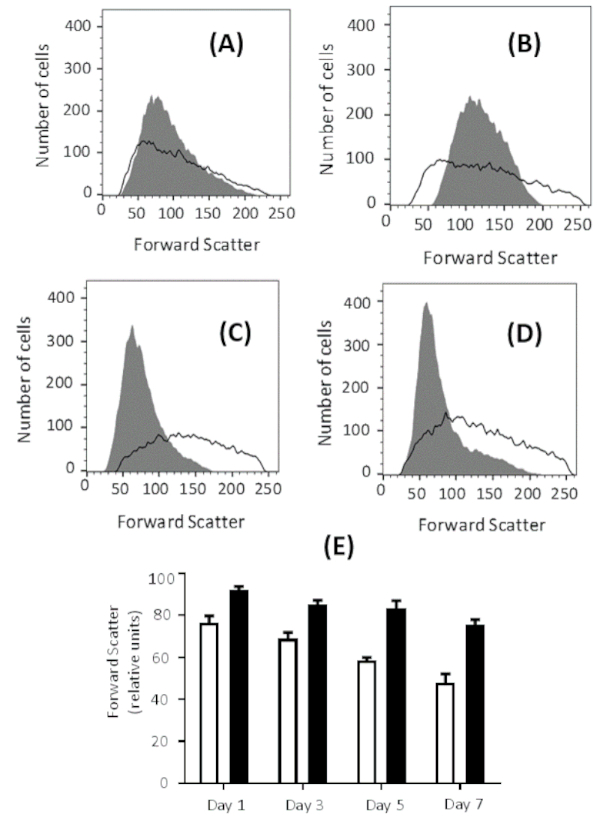
Figure 5: Effect of energy depletion on cell size. Forward scatter histogram for erythrocytes treated with 1 µM ionomycin at 37 °C for 2 h at 0.4% hematocrit, after pre-incubation in glucose-free Ringer solution (gray) and Ringer solution (black line) for (A) 1 day, (B) 3 days, (C) 5 days, and (D) 7 days. The forward scatter histogram over time indicates erythrocyte shrinkage in glucose-free buffer. (E) Arithmetic means ± SD (n = 3) of forward scatter intensities of erythrocytes treated with 1 µM ionomycin at 37 °C for 2 h at 0.4% hematocrit, after pre-incubation in Ringer solution (black bars) and glucose-free Ringer solution (white bars) from 1 to 7 days. Please click here to view a larger version of this figure.
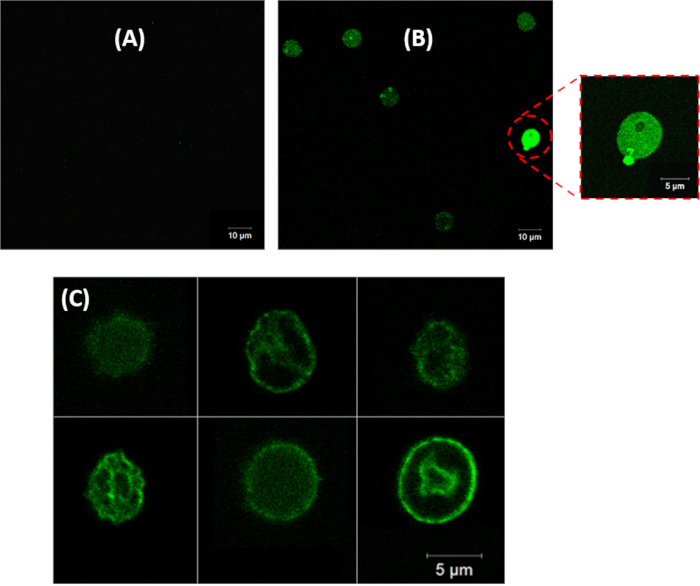
Figure 6: Confocal fluorescence microscopy images of erythrocytes treated with (A) 0 µM, (B) and (C) 1 µM ionomycin at 37 °C for 2 h at 0.4% hematocrit. 40x objective magnification was used for images in panels A and B, and 100x objective magnification was used to take images for panel C. PS in healthy erythrocytes is located on the inner leaflet of the cell membrane, therefore there is no fluorescence signal in panel A. In panels B and C erythrocytes have been induced for eryptosis and there is a bright fluorescence signal resulting from the binding of annexin-V to PS translocated to the outer leaflet of the cell membrane. Please click here to view a larger version of this figure.
