Study of Protein Dynamics via Neutron Spin Echo Spectroscopy
Summary
The present protocol describes methods for investigating the structure and dynamics of two model proteins that have an important role in human health. The technique combines bench-top biophysical characterization with neutron spin echo spectroscopy to access the dynamics at time and length scales relevant for protein interdomain motions.
Abstract
Most human body proteins’ activity and functionality are related to configurational changes of entire subdomains within the protein crystal structure. The crystal structures build the basis for any calculation that describes the structure or dynamics of a protein, most of the time with strong geometrical restrictions. However, these restrictions from the crystal structure are not present in the solution. The structure of the proteins in the solution may differ from the crystal due to rearrangements of loops or subdomains on the pico to nanosecond time scale (i.e., the internal protein dynamics time regime). The present work describes how slow motions on timescales of several tens of nanoseconds can be accessed using neutron scattering. In particular, the dynamical characterization of two major human proteins, an intrinsically disordered protein that lacks a well-defined secondary structure and a classical antibody protein, is addressed by neutron spin echo spectroscopy (NSE) combined with a wide range of laboratory characterization methods. Further insights into protein domain dynamics were achieved using mathematical modeling to describe the experimental neutron data and determine the crossover between combined diffusive and internal protein motions. The extraction of the internal dynamic contribution to the intermediate scattering function obtained from NSE, including the timescale of the various movements, allows further vision into the mechanical properties of single proteins and the softness of proteins in their nearly natural environment in the crowded protein solution.
Introduction
Probing dynamics of soft matter with neutrons
Investigating the dynamical properties of proteins and peptides is a major part of biophysical research, and many well-developed methods exist today to access a wide range of energy landscapes1. Relating the experimentally revealed dynamics of the proteins to their biological function is a far more difficult task, requiring complex mathematical models and computer-aided dynamics simulations. The importance of neutron spectroscopy for the analysis of protein motions has been emphasized in several well-received and widely recognized studies1,2,3,4,5. Before exploring the diverse energy landscape of internal protein dynamics, a short overview of the dynamical processes in soft matter and how neutrons can access them is required.
The sensitivity of neutrons to isotopic configuration and the type of interactions they display with soft matter makes neutron scattering one of the most versatile investigation techniques6. There is a broad spectrum of correlation length scales and correlation times that neutrons can access, from nuclear excitations and atomic vibrations to collective motions and slow relaxation processes like isotropic rotations and diffusive motions. When investigating the scattered neutrons for their energy transfer, three main interactions can be distinguished: the elastic scattering, in which there is no energy exchange between incoming neutron and particle in the sample; the inelastic scattering, with a large, quantifiable energy exchange between neutron and particle; and the peculiar case of quasi-elastic scattering that designates a very small energy transfer compared to the incident neutron energy1,7. These interactions give precise information about the material investigated and form the theoretical basis of a wide variety of neutron scattering techniques.
In elastic scattering, the detector records the directions of the neutrons as a diffraction pattern, which shows the position of the sample atoms relative to one another. Information about the correlations of atomic positions is acquired (i.e., integrated intensity S(Q) concerning the momentum transfer Q, which pertains to structural information alone). This principle forms the basis of neutron diffraction8.
Complexity arises when the energy transfer is no longer zero due to excitations and internal fluctuations in the sample material. This forms the basis of neutron spectroscopy, in which the scattered neutrons are investigated as a function of both the energy transfer E and the momentum transfer Q. Dynamical and structural information is obtained. Neutron spectroscopy measures the same integrated intensity S(Q) for energy transfer (i.e., velocity change of the neutrons due to sample scattering, S(Q,ω) = S(Q, E), which is also referred to as the dynamic structure factor)9.
For calculating the scattering from a material, it is more adequate to use the pair correlation function7,10. In the diffraction case, the static pair correlation function G(r) gives the probability of finding the center of a particle at a given distance r from the center of another particle. The spectroscopy generalizes the static pair correlation function and includes energy/frequency/time in the scattering equation. The pair correlation function G(r) becomes a function of time G(r, t), which may be decomposed into a distinct atom pair correlation function GD(r, t), and a self-correlation function GS(r, t). These describe two types of correlations: pair-correlated motions of atoms that govern the coherent scattering, and self-correlation that governs the incoherent scattering10.
Coherent scattering is the scattering from "the average" and depends on the relative phase of the scattered waves. In the small-angle scattering regime, the scattered neutron waves from different scattering centers (different atoms) interfere constructively (have similar phases), and the collective motion of the atoms is observed with strong intensity enhancement. Coherent scattering essentially describes the scattering of a single neutron from all the nuclei in the sample10.
When no constructive interference occurs between the scattered neutron waves from different centers, a single atom is followed in time, and the self-correlation between the position of the atom at time t = 0 and the same atom at time t is observed. Thus, the information on the relative positions of atoms is lost, and the focus is only on local fluctuations. Scattering from local fluctuations governs incoherent scattering. Incoherent scattering is isotropic, contributes to the background signal, and degrades the signal-to-noise10,11.
Combining all of the above, we distinguish four major neutron scattering processes10: (1) elastic coherent (measures the correlations of atomic positions), (2) inelastic coherent (measures collective motions of atoms), (3) elastic incoherent (contributes to the background, reduces scattering intensity by Debye-Waller factor (DWF) and measures elastic incoherent structure factor (EISF), describing the geometry of diffusive motions in confined geometry, and (4) inelastic incoherent (measures single atom dynamics and self-correlation).
Dynamics processes that neutrons can access in biology range from the damping of low frequency atomic and molecular vibrations, the interaction of solvent molecules with bio-surfaces, and diffusion processes in the hydration layer of macromolecules and confined geometry, to short-range translational, rotational, and tumbling diffusive motions, and protein domains and allosteric motions1. The wide diversity of neutron methods and instruments for measuring protein dynamics is based on how the achromatization of the incident or outgoing neutron beam is achieved and how the energy analysis of the scattered neutrons is performed. From triple-axis to time-of-flight, backscattering, and spin-echo spectrometers, one can explore dynamical processes with characteristic times between 1 x 10-14 s and 1 x 10-6 s (femtoseconds to microseconds)12.
