Visualization of Estrogen Receptors in Colons of Mice with TNBS-Induced Crohn’s Disease using Immunofluorescence
Summary
The protocol presents a complete validated TNBS-induced murine model of Crohn's disease and methods for visualization of estrogen receptors by immunohistochemistry using immunofluorescence of formalin-fixed colon sections embedded in paraffin.
Abstract
Crohn's disease is the most diagnosed type of inflammatory bowel disease. Chronic inflammation developing in the intestine leads to peristalsis disorder and damage of intestinal mucosa and seems to be associated with an increased risk of colon neoplastic transformation. Accumulating evidence indicates that estrogens and estrogen receptors affect not only hormone-sensitive tissues, but also other tissues not directly related to estrogens, such as the lungs or colon. Here, we describe the protocol for the successful immunofluorescence staining of estrogen receptors in colon obtained from a murine model of TNBS-induced Crohn's disease. A detailed protocol for the induction of Crohn's disease in mice and intestine preparation is provided as well as a step-by-step immunohistochemical procedure using formalin-fixed paraffin-embedded intestine sections. The described methods are not only useful for estrogen receptor detection and estrogen signaling investigation in vivo but can also be applied to for other proteins which may be involved in the development of colitis.
Introduction
Crohn's disease (CD) is an inflammatory bowel disease (IBD) manifested as chronic intestine inflammation. The etiology of CD is poorly understood, but there are a few major factors that appear to be responsible for CD development, including intestinal microbiota, and genetic and environmental factors, such as diet or stress1. For a better understanding of the pathogenesis of Crohn's disease, several models of intestinal inflammation have been used2,3,4,5,6,7. In this article, we present results obtained from a 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced murine model of CD.
It has been documented that estrogens are capable of modulating chronic intestinal inflammation8,9,10,11,12. The biological activity of estrogens is mediated by cognate receptors, among which are nuclear estrogen receptors (ERs), i.e., ERα (gene ESR1) and ERβ (gene ESR2), as well as G protein-coupled estrogen receptor, i.e., GPER (gene GPER1), referred to as membrane-bound ER13,14. There are several methods for determining the level of estrogen receptors, but only a few can be used to visualize them in the intestine.
Immunohistochemistry (IHC) is a widely used method in clinical and basic studies for the detection of certain antigens in cells or tissues with fluorochrome-conjugated antibodies. IHC seems to be an important method in tissue structure visualization, as well as in the identification and localization of specific proteins, which may be crucial for understanding the development of colitis. Here, we present a complete and validated protocol for immunohistochemical visualization of estrogen receptors in the intestine using immunofluorescence.
Protocol
Animal studies were conducted with the consent of the Local Ethical Committee (28/ŁB29/2016) in accordance with Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, and institutional recommendations.
1. TNBS-induced murine model of Crohn's disease
NOTE: This protocol uses male BALB/C mice weighing 25-28 g. Animals are housed at a constant temperature (22-24 °C) and, relative humidity 55 ± 5%, and maintained in a 12 h light/dark cycle with free access to standard chow pellets and tap water ad libitum.
- Place the mouse into the induction chamber and close the lid tightly. Anesthetize the mouse briefly with isoflurane (25% O2 with O2 flow rate at 1.5-2 L/min).
NOTE: Respiratory rate should remain rhythmic and slower than normal and should not change in response to a noxious stimulus. - Instill 4 mg of TNBS in 0.1 mL of 30% ethanol in 0.9% NaCl or 0.1 mL of 30% ethanol in 0.9% NaCl as a vehicle control into the distal colon through a catheter.
NOTE: The catheter should be carefully introduced approximately 3 cm into the anus. - Monitor the mouse daily from day two to eight for clinical parameters including body weight, rectal bleeding, stool consistency and mortality.
- On day eight, euthanize the mouse by cervical dislocation.
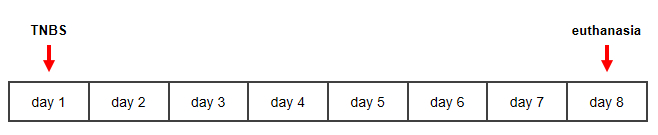
Figure 1: Timeline for TNBS-induced murine model of Crohn's disease. Please click here to view a larger version of this figure.