| Cell density /hematocrit | Ionomycin concentration | Buffer | Pre-incubation | Treatment time with ionomycin | Detection method | Reference |
| 1.65 x 108 cells/mL | 0.3 mM | Buffer A* | 36 h in buffer A | 1 h | Annexin V | 12 |
| 0.40% | 1 mM | Ringer solution | 48 h in Ringer | 1 h | Annexin V | 17 |
| 50% | 10 mM | Buffer B** | – | 3 h | Merocyanine 540 | 18 |
| 0.40% | 1 mM | Ringer solution | 48 h in Ringer | 1 h | Annexin V | 19 |
| 0.40% | 1 mM | Ringer solution | 48 h in Ringer | 1 h | Annexin V | 20 |
| 2% | 1 mM | Ringer solution | – | 4 h | Annexin V | 21 |
| 0.40% | 1 mM | Ringer solution | – | 0.5 h | Annexin V | 22 |
| 10% | 1 mM | Ringer solution | – | 3 h | Annexin V | 23 |
| 0.40% | 10 mM | Ringer solution | – | 0.5 h | Annexin V | 24 |
| 0.40% | 1 mM | Ringer solution | 48 h in Ringer | 0.5 h | Annexin V | 25 |
| 2 x 106 cells/mL | 1 mM | HEPES-buffered saline (HBS) | – | 0.5 h | Annexin V | 26 |
| *Buffer A: 10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2·6H2O, 10 mM glucose, and 1.8 mM CaCl2·2H2O | ||||||
| **Buffer B: 5 mM Tris, 100 mM KC1, 60 mM NaCl, and 10 mM glucose | ||||||
Table 1: Various protocols used in the literature to induce eryptosis using ionomycin.
Discussion
The goal of this procedure is to provide optimal values for ionophore concentration, treatment time, and extracellular glucose concentration, which are important factors in ensuring successful induction of eryptosis. A critical step in the protocol is the depletion of extracellular glucose, which, despite its importance, has not been sufficiently emphasized in the literature. The sugar content in normal Ringer solution (5 mM) has an inhibitory effect on eryptosis. Glucose depletion in the extracellular environment induces cellular stress and activates protein kinase C (PKC), resulting in the activation of calcium and potassium channels. This results in an increase in the entry of Ca2+ in the cytosolic space30,31,34 and ultimately activates the scramblase16, which increases eryptosis. Activation of potassium channel also results in potassium chloride leakage out of the cell, which leads to erythrocyte shrinkage35.
The procedure outlined above needs to be performed with specific attention to hemolysis. It is important to use an optimized ionophore concentration, which is high enough to induce eryptosis, and low enough to prevent hemolysis. Similarly, incubating erythrocytes with ionomycin for a short period of time results in low eryptosis while very long incubation may lead to cell membrane disruption and hemolysis. It should also be noted that while the presented protocol is highly reliable when performed on the same erythrocyte sample, cells from different individuals respond differently to ionomycin and there might be inter-subject variability between different samples.
Particular attention should be paid to data analysis from flow cytometry. The percentage eryptosis obtained from the flow cytometer indicates the percentage of cell population with PS on their outer leaflet. However, cells with different intensities of annexin-V binding cannot be distinguished based on this number. Annexin-V binds to the PS exposed on cell surface, with a very high affinity and high specificity to PS36,37,38. However, as shown in the microscopy images in this report, different cells show differences in annexin-V binding intensity. The cells with low PS on their membranes have low fluorescence intensities, whereas higher PS occupancy on cell membrane results in higher fluorescence intensities.