Oak Ridge National Laboratory, with its two renowned neutron sources, the Spallation Neutron Source – SNS13 and the High Isotope Flux Reactor – HFIR14, has one of the best suites of spectrometers for investigating dynamics in bio-materials. Some of the most eloquent examples include the use of the cold neutron chopper spectrometer (CNCS) at SNS15 to investigate the dynamical perturbation of hydration water around green fluorescent protein in solution16 or the sub-picosecond collective vibrations of several proteins17. A recurring problem of inelastic neutron scattering investigations is that some biological processes are too slow to be observed. Without extreme setups that lead to a huge loss of neutron intensity, time-of-flight spectrometers are limited to 10 µeV energy resolution, corresponding to a maximum time scale of ~200 ps10,11. This is not sufficient to observe large-scale motions in proteins. Therefore, instruments with higher energy resolution like the backscattering spectrometers are often needed. Combining the time-of-flight and backscattering techniques has proven powerful for investigating the change in internal dynamics of Cytochrome P450cam (CYP101), an enzyme that catalyzes the hydroxylation camphor18.
Microscopic diffusivity measured by the backscattering spectrometer at SNS-BASIS19 was surprisingly well defined and could be separated into the diffusivity of water (hydration, cytoplasmic, and bulk-like water) and the diffusivity of cell constituents in planarian flatworms, the first living animal to be studied by neutron scattering20. Backscattering is a high-resolution spectroscopic technique, but it is also limited to several µeV = several nanoseconds, while the slow dynamics in biomaterials also manifest as the survival time of correlation between atomic position or spin orientations (e.g., relaxation processes, which regularly happen in the time range of ten to hundreds of nanoseconds).
Neutron spin echo spectroscopy (NSE) is the only neutron scattering technique to reach such high resolution. Unlike other neutron techniques, NSE does not require achromatization of the beam since it uses the quantum mechanical phase of the neutrons, which is their magnetic moments. The manipulation of magnetic moments allows the use of a broad neutron beam wavelength distribution, while the technique is sensitive to very small neutrons velocity changes in the order of 1 x 10-4. NSE has been successfully used to investigate the slow dynamics of proteins in solution for many proteins. Among these many pioneer studies, we acknowledge the study of the segmental flexibility of pig immunoglobulin21; the coupled domain motions in Taq polymerase22; the domain motions in the tetramer of yeast alcohol dehydrogenase23; the change of conformation in phosphoglycerate kinase upon substrate binding3; the activation of domain motions and the dynamic propagation of allosteric signals in the Na+/H+ exchange regulatory cofactor 1 (NHERF1) protein4,24,25; the dynamics of a compact state of mercuric ion reductase26; and the diffusion of hemoglobin in red blood cells27. Two more recent studies in protein dynamics have exposed the flexibility of human antibody Immunoglobulin G (IgG) as an entropic spring28 and the characteristics of solvent contribution to the dynamics of intrinsically disordered myelin basic protein (MBP)5.
The present article explains the basic principles of NSE, the multiple preparatory methods recommended for a thorough protein dynamics investigation, as well as the methodology and the experimental protocol for NSE data acquisition at the NSE spectrometer at SNS, SNS-NSE. The protocol characterizes two proteins: IgG, a regular human antibody protein, and the intrinsically disordered protein MBP. The biophysical implications, the research relevance of the examples, and the limitations of the technique are discussed briefly.
NSE spectroscopy, the method for slow dynamics measurements
NSE is a polarized technique that uses neutron time-of-flight to measure the exchange of energy (loss of polarization) due to the quasi-elastic interaction between neutrons and atoms in a sample. At the core of NSE spectroscopy lie two basic principles: (1) the ability of the neutron spin to precess in the magnetic field with a frequency proportional to magnetic strength 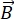 , namely the Larmor frequency29, and (b) the spin-echo or Hann echo, representing the manipulation and refocusing of the polarization signal when applying a series of radiofrequency pulses30.
, namely the Larmor frequency29, and (b) the spin-echo or Hann echo, representing the manipulation and refocusing of the polarization signal when applying a series of radiofrequency pulses30.
The basics of the NSE process can be summarized in a few simple steps6,11 using Figure 1. (1) The neutron beam produced by the source (position 1) is polarized (position 2), guided, and transported (position 3), and arrives at the entrance of the NSE spectrometer, where it gets rotated by 90° by the first pi-half flipper (position 4). (2) The polarized beam (e.g., neutron magnetic moments) becomes perpendicular to the first magnet's magnetic field lines (first precession zone, position 5) and starts to precess. (3) At the end of the magnet, neutron spins accumulate a certain precession angle proportional to the magnetic field strength and the time-of-flight spent inside (basically inversely proportional to the neutron velocity). The individual neutron velocities are encoded within their precession angle at the end of the first precession zone. (4) Close to the sample position, the pi-flipper (position 6) reverses the orientation of the spin by 180°, changing the sign of the precession angle. (5) The neutrons interact with the sample's molecules (position 7) and get scattered. (6) The scattered neutrons enter and precess in the second precession zone (position 8) but become reversed-oriented. (7) Another pi-half flipper (position 9) is used to rotate the orientation of the spin from perpendicular to the horizontal direction. This will stop the precession, translating the precession angle φ into polarization proportional to cos(φ). (8) The analyzer (position 10) selects the neutrons based on one orientation. If the interaction with the sample is elastic, the neutron's velocity will not change. The neutrons will spend an identical amount of time flying in the first and second precession zones, and the accumulated precession angles are fully recovered. The full polarization is restored on the detector (position 11) as an echo of the original polarization (i.e., spin-echo). (9) However, in NSE, the scattering is quasi-elastic, so a small energy exchange between neutrons and sample molecules leads to different neutron velocities after scattering by the sample. Due to the different velocities, the neutrons will spend an additional time flying through the second precession zone and will not have properly recovered their precession angle. A partial polarization is retrieved on the detector, and the loss of polarization due to spin relaxation is proportional to the cos-Fourier-transform of the spectral function S(Q, ω), the intermediate scattering function F(Q, t). (10) The time parameter of the function F(Q, t) is proportional to the precession magnetic field strength. Scanning the loss of polarization as a function of magnetic field strength yields, therefore, a relaxation function that depends on the dynamical processes within the sample.
