Robot Assisted Distal Pancreatectomy with Celiac Axis Resection (DP-CAR) for Pancreatic Cancer: Surgical Planning and Technique
Summary
We present our operative approach to robot assisted distal pancreatectomy, splenectomy, and celiac axis resection (DP-CAR), demonstrating that the procedure is safe and feasible with proper planning, patient selection, and surgeon experience.
Abstract
Malignant pancreatic tumors involving the celiac artery can be resected with a distal pancreatectomy, splenectomy and celiac axis resection (DP-CAR), relying on collateral flow to the liver through the gastroduodenal artery (GDA). In the current manuscript, the technical conduct of robotic DP-CAR is outlined. The greater curve of the stomach is mobilized with care to avoid sacrificing the gastroepiploic vessels. The stomach and liver are retracted cephalad to facilitate dissection of the porta hepatis. The hepatic artery (HA) is dissected and encircled with a vessel loop. The gastroduodenal artery (GDA) is carefully preserved. The common HA is clamped and triphasic flow in the proper HA via the GDA is confirmed using intra-operative ultrasound. A retropancreatic tunnel is made over the superior mesenteric vein (SMV). The pancreas is divided with an endovascular stapler at the neck. The inferior mesenteric vein (IMV) and splenic vein are ligated. The HA is stapled proximal to the GDA. The entire specimen is retracted laterally with further dissection cephalad to expose the superior mesenteric artery (SMA). The SMA is then traced back to the aorta. The dissection continues cephalad along the aorta with the bipolar energy device used to divide the crural fibers and celiac nerve plexus. The specimen is mobilized from the patient’s right to left until the origin of the celiac axis is identified and oriented towards the left. The trunk is circumferentially dissected and stapled. Additional dissection with hook cautery and the bipolar energy device fully mobilizes the pancreatic tail and spleen. The specimen is removed from the left lower quadrant extraction site and one drain is left in the resection bed. A final intra-operative ultrasound of the proper HA confirms pulsatile, triphasic flow in the artery and liver parenchyma. The stomach is inspected for evidence of ischemia. Robotic DP-CAR is safe, feasible and when used in conjunction with multi-modality therapy, offers potential for long-term survival in selected patients.
Introduction
Pancreatic cancers involving the body and tail of the pancreas are traditionally surgically managed with a distal pancreatectomy and splenectomy. Approximately 30% of pancreatic cancers present in a locally advanced stage with involvement of structures beyond the pancreas1. A subset of these patients present with involvement of the celiac axis or proximal hepatic artery without involvement of the aorta. In this circumstance, an aggressive pre-operative strategy involving neo-adjuvant chemotherapy of FOLFIRINOX2,3 or Gemcitabine-Abraxane4 with potential neoadjuvant radiation prior to surgical resection with a modified version of the original Appleby procedure is considered5. The procedure involves resecting the celiac axis at its origin and relying on collateral flow to hepatic artery proper through the GDA. While this aggressive approach for locally advanced pancreatic cancer is performed only in highly selected patients, there is suggestion of potential oncologic benefit in retrospective series6,7,8.
The robotic surgical platform offers numerous technical advantages compared with open and laparoscopic techniques, including enhanced three-dimensional visualization, instrument wrist articulation and the ability for the operating surgeon to control multiple instruments and the camera. Additionally, limited retrospective case series of patients undergoing robotic pancreatic surgery have suggested decreased intra-operative blood loss, decreased peri-operative pain, lower pancreatic fistula rates and improved recovery when compared with open pancreatic resections9,10,11,12,13,14. These technical and clinical benefits along with increased robotic training have led to an expansion of the robotic approach in pancreatic surgery, demonstrating the versatility of the platform to perform a variety of pancreatic resections and procedures, including pancreaticoduodenectomy and distal pancreatectomy with and without splenic preservation. Herein, we will provide the pre-surgical and surgical evaluation and decision making that is involved in proper selection of patients as well as detail the patient characteristics, pre-operative management, and a detailed review of the surgical technique of the DP-CAR performed with the robotic platform on a singular patient in our practice.