The protocol presented in this paper can be modified by increasing the extracellular Ca2+ concentration. In this protocol, ionomycin was used to induce eryptosis in the presence of 1 mM CaCl2; higher Ca2+ concentrations might lead to enhanced intracellular calcium levels and may induce more eryptosis. In addition, different calcium ionophores, such as selectophore and calcimycin, might have different ability to enhance the intracellular concentration of Ca2+, compared to ionomycin, and could result in different eryptosis values. However, consistent eryptosis of erythrocytes can be achieved using ionomycin with the outlined protocol and can be used in the laboratory to examine the molecular mechanisms of eryptosis, mimic diseased conditions39,40 in vitro, and screen potential therapeutics that inhibit eryptosis, among other applications.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH grant R15ES030140 and NSF grant CBET1903568. Financial support from the Russ College of Engineering and Technology and the Department of Chemical and Biomolecular Engineering at Ohio University is also acknowledged.
Materials
| 96-well plate | Fisher Scientific | 12-565-331 | |
| Annexin V Alexa Fluor 488 – apoptosis kit | Fisher Scientific | A10788 | Store at 4 °C |
| BD FACSAria II flow cytometer | BD Biosciences | 643177 | |
| CaCl2 | Fisher Scientific | C79-500 | |
| Centrifuge | Millipore Sigma | M7157 | Model Eppendorf 5415C |
| Confocal fluorescence microscopy | Zeiss, LSM Tek Thornwood | Model LSM 510, Argon laser excited at 488 nm for taking images | |
| Cover glasses circles | Fisher Scientific | 12-545-100 | |
| Disposable round bottom flow cytometry tube | VWR | VWRU47729-566 | |
| DMSO | Sigma-Aldrich | 472301-100ML | |
| DPBS | VWR Life Science | SH30028.02 | |
| Glucose monohydrate | Sigma-Aldrich | Y0001745 | |
| HEPES Buffer (1 M) | Fisher Scientific | 50-751-7290 | Store at 4 °C |
| Ionomycin calcium salt | EMD Milipore Corp. | 407952-1MG | Dissolve in DMSO to reach 2 mM. Store at -20 °C |
| KCl | Fisher Scientific | P330-500 | |
| MgSO4 | Fisher Scientific | M65-500 | |
| Microcentrifuge tube | Fisher Scientific | 02-681-5 | |
| NaCl | Fisher Scientific | S271-500 | |
| Plain glass microscope slides | Fisher Scientific | 12-544-4 | |
| Synergy HFM microplate reader | BioTek | ||
| Whole blood in ACD | Zen-Bio | Store at 4 °C and warm to 37 °C prior to use |
References
- Bratosin, D., et al. Programmed Cell Death in Mature Erythrocytes: A Model for Investigating Death Effector Pathways Operating in the Absence of Mitochondria. Cell Death and Differentiation. 8 (12), 1143-1156 (2001).
- Lang, E., Lang, F. Mechanisms and Pathophysiological Significance of Eryptosis, the Suicidal Erythrocyte Death. Seminars in Cell and Developmental Biology. 39, 35-42 (2015).
- Garnier, M., et al. Erythrocyte Deformability in Diabetes and Erythrocyte Membrane Lipid Composition. Metabolism. 39 (8), 794-798 (1990).
- Verkleij, A. J., et al. The Asymmetric Distribution of Phospholipids in the Human Red Cell Membrane. A Combined Study Using Phospholipases and Freeze-Etch Electron Microscopy. Biochimica et Biophysica Acta (BBA) Biomembranes. 323 (2), 178-193 (1973).
- de Back, D. Z., Kostova, E. B., van Kraaij, M., van den Berg, T. K., van Bruggen, R. Of Macrophages and Red Blood Cells; A Complex Love Story. Frontiers in Physiology. 5, 9 (2014).