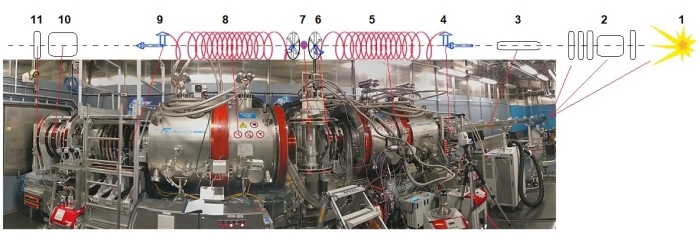
Figure 1: Photograph of the NSE spectrometer at SNS (SNS-NSE) and neutron fly path schematic with the most important functional components. From right to left: 1 = neutron source; 2 = choppers-bender-polarizer-secondary shutter system; 3 = beam transport guides; 4 = pi/2 flipper for first 90° spin-turn; 5 = first precession zone; 6 = pi flipper for 180° spin-turn; 7 = sample area and sample environment (here, the cryo-furnace is shown); 8 = second precession zone; 9 = pi/2 flipper for second 90° spin-turn; 10 = analyzer; 11 = detector. (Note that portions of 3, as well as 2 and 1 ,are situated behind the blue wall inside shielding; the choppers are replaced by a velocity selector for reactor-based NSE). Please click here to view a larger version of this figure.
Protocol
The present work characterizes two proteins: a regular human antibody protein IgG, and the intrinsically disordered MBP. The lyophilized form of the proteins was obtained from commercial sources (see Table of Materials).
1. Protein sample preparation
- Prepare a 50 mM sodium phosphate + 0.1 M NaCl buffer by weighing and dissolving the respective solid reagents in heavy water (D2O) (see Table of Materials). This is the deuterated buffer solvent for IgG.
- Adjust the pH of the buffer solution to 6.6.
- Prepare a 20 mM sodium phosphate + 6 M urea buffer by weighing and dissolving the respective solid components in heavy water (D2O). This is the deuterated buffer solvent for MBP.
- Adjust the pH of the buffer solution to 4.7.
- Filter the buffer solvents using 0.2 µm pore size filters (see Table of Materials).
- Weigh and dissolve the purified protein lyophilized powders in the deuterated solvents for the respective proteins (Steps 1.1.-1.2.) at high protein concentration (~50 mg/mL).
- Load the protein solution in dialyze baskets with dialysis membranes of 3.5 K MWCO, and dialyze against the filtered buffer for 24 h at 10 °C for MBP and 25 °C for IgG (see Table of Materials) by slightly shaking the tubes to create a diffusion gradient.
- Dilute the protein solution using the dialysis buffer in a series of concentrations: 1, 2, 5, 10, and 50 mg/mL.
- Determine the exact concentrations using a Nanodrop spectrophotometer (see Table of Materials).
2. Preliminary sample characterization by dynamic light scattering (DLS)
- Load 80 µL of each protein solution from the concentration series prepared above (Step 1.6.) into the DLS disposable cell (see Table of Materials) and determine the diffusion coefficients, averaging over 10 acquisitions.
- Plot the translational diffusion coefficients as a function of protein concentration and extrapolate to zero concentration.
- Load each protein solution from the concentration series into the capillary tubes of a viscometer (see Table of Materials) and measure the dynamic viscosity.
- Plot the dynamic viscosities measured as a function of protein concentration and extrapolate to zero concentration.
NOTE: The extrapolation of DLS diffusion to zero concentration yields the value of translational diffusion for one single protein. The extrapolation of dynamic viscosity to zero concentration must yield the dynamic viscosity value measured experimentally for the buffer solution.
3. Collection of small-angle scattering (neutron or x-ray)
- Measure small-angle neutron scattering (SANS) and/or small-angle x-ray scattering (SAXS) (see Table of Materials) on four protein concentrations, preferably 2 mg/mL, 5 mg/mL, 10 mg/mL, and 50 mg/mL.
- Normalize SANS and SAXS spectra by protein concentration.
- Fit protein form factor P(Q) to the SANS and SAXS spectra using ensemble optimization31 and/or SasView32 software.
- Calculate structure factor S(Q, c) by dividing SANS and SAXS signal with P(Q) for each concentration.
NOTE: Readers interested in how to measure and interpret small-angle scattering data as support for NSE measurements are encouraged to thoroughly consult the references23,28,31,32,33.
4. Measurement of NSE
- Set up for the experiment and mount the sample following the steps below.
- Select the thickness of the cell for sample loading based on the concentration of the protein solution, the temperature needed for measurement, and the amount of solution available.
NOTE: The present study used top loader transparent quartz containers of 40 mm x 30 mm x 4 mm. - Clean the cell repeatedly, alternating between phosphate-free dish detergent (see Table of Materials), deionized water, and 70% ethanol.
- Dry the cell in the convection oven; do not exceed 80 °C for the quartz cells.
- Load 4 mL of protein solution into the cell and close with caps. Use wax film or any sealant (see Table of Materials) to seal the sample cells.
NOTE: In the present study, 4.8 mL of solution at ~50 mg/mL was used to obtain sufficient scattering intensity. - Load 4 mL of dialysis buffer into an identical container as the protein sample and seal.