Protocol
All aspects of this protocol fall within our institutions ethical guidelines of the human research ethics committee
1. Pre-operative planning
- Evaluate the patient pre-operatively.
NOTE: Patients present generally with vague abdominal complains and will be diagnosed primarily by imaging studies. This patient is a 65 year old Caucasian female presented with vague abdominal pain and underwent several CT imaging studies, eventually resulting in a diagnosis of pancreatic mass involving the body of the pancreas and abutting the common hepatic and splenic arteries (Figure 1). - Preoperative Imaging and Biopsy
- Proceed initially with cross-sectional imaging to diagnose the mass and identify anatomical relationships, arterial/venous involvement, and any aberrant arterial and venous anatomy. Once identified, proceed with biopsy for tissue diagnosis, if the mass is accessible. This patient's lesion was biopsied during EUS and confirmed as pancreatic ductal adenocarcinoma.
- Carefully note and consider any aberrant arterial anatomy or portosplenic confluence aberrancies and involvement of additional structure outside of the celiac axis prior to pursuing surgical resection.
- Consider preoperative therapy.
NOTE: Many different preoperative treatment protocols are available for treatment of locally advanced pancreatic cancer. Patient tolerance and standards of practice at various institutions can guide therapy. In the case of our patient, she was initially started on FOLFIRINOX, but after significant intolerance requiring hospitalization, she was ultimately transitioned to gemcitabine/nab-paclitaxel and completed 6 months of treatment. - Repeat imaging and serum studies.
- Consider follow up imaging to evaluate treatment response prior to moving forward with resection. Serum studies, such as CA 19-9, will additionally help evaluate treatment response. In our patient, imaging demonstrated a promising treatment response as well as a 94% reduction in her serum CA 19-9 levels. However, she continued to have persistent soft tissue infiltration of her celiac axis on repeat imaging (Figure 2). As a result, she was scheduled for a robotic DP-CAR.
2. Initial Operative Steps: Diagnostic Laparoscopy and Robot Docking
- Begin with a diagnostic laparoscopy to ensure no evidence of metastatic disease.
- Once no metastatic disease is confirmed, place the remainder of the ports and dock the robot from the right side of the operating table.
NOTE: Several variations of port placement have been successfully utilized. However, our approach utilizes 4 robotic ports across the upper abdomen, two assistant ports and a liver retractor. A skilled bedside assistant is critical to successful completion of the procedure, utilizing the two assistant ports for operating the bipolar vessel sealing device, suction, and endovascular stapler.
3. Robot-Assisted Dissection
- Open lesser sac and mobilize stomach along greater curve.
- Following robot docking, proceed with opening the lesser sac with electrocautery and bipolar vessel sealer.
- Completely mobilize the greater curve of the stomach with care to avoid sacrificing the gastroepiploic vessels (Figure 3).
- Cauterize the short gastric arteries with vessel sealing device to the level of the diaphragm. Then retract the stomach and liver edge cephalad to allow for dissection of the porta hepatis.
- Identify hepatic arterial anatomy and assess for adequate retrograde flow into proper hepatic artery and liver through the gastroduodenal artery.
- After cephalad retraction of the stomach and liver, identify the porta hepatis. Dissect and isolate the hepatic artery node (Station 8a) for removal and permanent pathological evaluation.
- At this time, carefully dissect and identify the gastroduodenal artery (GDA). Evaluate for adequate retrograde flow through the GDA into the hepatic circulation with intra-operative Doppler ultrasound.
- Identify pulsatile flow in the liver and GDA before and after clamping of the common hepatic artery (Figure 4).
- Dissect the inferior border of the pancreas and create a retropancreatic tunnel.
- After identification of adequate retrograde flow through the GDA, dissect along the inferior border of the pancreas. Continue dissection with electrocautery and bipolar vessel sealer to identify the superior mesenteric vein.
- Create a retropancreatic tunnel over the vein with a sufficient margin from the tumor (Figure 5).
- Divide the pancreas and continue the retropancreatic dissection to divide the splenic vein and coronary vein.
- Divide the pancreas with an endovascular stapler. Mobilize the inferior and posterior border of the pancreas laterally with electrocautery and bipolar assisted dissection.