- Fadok, V. A., et al. A Receptor for Phosphatidylserine-Specific Clearance of Apoptotic Cells. Nature. 405 (6782), 85-90 (2000).
- Henson, P. M., Bratton, D. L., Fadok, V. A. The Phosphatidylserine Receptor: A Crucial Molecular Switch. Nature Reviews Molecular Cell Biology. 2 (8), 627-633 (2001).
- Messmer, U. K., Pfeilschifter, J. New Insights into the Mechanism for Clearance of Apoptotic Cells. BioEssays. 22 (10), 878-881 (2000).
- Basu, S., Banerjee, D., Chandra, S., Chakrabarti, A. Eryptosis in Hereditary Spherocytosis and Thalassemia: Role of Glycoconjugates. Glycoconjugate Journal. 27 (9), 717-722 (2010).
- Kuypers, F. A., et al. Detection of Altered Membrane Phospholipid Asymmetry in Subpopulations of Human Red Blood Cells Using Fluorescently Labeled Annexin V. Blood. 87 (3), 1179-1197 (1996).
- Lang, F., Lang, E., Fller, M. Physiology and Pathophysiology of Eryptosis. Transfusion Medicine and Hemotherapy. 39 (5), 308-314 (2012).
- Wróbel, A., Bobrowska-Hägerstrand, M., Lindqvist, C., Hägerstrand, H. Monitoring of Membrane Phospholipid Scrambling in Human Erythrocytes and K562 Cells with FM1-43 – a Comparison with Annexin V-FITC. Cellular and Molecular Biology Letters. 19 (2), 262-276 (2014).
- Mohandas, N., Gallagher, P. G. Red Cell Membrane: Past, Present, and Future. Blood. 112 (10), 3939-3948 (2008).
- Barber, L. A., Palascak, M. B., Joiner, C. H., Franco, R. S. Aminophospholipid Translocase and Phospholipid Scramblase Activities in Sickle Erythrocyte Subpopulations. British Journal of Haematology. 146 (4), 447-455 (2009).
- Pretorius, E., Du Plooy, J. N., Bester, J. A. A Comprehensive Review on Eryptosis. Cellular Physiology and Biochemistry. 39 (5), 1977-2000 (2016).
- Suzuki, J., Umeda, M., Sims, P. J., Nagata, S. Calcium-Dependent Phospholipid Scrambling by TMEM16F. Nature. 468 (7325), 834-838 (2010).
- Bhuyan, A. A. M., Haque, A. A., Sahu, I., Coa, H., Kormann, M. S. D., Lang, F. Inhibition of Suicidal Erythrocyte Death by Volasertib. Cellular Physiology and Biochemistry. 43 (4), 1472-1486 (2017).
- Chandra, R., Joshi, P. C., Bajpai, V. K., Gupta, C. M. Membrane Phospholipid Organization in Calcium-Loaded Human Erythrocytes. Biochimica et Biophysica Acta. 902 (2), 253-262 (1987).
- Alzoubi, K., Calabrò, S., Egler, J., Faggio, C., Lang, F. Triggering of Programmed Erythrocyte Death by Alantolactone. Toxins (Basel). 6 (12), 3596-3612 (2014).
- Jacobi, J., et al. Stimulation of Erythrocyte Cell Membrane Scrambling by Mitotane. Cellular Physiology and Biochemistry. 4 (33), 1516-1526 (2014).
- Totino, P. R. R., Daniel-Ribeiro, C. T., Ferreira-da-Cru, M. Refractoriness of Eryptotic Red Blood Cells to Plasmodium Falciparum Infection: A Putative Host Defense Mechanism Limiting Parasitaemia. PLoS One. 6 (10), e26575 (2011).
- Borst, O., et al. Dynamic Adhesion of Eryptotic Erythrocytes to Endothelial Cells via CXCL16/SR-PSOX. American Journal of Physiology – Cell Physiology. 302 (4), C644-C651 (2011).