2. Separation and macroscopic evaluation of colon
NOTE: One day before colon separation, dilute 100 µL of antibiotic in 1 mL of phosphate buffer saline (PBS) and leave at 4 °C overnight.
- Clean the skin over the abdomen using 75% ethanol and sterile gauze.
- Cut the abdominal wall from breastbone to anus using sterile scissors and tweezers.
- Cut off the colon as close as possible to the anus and cecum.
- Place the colon on the Petri dish. Cut the colon along from the anus into the cecum end. Clean and wash the colon 2-4 times in cold antibiotic-PBS solution.
- Perform macroscopic evaluation using a caliper according to Table 1.
NOTE: Tissue adhesion* and erythema/hemorrhage#, fecal blood# and diarrhea# are subject to visual assessment. *Tissue adhesion evaluate using a three-point scale (0: colon without tissue adhesion, 1: colon with moderate tissue adhesion, 2: colon with extensive tissue adhesion); #based on absence (0) or presence (1) of erythema/hemorrhage, fecal blood and diarrhea.
| Adhesion* | Erythema/ hemorrhage# | Fecal blood# | Diarrhea# | Length of ulcer | Colon thickness | Colon length |
| points (0 – 2) | points (0 – 1) | points (0 – 1) | points (0 – 1) | cm/points | mm/points | cm/points |
| 0 – absent | 0 – absent | 0 – absent | 0 – absent | 0.5 cm = 0.5 point | n mm = n points | 0 – <10% shorter than the control |
| 1 – moderate | 1 – present | 1 – present | 0.5 – slight/loose stool | 1 – from 10 to 20% shorter than the control | ||
| 2 – present | 1 – present | 2 – over 20% shorter than the control |
Table 1: Macroscopic scoring of the intestine of mice with TNBS-induced model of Crohn's disease.
- Convert the length of the ulcer in centimeters to a point scale, i.e., every 0.5 cm of ulcer is counted as 0.5 point. Convert the thickness of the colon in millimeters to a point scale, i.e., every n mm corresponds to n points.
- Convert the length of the colon in centimeters on a three-point scale. The length of colon obtained from each mouse with TNBS-induced Crohn's disease is evaluated in relation to the average colon length for the control group (0: <10% shorter than the control, 1: from 10 to 20% shorter than the control, 2: over 20% shorter then the control).
- Calculate the total macroscopic score according to the equation: Total macroscopic score = adhesion (points) + erythema/hemorrhage (points) + fecal blood (points) + diarrhea (points) + length of ulcer (points) + colon thickness (points) + colon length (points).
3. Colon sample preparation
- Cut the colon into 1-2 cm fragments and place each on sponge in an appropriately labeled histological cassette.
NOTE: Sponges for histological cassettes prevent colon folding during dehydration and incubation in liquid paraffin. - Place the colon fragment in 4% formaldehyde and incubate for at least 24 h at 4 °C.
- Prepare and program the tissue processor for 1 h of incubation in 50%, 70%, 90%, 95%, 100% ethanol, xylene/100% ethanol (1:1; v/v), and xylene only, as well as for at least 3 h of incubation in liquid paraffin.
NOTE: Dehydration must be performed in increasing concentrations of ethanol and xylene, but the concentration of ethanol can be modified. The xylene/ethanol mixture is recommended but not required. - Transfer the colon fragment to a histological box and place in the pre-programmed tissue processor.
- Run the tissue processor.
- After incubation steps, place the colon fragment in a metal mold so that the two ends of the colon are in an upright position and fill one third of the mold with liquid paraffin.
- Place the mold in the cooling area (-5 °C) for a few seconds, and then move the mold to the warming area (70 °C). Place in the bottom part of the histological box and cover the entire colon fragment with liquid paraffin.
- Leave the metal mold with the colon fragment in paraffin for a few minutes in the cooling area. Remove the metal mold from the paraffin block and incubate for at least 24 h at 4 °C.
- Remove excess paraffin from the block and insert it into a fully automated rotary microtome.
NOTE: The paraffin block may be stored at -20 °C for a few minutes before this step. - Cut the colon fragment into 5 µm sections.
- Transfer the colon section to a water bath preheated to 40 °C.