- Divide the splenic vein and coronary vein to assist in visualization of arterial anatomy and retraction. Additionally, the inferior mesenteric vein may be ligated if it inserts into the splenic vein.
- Divide the hepatic artery and expose the superior mesenteric artery.
- Turn attention back to the hepatic artery and continue dissection to fully delineate the anatomy if required. Divide the hepatic artery proximal the gastroduodenal artery (Figure 6).
- Once the hepatic artery is divided, retract the specimen laterally and continue to mobilize the pancreas to expose the superior mesenteric artery (SMA).
- Identify and trace the superior mesenteric artery to its root on the aorta.
- Once the SMA is identified, dissect cephalad along the superior border to trace it back to the origin on the aorta (Figure 7). Continue the dissection cephalad with electrocautery and bipolar cautery through dense connective tissue.
- Dissect cephalad from the root of the SMA through dense connective tissue, nerve bundles, and lymphatic tissue until muscle fibers from the diaphgramatic crura are identified.
- During this en bloc mobilization of the tissue overlying the superior mesenteric artery and celiac axis as well as the final lateral dissection, sample lymph node stations 14, 16 and 18.
- Ligate the left gastric artery near its origin with an endovascular stapler to maximize collateral blood flow to the stomach.
- Expose and divide the celiac axis.
- Continue retracting the sample to the patient's left and dissect through the crural muscle fibers until the origin of the celiac axis is visualized (Figure 8). It is of utmost importance to maintain sufficient lateral retraction on the specimen to rotate the celiac axis to the left of the patient. This will facilitate ligation of the celiac trunk by providing a favorable angle for the endovascular stapling device.
- Continue retroperitoneal dissection laterally to fully mobilize pancreas and spleen and remove specimen.
- Continue the retroperitoneal dissection laterally with a combination of hook and bipolar cautery. Remove the specimen through the left lower quadrant incision, which can be extended if necessary. A detailed view of the final resection bed vascular anatomy is available in the supplementary figures (Figure 9).
- Complete a final intra-operative ultrasound of the proper hepatic artery continues to demonstrate pulsatile, triphasic flow in the artery and parenchyma of the liver. Evaluate the stomach for external signs of ischemia.
- Once a final inspection is completed, undock the robot, and close the fascia and skin. The procedure is complete.
Representative Results
The duration of the procedure was 228 minutes with a blood loss of 50 mL. Post-treatment final pathology revealed a moderately differentiated (G2) ypT1c ductal adenocarcinoma. No nodal involvement was noted (0/21 total nodes). The circumferential resection margin was negative. The patient's post-operative course was uncomplicated. Her drain amylase levels post-operatively were in the normal range and the drain was removed on post-operative day 3. She was discharged home on post-operative day 4 tolerating a regular diet. Her follow appointments in clinic demonstrated that her recovery was progressing well.
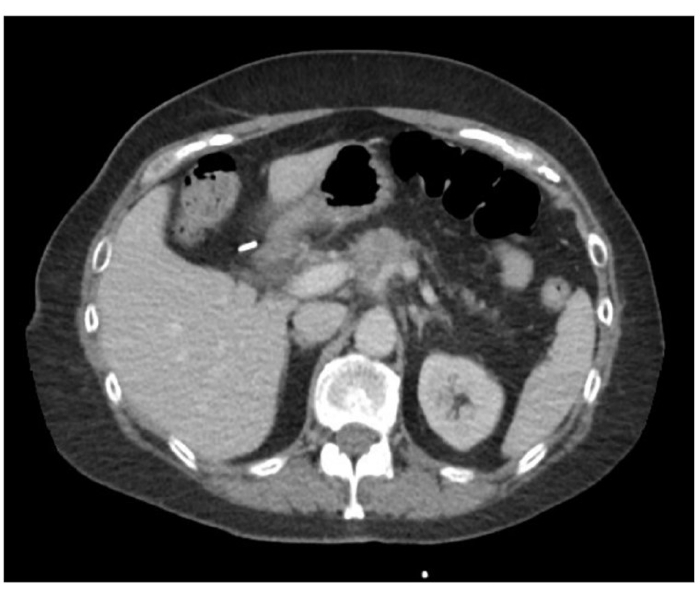
Figure 1: Pre-treatment CT imaging demonstrating locally advanced body of pancreas mass involving celiac axis and splenic vein. Please click here to view a larger version of this figure.