- Transport samples to the beamline, close the shutter, and enter the spectrometer enclosure cave area34.
- Mount the sample cell on the aluminum sample holder by tightening the screws and the holding plates (Figure 2, left panel).
NOTE: One can mount two sample cells at the same time, given that the same measurement protocol is needed for all samples. - Mount the graphite sample and/or Al2O3 powder sample loaded in an identical container as the protein sample. These are standards provided by the SNS-NSE beamline support.
- Place the sample holder by gently sliding it into the can of the temperature forcing system (TFS, see Table of Materials).
NOTE: TFS is the most used sample environment at SNS-NSE and pumps dry air into the sample canister to achieve the desired temperature (Figure 2, middle and right panels). - Close the TFS lid and set the temperature to the desired value by accessing the interactive screen of the TFS.
- Mount the neutron camera (see Table of Materials) for the alignment of the samples to the beam.
- Sweep the instrument enclosure, evacuate, close the doors, and open the beam shutter.
NOTE: Sample cells are provided by the SNS-NSE beamline support. For available sample cells and the sample environment library, please refer to the SNS-NSE beamline web page7,35.
- Select the thickness of the cell for sample loading based on the concentration of the protein solution, the temperature needed for measurement, and the amount of solution available.
- Collect the NSE data following the steps below.
- Align the sample in the neutron beam using the neutron camera and the four independent sample apertures.
- Open the SNS-NSE data collection software36 and collect sample statistics by running diffraction scans for the desired scattering angles and wavelength.
- Set up the measurement parameters based on the statistics collected for each sample by editing the measurement macros provided by the assisting Instrument Scientist.
- Start scanning by typing the protocol name at the command prompter and acquire echoes for the sample.
- Start scanning and acquire echoes also for the elastic reference and the buffered solvent. Perform intermittent beam shutter operation for the sample change.
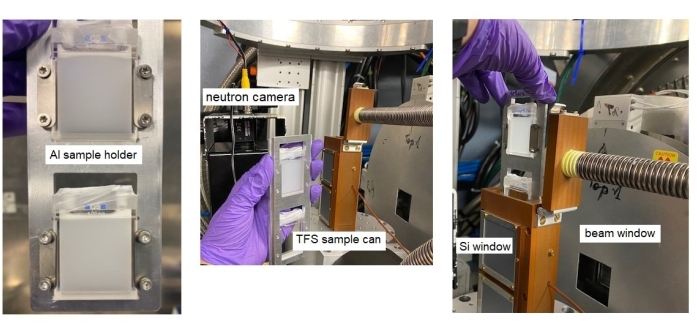
Figure 2: NSE measurement system. Left panel: protein solution samples in quartz container mounted with screws and plates on the aluminum (Al) sample holder. The Al sample holder offers the possibility to mount two samples simultaneously inside the sample environment. Middle panel: The temperature forcing system (TFS) sample can be mounted at the sample stage the neutron beam window is on the right, while the neutron camera used for alignment is visible on the left side. Right panel: placing the sample holder with two samples into the sample can work with silicon (Si) windows. Please click here to view a larger version of this figure.
5. NSE data reduction
NOTE: SNS-NSE is equipped with a dedicated software named DrSpine (data reduction for spin-echo)37,38 available on the ORNL Neutron Sciences Remote Analysis Cluster, a Quick User Guide, and built-in help support.
- Log in to Neutron Sciences Remote Analysis Cluster (see Table of Materials) with the ORNL user credentials, and press the Launch Session button.
- Set up the data reduction software following the steps below.
- In the user directory, open a terminal window and type: source/SNS/software/nse/etc/setup_nse.sh.
- Next, type: drspine_create_env.sh.
- Create a folder for the data reduction in the home directory and copy the provided scripts and macros from the shared directory.
- Edit, rename, and save the reduction macro provided accordingly.
- Type drspine at the command prompter and press enter to start the software reduction environment.
- Type "the name of the reduction macro" edited at Step 5.4. in the command prompter within the software environment and press Enter.
6. NSE data fitting
- Edit the python script "stapler-drspine.py", provided by the assisting Instrument Scientist, with the names of the reduced file data.
NOTE: The python script is freely available to users of the instrument. - Edit the function to fit from the library provided.
- Type the name of the edited script "stapler-drspine.py" at the command prompter and press Enter to read, fit, and plot reduced NSE data.
NOTE: The Instrument Scientist will provide a template for the reduction macro and the "stapler-drspine.py" python script that can read and fit NSE reduced data. The final reduced NSE data are in ASCII format and can be read by various preferred software.
Representative Results
IgG protein from human serum and bovine MBP proteins were reconstituted at high concentrations (~50 mg/mL) in D2O-base buffers. Since the proteins were dissolved in high concentrations, the solutions obtained were crowded proteins solutions. The dynamics investigated using NSE suffer from the crowded environment that the proteins reside in (structure factor interactions and hydrodynamics effects)5,28,39. DLS was performed on a concentration series for each protein to account for crowding effects. The extrapolation to zero concentration of the translational diffusion coefficients measured by DLS yields the value of translational diffusion for a single protein. The dynamic viscosity as a function of protein concentration must also be measured prior to NSE to account for the hydrodynamic interactions. The extrapolation to zero concentration of the dynamic viscosities as a function of concentration needs to yield the value that can be experimentally measured for the buffer solution of each prepared protein sample.