- Tagami, T., Yanai, H., Terada, Y., Ozeki, T. Evaluation of Phosphatidylserine-Specific Peptide-Conjugated Liposomes Using a Model System of Malaria-Infected Erythrocytes. Biological and Pharmaceutical Bulletin. 38 (10), 1649-1651 (2015).
- Mahmud, H., et al. Suicidal Erythrocyte Death, Eryptosis, as a Novel Mechanism in Heart Failure-Associated Anaemia. Cardiovascular Research. 98 (1), 37-46 (2013).
- Signoretto, E., Castagna, M., Lang, F. Stimulation of Eryptosis, the Suicidal Erythrocyte Death by Piceatannol. Cellular Physiology and Biochemistry. 38 (6), 2300-2310 (2016).
- Lange, Y., Ye, J., Steck, T. L. Scrambling of Phospholipids Activates Red Cell Membrane Cholesterol. 生物化学. 46 (8), 2233-2238 (2007).
- Lang, F., et al. Eryptosis, a Window to Systemic Disease. Cellular Physiology and Biochemistry. 22 (6), 373-380 (2008).
- Gil-Parrado, S., et al. Ionomycin-Activated Calpain Triggers Apoptosis. A Probable Role for Bcl-2 Family Members. Journal of Biological Chemistry. 277 (30), 27217-27226 (2002).
- Liu, C. M., Hermann, T. E. Characterization of Ionomycin as a Calcium Ionophore. Journal of Biological Chemistry. 253 (17), 5892-5894 (1978).
- Klarl, B. A., et al. Protein Kinase C Mediates Erythrocyte “Programmed Cell Death” Following Glucose Depletion. American Journal of Physiology – Cell Physiology. 290 (1), C244-C253 (2006).
- Danilov, Y. N., Cohen, C. M. Wheat Germ Agglutinin but Not Concanavalin A Modulates Protein Kinase C-Mediated Phosphorylation of Red Cell Skeletal Proteins. FEBS Letters. 257 (2), 431-434 (1989).
- Nazemidashtarjandi, S., Farnoud, A. M. Membrane Outer Leaflet Is the Primary Regulator of Membrane Damage Induced by Silica Nanoparticles in Vesicles and Erythrocytes. Environmental Science Nano. 6 (4), 1219-1232 (2019).
- Jaroszeski, M. J., Heller, R. . Flow Cytometry Protocols. , (2003).
- Ghashghaeinia, M., et al. The Impact of Erythrocyte Age on Eryptosis. British Journal of Haematology. 157 (5), 1365 (2012).
- Repsold, L., Joubert, A. M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. Biomed Research International. 2018 (5), 9405617 (2018).
- Tait, J. F., Gibson, D., Fujikawa, K. Phospholipid Binding Properties of Human Placental Anticoagulant Protein-I, a Member of the Lipocortin Family. Journal of Biological Chemistry. 264 (14), 7944-7949 (1989).
- Andree, H. A. M., et al. Binding of Vascular Anticoagulant α (VACα) to Planar Phospholipid Bilayers. Journal of Biological Chemistry. 265 (9), 4923-4928 (1990).
- Tait, J. F., Gibson, D. F., Smith, C. Measurement of the Affinity and Cooperativity of Annexin V-Membrane Binding under Conditions of Low Membrane Occupancy. Analytical Biochemistry. 329 (1), 112-119 (2004).
- Jiang, P., et al. Eryptosis as an Underlying Mechanism in Systemic Lupus Erythematosus-Related Anemia. Cellular Physiology and Biochemistry. 40 (6), 1391-1400 (2016).
- Chakrabarti, A., Halder, S., Karmakar, S. Erythrocyte and Platelet Proteomics in Hematological Disorders. Proteomics – Clinical Applications. 10 (4), 403-414 (2016).