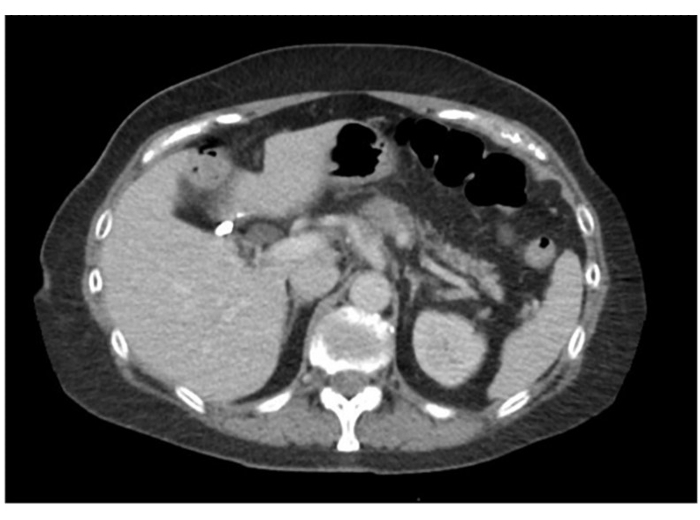
Figure 2: Post-treatment CT imaging demonstrating locally advanced body of pancreas mass involving celiac axis and splenic vein with persistent soft tissue infiltration surrounding celiac cells. Please click here to view a larger version of this figure.
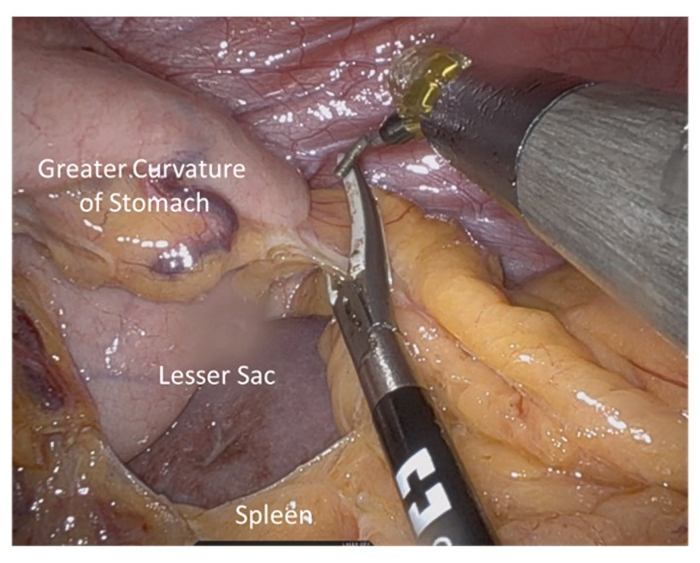
Figure 3: Opening of lesser sac and mobilization of greater curve. Short gastric vessels are ligated with care taken to preserve gastroepiploic vessels. Please click here to view a larger version of this figure.
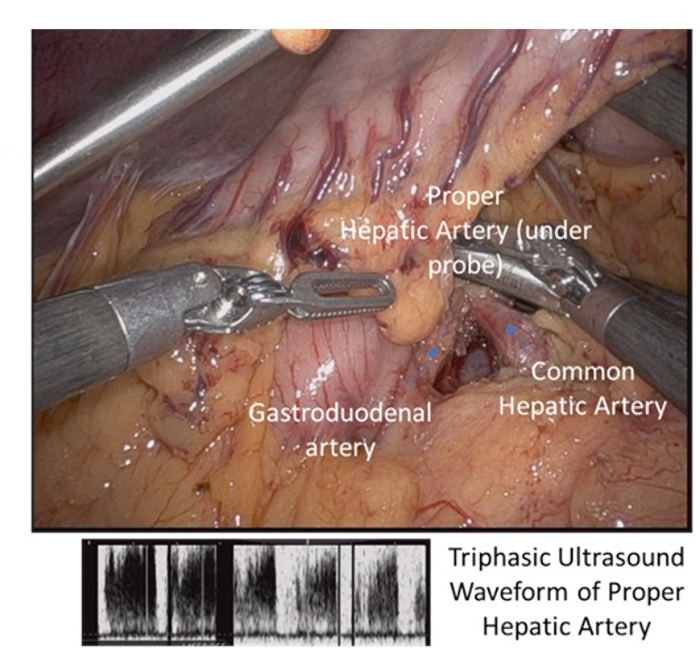
Figure 4: Dissection of porta hepatis with identification of common and proper hepatic artery and gastroduodenal artery with triphasic ultrasound signal. Please click here to view a larger version of this figure.