The shape and structure of proteins in concentrated solution were assessed prior to any NSE experiment to gain insights on the protein form factor and the strength of the structure factor, which might influence how the proteins move in solution. Small-angle scattering was the method of choice for these investigations. In the present study, the structural conformation of IgG protein in solution was observed by SAXS, and the structure of MBP was assessed by SANS. The scattering intensity (Q) measured (Steps 3.3.-3.4.) is proportional to the product between form factor P(Q) and the structure factor S(Q,c) weighted by the number of particles (Equation 1)5,28,39:
I (Q) = N ∙ P (Q) ∙S (Q, c) (1)
SAXS was measured on a Kratky-type SAXS instrument40 with an X-ray wavelength of 0.154 nm for IgG, and SANS was measured41 for MBP with a neutron wavelength of 4.5 Å. The experimental small-angle intensities I(Q) were background and solvent corrected, scaled by solution concentration, and the form factor was calculated using freely available software packages like Ensemble modeling-EOM and SasView24,25,29,39,42,43. Further, the structure factor for each protein was obtained from Equation 1.
For the present study, the NSE experiments were performed with two spin-echo spectrometers: the SNS-NSE instrument34 in Figure 1 and the Phoenix-J-NSE instrument44. Incident wavelengths between 8 Å – 12 Å, with Fourier times between 0.1 ns ≤ tmax ≤ 130 ns for several Q measurements between 0.05 Å−1– 0.2 Å−1, were measured. The difference between the two spectrometers is a vital point in the understanding of NSE science, and they represent both the old concept of a so-called "classic spin echo" for the static neutron source of a reactor and the new "time-of-flight concept" for the pulsating neutron source. The Phoenix-J-NSE spectrometer is a classic-type NSE, in which a velocity selector selects a particular wavelength. To allow variations in the neutron velocities, a ±10% wavelength bandwidth is introduced. Despite the very narrow energy band used for a single scan, the situation of the Phoenix-J-NSE spectrometer at a high flux reactor source ensures space-vector and correlations time ranges are covered in relatively short measurement times. The SNS-NSE spectrometer is the new-generation, ultra-high resolution, choppers neutron spectrometer, with a wavelength span of 2 Å < λ < 14 Å and a simultaneous wavelength bandwidth of 2.4 Å – 3.6 Å, depending on the position of the spectrometer. Due to this wide bandwidth, high data collection efficiency is accomplished, allowing nearly gapless coverage of a broad wave-vector time range with only few scattering angle settings. The selection of wavelength band in the SNS-NSE is made by a chopper system consisting of four choppers. Both NSE spectrometers discussed here have precession fields based on superconducting technology with high magnetic field homogeneity, novel state-of-the-art field correction elements for stray field corrections, and novel polarizing benders41,42.
The NSE spectra were measured at 10 °C for MBP protein and 25 °C for IgG1 protein. The coherent intermediate scattering intensity I(Q, t) / I(Q, t = 0) measured by NSE is the contributive result of all the dynamic processes in the sample that happen within the investigated time scale. This contains the internal protein dynamics, the overall translational diffusion, the rotational and tumbling diffusion, and the segmental motions within the protein molecule itself. A simplified model assumes that the internal dynamics and the translational and rotational diffusions are totally decoupled5,45,46,47,48 and can be characterized separately. For the single particle, the coherent scattering function can be written as the product (Equation 2)23,48:
F (Q, t) = Ftrans(Q, t) ∙Frot(Q, t) ∙Fint(Q, t) (2)
where the translational diffusion term for a single rigid protein is an exponential function of the translational diffusion coefficient DT (Equation 3)23,48:
Ftrans(Q, t) = exp(−Q2∙ DT∙ t) (3)
For large concentrations of proteins, the translational diffusion coefficient DT is influenced by protein-protein interactions, quantified by the structure factor S(Q) and by hydrodynamic interactions described by the hydrodynamic function HT(Q) (Equation 4)23,48:
DT(Q) = DT0 ∙HT(Q, c) / S(Q, c) (4)
In Equation 4, DT0 is the extrapolated diffusion value to zero concentration from the DLS measurements (Step 2.2.). HT is calculated as HT = 1 –c∙ [η], where c is the protein concentration, [η] is the intrinsic viscosity calculated by [η] = (η – η0) /η0 ∙ c, and η0 is the measured dynamic viscosity (Steps 2.3.-2.4.). The structure factor S(Q, c) was obtained from SAXS measurements for the IgG protein and SANS measurements for the MBP protein.
Figure 3 displays examples of intermediate scattering functions I(Q, t) / I(Q, t = 0), as measured by NSE for IgG and MBP proteins. A clear deviation from a simple diffusion-like relaxation process was observed on the short Fourier time scale, <25 ns, indicating the accessibility of protein internal dynamics by NSE and the need for a more complex model to describe the observed dynamical processes.
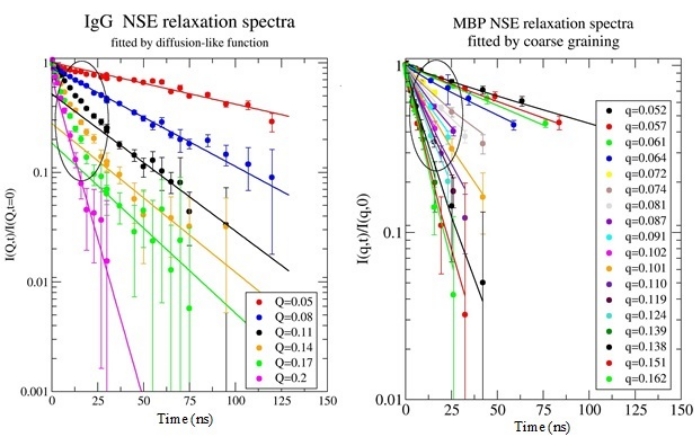
Figure 3: NSE relaxation spectra for IgG and MBP proteins. The IgG data are shown here fitted just by a single-exponential function to reveal the deviation from diffusion at shorter Fourier times. The deviation was observed for both proteins. In contrast, the MBP data are shown here in the full-relaxation range, fitted by the model developed byBiehl45. The model implies coarse-graining and enables the simultaneous fit of short, intermediate, and long Fourier time regimes. Please click here to view a larger version of this figure.