- Use the labeled glass slide to remove the colon section from the water bath.
NOTE: The colon sections float on the water. Put the labeled glass slide in the water under the colon section and withdraw the glass slide carefully. - Leave the glass slide for 24 h at room temperature. For long term storage, keep the glass slide at 4 °C after 24 h of incubation at room temperature.
4. Immunohistochemistry with immunofluorescence staining
NOTE: Do not allow the colon section to dry at any step during the procedure.
- Remove paraffin by incubating the glass slide in xylene for 5 min. Repeat this step three times.
- Place the glass slide in xylene/100% ethanol (1:1; v/v) for 5 min. Repeat this step three times.
- Rehydrate the colon section in a series of decreasing ethanol concentrations, i.e., 70%, 50%, 30% and 10% ethanol for 5 min. Repeat each step three times.
- Rinse the glass slide under running water for 5 min.
- Preheat antigen retrieval buffer (10 mM sodium citrate; 0.05% Tween 20, pH 6.0) to 95-98 °C and heat the glass slide in boiling antigen retrieval solution for 10 min.
NOTE: The antigen retrieval step is optional but recommended. The unmasking solution should be optimized depending on the antibody used in the experiment. - Draw a circle around the colon section using a hydrophobic pen.
NOTE: This step is optional but recommended. The hydrophobic pen prevents waste of reagents by keeping the liquid pooled in a small volume inside marked the circle. - Incubate the section in 3% water solution of hydrogen peroxidase for 10 min.
- Wash in washing solution (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.05% Tween 20) for 5 min.
- Incubate in blocking solution (5% normal goat serum; 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.05% Triton X-100) for 1 h at room temperature.
NOTE: In the blocking solution, the normal serum must be from the same species as the secondary antibody. In stages where incubation is required, place the glass slide in a humidity chamber to prevent excessive evaporation. - Remove the blocking solution and add 20-50 µL of primary antibody against ERα, ERβ or GPER diluted in 1% bovine serum albumin with 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Triton X-100.
NOTE: Recommended dilutions of primary antibodies are shown in Table 2.
| Antibody type | Antibody against | Clonality | Host species | Species reactivity | Dilution |
| Primary | ERα | Polyclonal | Rabbit | Human | 1:100 |
| Mouse | |||||
| Turtle | |||||
| Capybara | |||||
| ERβ | Polyclonal | Rabbit | Human | ||
| Monkey | |||||
| Rat | |||||
| Mouse | |||||
| Sheep | |||||
| Pig | |||||
| GPER | Polyclonal | Rabbit | Human | ||
| Rat | |||||
| Mouse | |||||
| Secondary | DyLight 650 | Polyclonal | Goat | Rabbit | 1:250 |
Table 2: Characteristics of antibodies.
- Incubate with primary antibody overnight at 4 °C in darkness.
- Remove the antibody solution and wash in washing solution (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.05% Tween 20) for 5 min. Repeat this step three times.
- Add 20-50 µL of DyLight 650 secondary antibody diluted in 1% bovine serum albumin (containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Triton X-100). Incubate with secondary antibody conjugated with dye for 1 h at room temperature in darkness.
NOTE: The recommended dilution of the secondary antibody is shown in Table 2. - Remove the antibody solution and wash in washing solution (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) for 5 min. Repeat this step three times.
- Add 2% DiOC6(3) diluted in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl and incubate for 10 min at room temperature in darkness.
- Remove the solution and wash in washing solution (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) for 5 min. Repeat this step three times.
- Add a few drops of glycerol-based liquid with DAPI directly on the colon section and cover carefully with a cover slide. Incubate the colon section for least 24 h at 4 °C.
NOTE: Avoid air bubbles when covering the tissue with the cover slide. - Analyze the colon section under confocal microscope featuring 20x or 63x objectives and oil immersion using dedicated software.
NOTE: Table 3 lists characteristics of the fluorochromes used in this study.
| Fluorochome type | Wavelength (nm) | Dye | |
| Excitation | Emission | ||
| DAPI | 405 | 460 – 480 | Blue |
| DiOC6 (3) | 485 | 538 – 595 | Green |
| DyLight 650 | 654 | 660 – 680 | Red |
Table 3: Characteristics of fluorochromes.