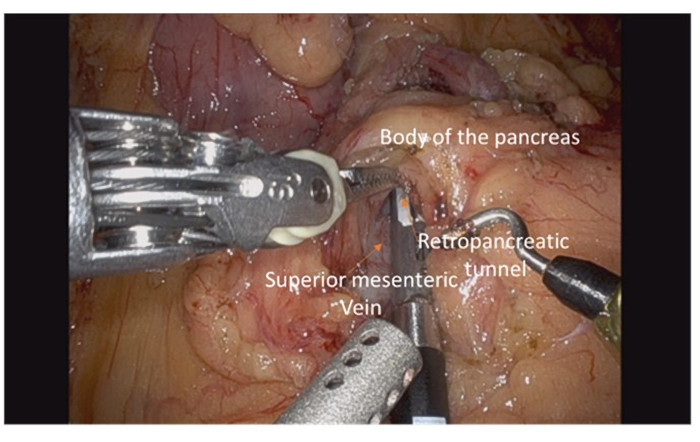
Figure 5: Dissection of inferior border of pancreas with identification of superior mesenteric vein and creation of retropancreatic tunnel overlying vein. Please click here to view a larger version of this figure.
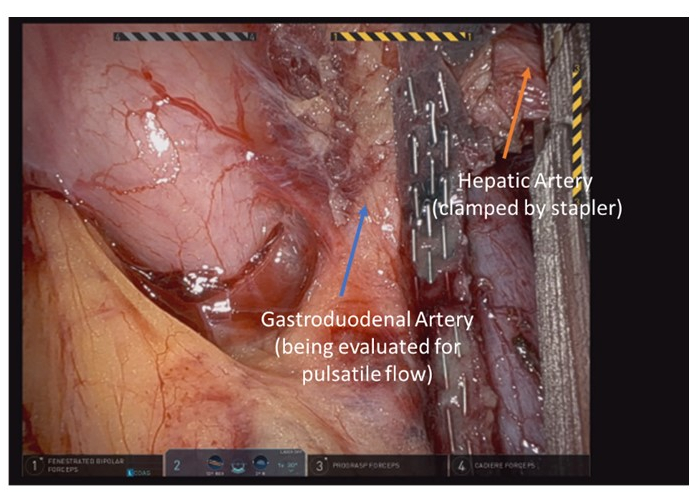
Figure 6: Final identification of arterial Following division landmarks and adequate flow prior to ligation of common hepatic artery. Please click here to view a larger version of this figure.
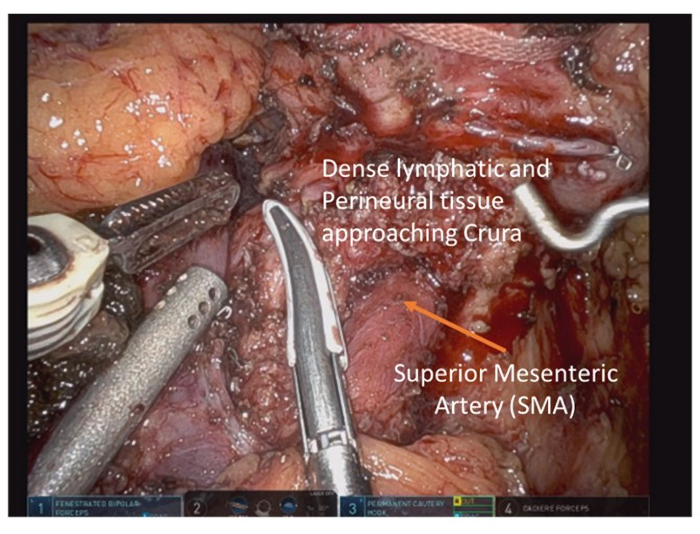
Figure 7: Following division of pancreas, the superior mesenteric artery is exposed and dissection proceeds cephalad to its root on the aorta. Please click here to view a larger version of this figure.