Therefore, the scattering functions were fitted by atomic modeling of both proteins using models developed by Biehl, described in references18,25,38,43,44,46. The intermediate scattering function was calculated by coarse-graining to a couple of hundred individual grains, as opposed to all-atom simulation, to reduce computational load and time, and the MMTK49 software library (freely accessible) was used to access atomic coordinates and implement trans-rotational motions on protein domains and fragments. The results of the intermediate scattering function calculation for both proteins were in excellent agreement with the experimental NSE data and proved that the slower dynamics observed at long Fourier times can be attributed to the overall translational and rotational diffusion processes, while the fast dynamics observed at short time scales can be attributed to the dynamics of protein domains.
Discussion
NSE spectroscopy delivers a unique and detailed view of the dynamics of proteins, which other spectroscopic techniques cannot produce. Measurements over an extended time scale provide observations of both the proteins' translational and rotational diffusion, as presented here. The segmental dynamics and other internal oscillations reveal themselves as a strong decay of the coherent scattering function S(Q, t) at a short time scale and are well separated from the overall diffusional relaxation processes. The main limitations of the NSE technique are the extended measurement time and the large sample volumes needed to acquire a good statistical signal. This can pose a challenge to measuring relevant macromolecular systems that exhibit low concentrations of the mobile species and reduced lifetimes.
Internal dynamics of IgG
IgG protein has a Y-shaped structure specific to antibodies that can be approximated by three large fragments connected by flexible linkers. A mathematical model of three sizable particles residing in harmonic potential was assumed for this kind of structure. The linkers were approximated by elastic springs that give a fixed equilibrium position but allow fluctuations as a form of Brownian motion. Three degrees of freedom were permitted for each fragment around the relative equilibrium28. The internal dynamics represented by Fint(Q, t) in Equation 2 were described by the Ornstein-Uhlenbeck process50,51. The model allows the calculation of the mean square displacement of the fragments in the potential dip, the timescale of different motions, the friction, and the force constants that occur. The amplitude of the internal motion was approximated by normal modes analysis, considering different degrees of motion for each fragment. An extensive description of the model and the resulting mathematical parameters is out of scope here but can be found in detail in reference28. In this case study of IgG, a clear signature of the internal dynamics was observed on the timescale of several nanoseconds. The calculated spring constant of the linker appears to be realistically comparable to an entropic spring of similar length. The observed effective friction seems close to the friction of a free unbound IgG fragment. The pre-existing equilibrium hypothesis was validated, with assumed coexisting "open" or "closed" configurations of IgGs that are in equilibrium exhibiting different binding sites and binding specificities28. This information may be relevant for understanding the mechanics of antibodies and whether that mechanical behavior can be used to improve biocompatibility and bioavailability.
Internal dynamics of MBP
MBP structure has a compact globular core that allows strong stretching and bending motions with its flexible random coil ends. MBP dynamics were interpreted using flexible polymer models with and without the addition of internal friction28,39. As in the case of IgG, the observations of the internal dynamics of MBP can be reconciled within the theory of Brownian motion, in which a larger amplitude of motion results in longer relaxation time with influences from the internal friction within the protein chain. The increased relaxation time with a larger amplitude of motions but smaller friction can be described using the same Ornstein-Uhlenbeck process50,51 of Brownian motion in a harmonic potential. Internal motions with large amplitudes but slow relaxation times become active upon full denaturation of MBP from its native state by reducing the restoring forces of chain configuration (dihedral potentials) and by smoothing the local energy barriers. Detailed descriptions of the MBP internal dynamics in the native and denatured state can be further found in references28,39. In the study of MBP, the investigation using NSE revealed a dynamical behavior pointing toward intermediary compactness of the protein between random coil polymers and globular proteins. The significant contribution of internal protein dynamics with a relaxation rate of several nanoseconds was governed by low-frequency collective stretching and bending motions5. Description of the native MBP dynamics by models from polymer theory breakdown was provided due to a large value of protein internal friction. In denatured MBP, the internal friction within the protein chain is reduced, and the polymer chain character of the relaxation spectrum prevails. The high flexibility of the structural ensemble motions might help increase the accessible protein surface, thereby facilitating the interaction with different binding partners.
Dynamical models
While NSE as a technique is experimentally unique in assessing low-frequency harmonic motions in biological macromolecules and molecular subunits, the analysis of the intermediate scattering function requires a comparison with neutron spectra derived from various mathematical models. The model presented here and developed by Biehl et al.28 for concentrated protein solutions uses an elastic network approximation, in which the intermediate scattering function is a convolution of proper modular functions, and Brownian oscillators represent the internal dynamics. This is one of the very few available models for analyzing the segmental dynamics of proteins. Another well-established model for dilute protein solutions is the model proposed by Bu et al.52, which is a robust model that uses statistical mechanics and does not require complex fits or multiple parameters. A similar approach based on dynamic decoupling approximation can also be applied to dilute systems to characterize the effective diffusion as a sum of self-translational and internal motions53. Several of our references can provide comprehensive reporting on these models and their limitations. We strongly recommend Fitter et al.1 and Liu et al.54.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research used resources at the Spallation Neutron Source (BL-15, BL-6, Biology and Chemistry labs), a DOE Office of the Science User Facility operated by the Oak Ridge National Laboratory. This research also used resources at the MLZ-FRM2 reactor Garching (KWS-2, Phoenix-J-NSE) and the JCNS1 at Forschungszentrum Jülich GmbH, Germany. The author acknowledges Dr. Ralf Biehl and Dr. Andreas Stadler for their help with modeling and their contribution to both IgG and MBP proteins research, Dr. Piotr A. Żołnierczuk for NSE data reduction support, Dr. Changwoo Do for support with SANS measurements, and Rhonda Moody and Dr. Kevin Weiss for SNS biochemistry lab support.