Representative Results
Macroscopic characteristics of colons in mice with TNBS-induced Crohn's disease
Representative images of colons taken from control and TNBS-treated mice are shown in Figure 2. In mice with a TNBS-induced model of Crohn's disease, the length of the colon is reduced while the width of the colon is increased.
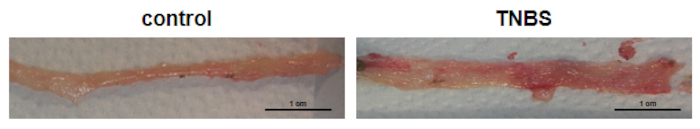
Figure 2: Representative colon obtained from the control mice (control) and TNBS-treated mice (TNBS). Please click here to view a larger version of this figure.
The evaluated macroscopic parameters are given in Table 1. Administration of TNBS to mice leads to an increase in the total colonic macroscopic score (Figure 3A) and inflammation length (Figure 3B) relative to the control mice.
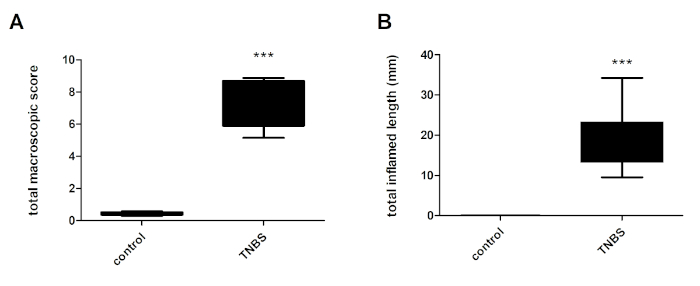
Figure 3: Total macroscopic score of the colon (A) and total colonic inflammation length (B) in control mice (control) and TNBS-treated mice (TNBS). Ten mice per group. Statistical analysis was performed using One-Way ANOVA followed by Newman-Keuls post-hoc test. Data are presented as means ± SEM; ***p < 0.001 TNBS vs. control. Please click here to view a larger version of this figure.
Estrogen receptor antibody validation
Validation of the specificity of the estrogen receptor antibodies used in the study was performed using MCF-7 cells. MCF-7 cells were chosen based on previous studies wherein several independent researchers found that estrogen receptors are present at the mRNA and protein levels. As shown in Figure 4, the antibodies used in the study allow the detection of both nuclear estrogen receptors, i.e., ERα (Figure 4A) and ERβ (Figure 4B), as well as the membrane-bound estrogen receptor, i.e., GPER (Figure 4C) in MCF-7 cells. Nuclear estrogen receptors are localized in the cytoplasm and nuclei, and the signal from GPER staining is only present in the cytoplasm of MCF-7 cells.
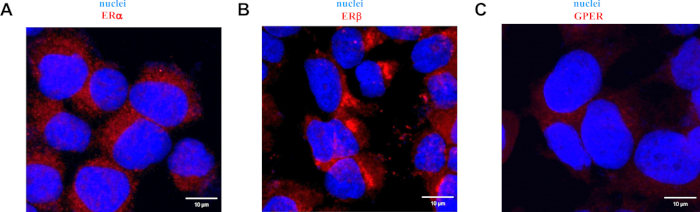
Figure 4: Representative images of immunofluorescence staining of ERα (A), ERβ (B) and GPER (C) in the MCF-7 cells. Detailed description at the top of the images. Scale bars: 10 µm. Please click here to view a larger version of this figure.
In addition to the positive control, a negative control was also performed, in which only the secondary antibody was used. Figure 5 shows an image of MCF-7 cells stained only with secondary antibody conjugated with fluorochrome and glycerol-based liquid with DAPI.
Figure 5: Representative image of immunofluorescence staining of DyLight 650 in the MCF-7 cells. Additional description is available above the image. Scale bars: 20 µm. Please click here to view a larger version of this figure.
Estrogen receptor localization in the TNBS-induced murine model of Crohn's disease
A strong cytoplasmic signal of ERα was found in the colon section obtained from control mice and mice with TNBS-induced Crohn's disease (Figure 6A). However, it appears that only in the intestine obtained from control mice had ERα localized in the goblet cell cytoplasm. Confocal microscopy also revealed cytoplasmic localization of ERβ in the colon section of both control and TNBS-treated mice (Figure 6B). Similarly, cytoplasmic localization of GPER was documented in the colon section obtained from control mice and TNBS-treated mice (Figure 6C).