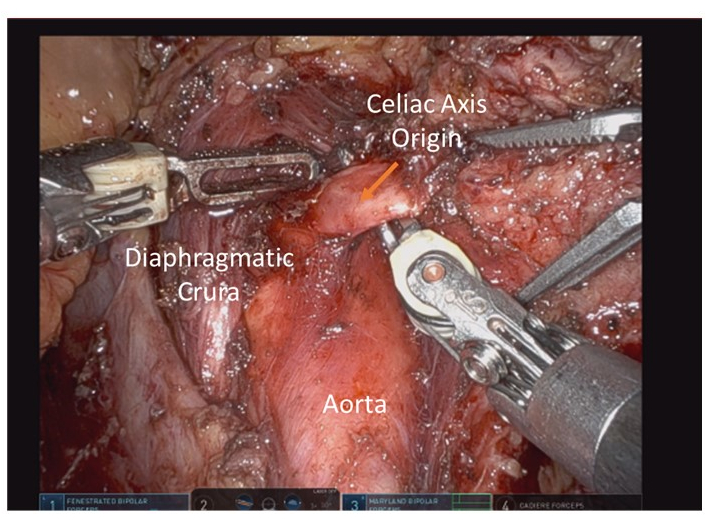
Figure 8: Celiac axis origin exposed and oriented to patient left by laterally retracting the specimen prior to division. Please click here to view a larger version of this figure.
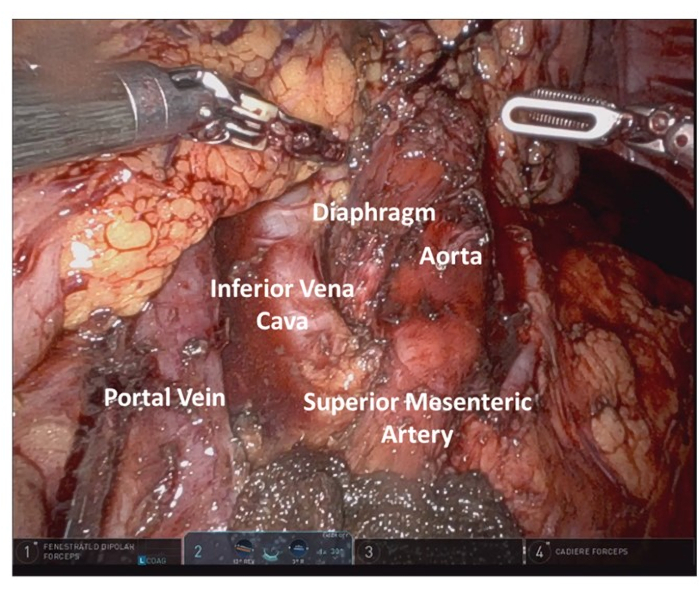
Figure 9: Final resection bed anatomy highlight. Please click here to view a larger version of this figure.
Discussion
With proper pre-operative planning, patient selection, and surgeon experience, it is clinically feasible and safe to approach locally advanced pancreatic tumors of the body/tail of the pancreas with celiac involvement with robot assisted distal pancreatectomy, splenectomy, and celiac axis resection. Proper patient selection requires comprehensive pre-operative planning with cross-sectional imaging to identify the tumor and its anatomical relationship to surrounding vascular structures. At this time, it is also imperative to identify any anomalous arterial or venous circulation that would imperil the attempted resection or make it infeasible.