Materials
| Bovine MBP protein solution | Sigma-Aldrich | M1891 | lyophilized powder reconstituted in D2O |
| D2O – heavy water | Sigma-Aldrich | Product No. 151882 | liquid |
| Dionized water | in house | – | for washing / cleanning cells |
| DLS instrument | Zetasizer Nano ZS, FZ-Jülich | – | dynamic light scattering instrument |
| Elastic scattering standards | SNS-NSE, ORNL | – | Al2O3 and Graphite powders |
| Ethanol | Sigma-Aldrich | 65350-M | 70% ethanol for cleaning cells |
| IgG protein solution | Sigma-Aldrich | I4506 | lyophilized powder reconstituted in D2O |
| KWS-2 instrument | JCNS outstation at the MLZ, Garching, Germany | – | small angle neutron instrument |
| Liquinox dish detergent | Alconox | – | Phosphate-free liquid lab glassware cleaner |
| Na2HPO4·7H2O | Sigma-Aldrich | Product No.S9390 | disodium phosphate heptahydrate salt |
| NaCl | Sigma-Aldrich | Product No.S9888 | sodium chloride salt |
| NaH2PO4·H2O | Sigma-Aldrich | Product No. S9638 | monosodium phosphate monohydrate salt |
| Nanodrop spectrophotometer | Thermo Scientific | Catalog number: ND-2000 | NanoDrop 2000/2000c Spectrophotometer |
| Neutron alignment camera | NeutronOptics, Grenoble | NOG210222 | 100 x 100 mm camera with Sony IMX249 CMOS sensor |
| Parafilm M – wax parafilm | Bemis | Parafilm M – 5259-04LC PM996 | all-purpose laboratory film in cardboard dispenser |
| Phoenix-J-NSE Spectrometer | JCNS outstation at the MLZ, Garching, Germany | – | neutron spectrometer |
| SasView | https://www.sasview.org/ | ||
| SAXSpace, Anton Paar instrument | FZ-Jülich | – | small angle x-ray instrument |
| Slide-A-Lyzer dialysis membranes | Thermo Scientific | 88400-88405 | Slide-A-Lyzer mini dialysis devices tubes of 3.5 K MWCO |
| SNS Remote Analysis Cluster | Neutron Science Remote Analysis (sns.gov) | https://analysis.sns.gov | |
| SNS-NSE spectrometer | ORNL, Oak Ridge, TN, USA | – | neutron spectrometer |
| Sterile syringe filters | VWR | N.A. PN:28145-501 | 0.2 µm pore size filters |
| Temperature Forcing System (TFS) | SP Scientific | Part Number 100004055 | sample environment equipment |
| Urea -d4 | Sigma-Aldrich | Product No. 176087 | deuterated Urea salt |
| Viscometer | FZ-Jülich | – | falling ball viscometer |
References
- Fitter, J., Gutberlet, T., Katsaras, J. . Neutron Scattering in Biology: Techniques and Applications. , (2006).
- Stadler, A., Monkenbusch, M., Biehl, R., Richter, D., Ollivier, J. Neutron spin-echo and TOF reveals protein dynamics in solution. Journal of the Physical Society of Japan. 82, (2013).
- Inoue, R. Large domain fluctuations on 50-ns timescale enable catalytic activity in phosphoglycerate kinase. Biophysical Journal. 99 (7), 2309-2317 (2010).
- Callaway, D. J. E., et al. Controllable activation of nanoscale dynamics in a disordered protein alters binding kinetics. Journal of Molecular Biology. 429 (7), 987-998 (2017).
- Stingaciu, L. R., Biehl, R., Changwoo, D., Richter, D., Stadler, A. M. Reduced internal friction by osmolyte interaction in intrinsically disordered myelin basic protein. Journal of Physical Chemistry Letters. 11 (1), 292-296 (2020).
- Monkenbusch, M., Richter, D. High resolution neutron spectroscopy-a tool for the investigation of dynamics of polymers and soft matter. Comptes Rendus Physique. , (2007).
- Richter, D., Monkenbusch, M., Schwahn, D. Neutron Scattering. Polymer Science: A Comprehensive Reference, 10 Volume Set. , (2012).
- Wilson, C. C. . Single Crystal Neutron Diffraction From Molecular Materials. , (2000).
- Marshall, W. . Theory of thermal neutron scattering. , (1971).
- . Roger Pynn Introduction & Neutron Scattering "Theory" Available from: https://neutrons.ornl.gov/sites/default/files/intro_to_neutron_scattering.pdf (2004)
- Richter, D. Neutron scattering in polymer physics. Physica B: Condensed Matter. 276-278, 22-29 (2000).
- Harroun, T. A., Wignall, G. D., Katsaras, J. Neutron scattering for biology. Neutron Scattering in Biology. , (2006).
- . SNS Available from: https://neutrons.ornl.gov/sna (2020)
- . HFIR Available from: https://neutrons.ornl.gov/hfir (2020)
- . CNCS Available from: https://neutrons.ornl.gov/cncs (2020)
- Perticaroli, S., et al. Description of hydration water in protein (green fluorescent protein) solution. Journal of the American Chemical Society. 139 (3), 1098-1105 (2017).
- Perticaroli, S., Nickels, J. D., Ehlers, G., Sokolov, A. P. Rigidity, secondary structure, and the universality of the boson peak in proteins. Biophysical Journal. 106 (12), 2667-2674 (2014).
- Miao, Y., et al. Coupled flexibility change in cytochrome p450cam substrate binding determined by neutron scattering, NMR, and molecular dynamics simulation. Biophysical Journal. 103 (10), 2167-2176 (2012).
- Mamontov, E., Zamponi, M., Hammons, S., Keener, W. S., Hagen, M., Herwig, K. W. BASIS: A new backscattering spectrometer at the SNS. Neutron News. 19 (3), 22-24 (2008).
- Mamontov, E. Microscopic diffusion processes measured in living planarians. Scientific Reports. 9, 8708 (2018).