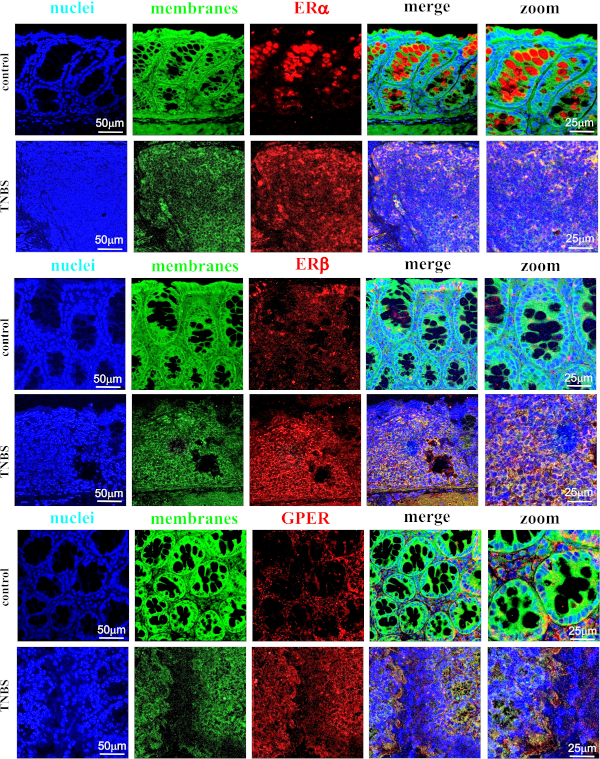
Figure 6: Representative images of immunofluorescence staining of ERα (A), ERβ (B) and GPER (C) in the colon section obtained from control mice (control) and TNBS-treated mice (TNBS). Additional description is available above each image. Scale bars: 50 µm; zoom scale bars: 25 µm. Please click here to view a larger version of this figure.
Discussion
There are numerous animal models for IBD pathophysiology examination, including genetic, immunological or spontaneous models, as well as chemically induced models15. Among the several types of animal models of colitis, chemically induced models such as the TNBS-induced model described in this protocol, are relatively inexpensive and easy to obtain. The TNBS-induced murine model of colitis has several clinical symptoms related to the pathological basis of CD. Animals with induced colitis are characterized by inconsistent stool formation, bloody diarrhea and loss of body weight. However, this does not mean that this model can be used to study CD etiopathogenesis exclusively. The TNBS-induced model is recommended and commonly used, for instance, for potential therapeutic screening. In the case of chemically induced colitis some critical points need to be highlighted. The TNBS has to be diluted in ethanol, which disturbs the mucosal barrier and allows TNBS to penetrate through the intestine wall and interact with high molecular weight proteins, leading to a cellular mediated immune response2,16,17. Both the TNBS dose and ethanol concentration should be optimized for the mouse strain and weight. Too high a TNBS dose and ethanol concentration may cause excessive mortality, which prevents further analysis. On the other hand, too low a TNBS dose and ethanol concentration may cause poor response and unnecessarily prolong the experiment.
The intestine obtained from mice with TNBS-induced colitis can be examined not only at the macroscopic level, as described in this protocol, but may also be used for biochemical and molecular analysis. One useful approach for studying both expression and localization is an immunohistochemical technique with the use of immunofluorescence. However, some critical steps must be included in the preparation and implementation of IHC for formalin-fixed paraffin-embedded murine colon sections. The first pivotal step, i.e., preparation of the colon determines the quality of the results. The fixation time, which depends on the tissue thickness has to be optimized. Another crucial stage is dehydration, which should be performed gently by multiple incubations in increasing concentrations of ethanol. Finally, the correct positioning of the colon in the mold is essential to generate the correct cross-sections. Tissue preparation is not the only important issue in immunohistochemistry. Although antigen retrieval is an optional step, in formalin-fixed paraffin-embedded sections it appears to be necessary. During fixation with formaldehyde, methylene bridges between proteins are generated and protein crosslinking masks antigen sites18. There are two principal methods, based on heat- or enzymatic-induced (trypsin, pepsin or proteinase K) antigen retrieval. Heat-induced antigen retrieval, carried out in sodium citrate buffer, ethylenediaminetetraacetic acid (EDTA) buffer or Tris-EDTA buffer, is more widely used because it does not affect cell morphology. The type of antigen uptake and the conditions should be adjusted experimentally. It should be noted that sometimes the antigen retrieval method is determined by the antibody used in the experiments. Permeabilization is a condition dependent on the examined antigen, and is required especially for intracellular proteins. There are several approaches that use solvents (acetone or methanol) and harsh (Triton X-100 or NP-40) as well as mild (Tween 20 or saponin) detergents. In the present protocol two detergents, were used simultaneously, i.e., Triton X-100 and Tween 20 depending on the step. It should be emphasized that permeabilization has to be optimized depending on the antibody used. Blocking of non-specific binding sites is particularly important during immunohistochemical analysis. The blocking solutions include normal serum, bovine serum albumin or even ready-to-use blocking solutions. The present protocol recommends the use of both normal serum and bovine serum albumin. As already mentioned in the protocol, the blocking solution should contain normal serum from the same species as the secondary antibody.
Finally, IHC detection by immunofluorescence as described in this protocol can be extended to staining other proteins in protein-protein interaction studies. When looking for colocalization of selected proteins, certain conditions must be fulfilled. Fluorochromes should be selected based on excitation and emission spectra. This step is crucial to eliminate spectral overlaps and must be perform at the planning stage of the experiment. In this protocol three fluorochromes, i.e., DyLight 650 secondary antibody, DAPI and DiOC6(3) are used. As shown in Table 3, DyLight 650 used for estrogen receptor detection is observed as a red dye with 654 nm excitation and emission at 660-680 nm. To stain cell nuclei and the internal membrane, DyLight 650 is used along with DAPI nuclear marker and DiOC6 (3) membrane marker. DAPI is observed as a blue dye with 405 nm excitation and emission at 460-480 nm. In turn, DiOC6(3) is observed as a green dye with 485 nm excitation and emission at 538-595 nm. Staining of the next protein should be performed after step 4.14., beginning with the blocking step (see step 4.9.). For two proteins, it is recommended to use staining antibodies from different species. This approach makes it possible to exclude binding of the dye-conjugated secondary antibody to the previously stained protein.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The work was published thanks to the financial support of the University of Lodz authorities: Vice-Rector for Scientific Research, Vice-Rector for National and International Cooperation and the Dean of the Faculty of Biology and Environmental Protection. Damian Jacenik was supported by grants (2017/24/T/NZ5/00045 and 2015/17/N/NZ5/00336) from National Science Centre, Poland.
Materials
| Animals | |||
| BALB/C mice | University of Lodz | NA | |
| Equipment | |||
| Caliper | VWR | 62379-531 | |
| Cardboard block | NA | NA | |
| Confocal microscope – TCS SP8 | Leica Biosystems | NA | |
| Fully automated rotary microtome – RM2255 | Leica Biosystems | NA | |