- Alpert, Y., Cser, L., Faragó, B., Franěk, F., Mezei, F., Ostanevich, Y. M. Segmental flexibility in pig immunoglobulin G studied by neutron spin-echo technique. Biopolymers. 24 (9), 1769-1784 (1985).
- Bu, Z., Biehl, R., Monkenbusch, M., Richter, D., Callaway, D. J. E. Coupled protein domain motion in Taq polymerase revealed by neutron spin-echo spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 102 (49), 17646-17651 (2005).
- Biehl, R., et al. Direct observation of correlated interdomain motion in alcohol dehydrogenase. Physical Review Letters. 101, 138102 (2008).
- Farago, B., Li, J., Cornilescu, G., Callaway, D. J. E., Bu, Z. Activation of nanoscale allosteric protein domain motion revealed by neutron spin echo spectroscopy. Biophysical Journal. 99 (10), 3473-3482 (2010).
- Bu, Z., Callaway, D. J. E. Dynamic propagation of long-range allosteric signals by nanoscale protein domain motion revealed by neutron spin echo spectroscopy. Biophysical Journal. 100 (3), 223 (2011).
- Hong, L., et al. Structure and dynamics of a compact state of a multidomain protein, the mercuric ion reductase. Biophysical Journal. 107 (2), 393-400 (2014).
- Longeville, S., Stingaciu, L. -. R. Hemoglobin diffusion and the dynamics of oxygen capture by red blood cells. Scientific Reports. 7, 10448 (2017).
- Stingaciu, L. R., Ivanova, O., Ohl, M., Biehl, R., Richter, D. Fast antibody fragment motion: Flexible linkers act as entropic spring. Scientific Reports. 6, 22148 (2016).
- Hahn, E. L. Nuclear induction due to free larmor precession. Physical Review. 77, 297 (1950).
- Hahn, E. L. Spin echoes. Physical Review. 80, 580 (1950).
- Svergun, D. I., Koch, M. H. J. Small-angle scattering studies of biological macromolecules in solution. Reports on Progress in Physics. 66 (10), 1735-1782 (2003).
- . Sasview Available from: https://www.sasview.org (2020)
- Zhao, J. K., Gao, C. Y., Liu, D. The extended Q-range small-angle neutron scattering diffractometer at the SNS. Journal of Applied Crystallography. 43, 1068-1077 (2010).
- Ohl, M., et al. The high-resolution neutron spin-echo spectrometer for the SNS with τ ≥ 1 µs. Physica B: Condensed Matter. 350, 147-150 (2004).
- . SNS-NSE web page Available from: https://neutrons.ornl.gov/nse (2022)
- Ohl, M., et al. The spin-echo spectrometer at the Spallation Neutron Source (SNS). Nuclear Instruments and Methods in Physics Research, Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 696, 85-99 (2012).
- Zolnierczuk, P. A., Holderer, O., Pasini, S., Kozielewski, T., Stingaciu, L. R., Monkenbusch, M. Efficient data extraction from neutron time-of-flight spin-echo raw data. Journal of Applied Crystallography. 52 (5), 1022-1034 (2019).
- . Dr Spine hub Available from: https://jugit.fz-juelich.de/nse/drspine (2022)
- Stadler, A. M., et al. Internal nanosecond dynamics in the intrinsically disordered myelin basic protein. Journal of the American Chemical Society. 136 (19), (2014).
- . FZJ Available from: https://www.fz-juelich.de/portal/EN/AboutUs (2022)
- . KWS2 Available from: https://miz-garching.de/kws-2 (2022)
- Moorhouse, M., Barry, P. The protein databank. Bioinformatics Biocomputing and Perl. , (2005).
- Tria, G., Mertens, H. D. T., Kachala, M., Svergun, D. I. Advanced ensemble modelling of flexible macromolecules using X-ray solution scattering. IUCrJ. 2 (2), 207-217 (2015).
- Holderer, O., Monkenbusch, M., Schätzler, R., Kleines, H., Westerhausen, W., Richter, D. The JCNS neutron spin-echo spectrometer J-NSE at the FRM II. Measurement Science and Technology. 90 (4), 043107 (2008).
- Biehl, R., Monkenbusch, M., Richter, D. Exploring internal protein dynamics by neutron spin echo spectroscopy. Soft Matter. 7 (4), 1299-1307 (2011).
- Stingaciu, L. R., Ivanova, O., Ohl, M., Biehl, R., Richter, D. Fast antibody fragment motion: Flexible linkers act as entropic spring. Scientific Reports. 6, 22148 (2016).
- Stadler, A. M., et al. Internal nanosecond dynamics in the intrinsically disordered myelin basic protein. Journal of the American Chemical Society. 136 (19), 6987-6994 (2014).
- Biehl, R., Richter, D. Slow internal protein dynamics in solution. Journal of Physics: Condensed Matter. 26 (50), 503103 (2014).
- Hinsen, K. The molecular modeling toolkit: A new approach to molecular simulations. Journal of Computational Chemistry. 21 (2), 79-85 (2000).
- Uhlenbeck, G. E., Ornstein, L. S. On the theory of the Brownian motion. Physical Review. 36 (5), 823 (1930).
- Wang, M. C., Uhlenbeck, G. E. On the theory of the Brownian motion II. Reviews of Modern Physics. 17, 323 (1945).
- Callaway, D. J. E., Bu, Z. Nanoscale protein domain motion and long-range allostery in signaling proteins-a view from neutron spin echo spectroscopy. Biophysical Reviews. 7 (2), 165-174 (2015).
- Liu, Y. Intermediate scattering function for macromolecules in solution probed by neutron spin echo. Physical Review E. 95, 020501 (2017).
- Liu, Y. Short-time dynamics of proteins in solutions studied by neutron spin echo. Current Opinion in Colloid & Interface Science. 42, 147-156 (2019).