| Glass slide | Thermo Scientific | J1800BMNT | |
| Heated Paraffin Embedding Module – EG1150 H | Leica Biosystems | NA | |
| Histological box | Marfour | LN.138747 | |
| Hydrophobic pen | Sigma-Aldrich | Z377821 | |
| Laboratory balance | Radwag | WL-104-0048 | |
| LAS X software | Leica Biosystems | NA | |
| Metal mold | Marfour | CP.5105 | |
| Sterile gauze | NA | NA | |
| Sterile scissor | NA | NA | |
| Sterile tweezer | NA | NA | |
| Tissue processor – TP1020 | Leica Biosystems | NA | |
| Reagents | |||
| 2, 4, 6-trinitrobenzene sulfonic acid | Sigma-Aldrich | 92822 | |
| Bovine serum albumin | Sigma-Aldrich | A3294 | |
| DiOC6 (3) | Sigma-Aldrich | 318426 | |
| DyLight 650 secondary antibody | Abcam | ab96886 | |
| ERα primary antibody | Abcam | ab75635 | |
| ERβ primary antibody | Abcam | ab3576 | |
| Ethanol | Avantor Performance Materials Poland | 396480111 | |
| Formaldehyde | Avantor Performance Materials Poland | 432173111 | |
| GPER primary antibody | Abcam | ab39742 | |
| Hydrochloric acid | Avantor Performance Materials Poland | 575283421 | |
| Hydrogen peroxidase | Avantor Performance Materials Poland | 885193111 | |
| isoflurane (forane) | Baxter | 1001936040 | |
| Normal goat serum | Gibco | 16210064 | |
| Paraffin | Leica Biosystems | 39602012 | |
| Petrie dish | Nest Scientific | 705001 | |
| Phosphate buffer saline | Sigma-Aldrich | P3813 | |
| Physiological saline | Sigma-Aldrich | 7982 | |
| Primocin (antibiotic) | Invitrogen | ant-pm-1 | |
| ProLong Diamond Antifage Mountant with DAPI (glycerol-based liquid with DAPI) | Invitrogen | P36971 | |
| Sodium chloride | Chempur | WE/231-598-3 | |
| Sodium citrate | Avantor Performance Materials Poland | 795780429 | |
| Tris | Avantor Performance Materials Poland | 853470115 | |
| Triton X-100 | Sigma-Aldrich | T8787 | |
| Tween 20 | Sigma-Aldrich | P9416 | |
| Xylene | Avantor Performance Materials Poland | BA0860119 |
References
- Zhang, Y. Z., Li, Y. Y. Inflammatory bowel disease: pathogenesis. World Journal of Gastroenterology. 20 (1), 91-99 (2014).
- Morris, G. P., et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 96 (3), 795-803 (1989).
- Okayasu, I., et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 98 (3), 694-702 (1990).
- Boirivant, M., Fuss, I. J., Chu, A., Strober, W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. Journal of Experimental Medicine. 188 (10), 1929-1939 (1998).
- Morrissey, P. J., Charrier, K., Braddy, S., Liggitt, D., Watson, J. D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. Journal of Experimental Medicine. 178 (1), 237-244 (1993).
- Kühn, R., Löhler, J., Rennick, D., Rajewsky, K., Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 75 (2), 263-274 (1993).
- Sundberg, J. P., Elson, C. O., Bedigian, H., Birkenmeier, E. H. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 107 (6), 1726-1735 (1994).
- Harnish, D. C., et al. Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. American Journal of Physiology Gastrointestinal and Liver Physiology. 286 (1), 118-125 (2004).
- Pierdominici, M., et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget. 6 (38), 40443-40451 (2015).
- Włodarczyk, M., et al. G protein-coupled receptor 30 (GPR30) expression pattern in inflammatory bowel disease patients suggests its key role in the inflammatory process. A preliminary study. Journal of Gastrointestinal and Liver Diseases. 26 (1), 29-35 (2017).
- Mohammad, I., et al. Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Science Signaling. 11 (526), eaap9415 (2018).
- Jacenik, D., et al. G protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn’s disease. Scientific Reports. 9 (1), 6749 (2019).
- Prossnitz, E. R., Barton, M. The G protein-coupled estrogen receptor GPER in health and disease. Nature Review Endocrinology. 7 (12), 715-726 (2011).
- Yaşar, P., Ayaz, G., User, S. D., Güpür, G., Muyan, M. Molecular mechanisms of estrogen-estrogen receptor signaling. Reproductive Medicine and Biology. 16 (1), 4-20 (2016).
- Pizarro, T. T., Arseneau, K. O., Bamias, G., Cominelli, F. Mouse models for the study of Crohn’s disease. Trends in Molecular Medicine. 9 (5), 218-222 (2003).
- Neurath, M. F., Fuss, I., Kelsall, B. L., Stüber, E., Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. Journal of Experimental Medicine. 182 (5), 1281-1290 (1995).
- Ikeda, M., et al. Simvastatin attenuates trinitrobenzene sulfonic acid-induced colitis, but not oxazalone-induced colitis. Digestive Diseases and Sciences. 53 (7), 1869-1875 (2007).
- Thavarajah, R., Mudimbaimannar, V. K., Elizabeth, J., Rao, U. K., Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. Journal of Oral Maxillofacial Pathology. 16 (3), 400-405 (2012).