Intra-operative findings may also shift our treatment approach in real time. Findings suggesting the continued involvement of the celiac root at the time of surgery would make resection infeasible. Therefore, high quality post-treatment imaging prior to surgery is also critically important to evaluate the degree of treatment response. Additionally, findings on inadequate retrograde arterial flow through the gastroduodenal artery to the proper hepatic artery or liver may require an arterial reconstruction, and pre-operative preparedness for this reconstruction is imperative. After resection, gastric ischemia or congestion due to division of major vascular structures is a potential and morbid sequela. However, despite division of the short gastric vessels and left gastric artery, the gastroepiploic circulation remains unviolated in the course of this dissection. These vessels are often adequate to ensure adequate perfusion of the stomach and therefore gastrectomy can be avoided. However, the stomach must be observed closely for ischemic changes prior to conclusion of this procedure.
The traditional operative approach to management of tumors involving the body and tail of the pancreas is a distal pancreatectomy. However, more recently, radical antegrade pancreaticosplenectomy (RAMPS) has been proposed as an alternative procedure that is increasingly utilized and has been suggested to offer an increased rate of negative tangential margins15. Despite these promising results, no large prospective studies yet exist that show a demonstrable improvement in overall survival or recurrence free survival when compared with traditional distal pancreatectomy. Additional prospective studies are needed to establish clear clinical guidelines for routine use of RAMPS during distal pancreactomy16.
The robot platform has seen ever increasing use in a variety of complex pancreatic resections. This protocol and video highlights one potential operative approach utilizing this platform for approaching a clinically challenging disease process.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 5U54GM104942-04 (BAB).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Materials
| Da Vinci Robotic Platform XI | Intuitive Surgical | ||
| Lightworks Video Editer | Lightworks | ||
| Studio 3 Video logging platform | Stryker |
References
- American Cancer Society. American Cancer Society (2016) Cancer Facts & Figures. American Cancer Society. , (2016).
- Suker, M., et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncology. 17 (6), 801-810 (2016).
- Faris, J. E., et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 18 (5), 543-548 (2013).
- Ueno, H., et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. Journal of Clinical Oncology. 31 (13), 1640-1648 (2013).
- Hirono, S., et al. Treatment Strategy for Borderline Resectable Pancreatic Cancer With Radiographic Artery Involvement. Pancreas. 45 (10), 1438-1446 (2016).
- Schmocker, R. K., et al. An Aggressive Approach to Locally Confined Pancreatic Cancer: Defining Surgical and Oncologic Outcomes Unique to Pancreatectomy with Celiac Axis Resection (DP-CAR). Annals of Surgical Oncology. , (2020).
- Klompmaker, S., et al. E-AHPBA DP-CAR study group. Outcomes and Risk Score for Distal Pancreatectomy with Celiac Axis Resection (DP-CAR), An International Multicenter Analysis. Annals of Surgical Oncology. 26 (3), 772-781 (2019).
- Ocuin, L. M., et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB. 18 (10), 835-842 (2016).
- Caba Molina, D., Lambreton, F., Arrangoiz Majul, R. Trends in Robotic Pancreaticoduodenectomy and Distal Pancreatectomy. Journal of Laparoendoscopic & Advanced Surgical Techniques A. 29 (2), 147-151 (2019).
- Kornaropoulos, M., et al. Total robotic pancreaticoduodenectomy: a systematic review of the literature. Surgical Endoscopy. 31 (11), 4382-4392 (2017).
- Peng, L., Lin, S., Li, Y., Xiao, W. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surgical Endoscopy. 31 (8), 3085-3097 (2017).
- Zureikat, A. H., et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Annals of Surgery. 264 (4), 640-649 (2016).
- Zureikat, A. H., et al. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Annals of Surgery. 273 (5), 966-972 (2021).
- Cai, J., et al. Robotic Pancreaticoduodenectomy Is Associated with Decreased Clinically Relevant Pancreatic Fistulas: a Propensity-Matched Analysis. Journal of Gastrointestinal Surgery. 24 (5), 1111-1118 (2020).
- Strasberg, S. M., Linehan, D. C., Hawkins, W. G. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. Journal of the American College of Surgeons. 204 (2), 244-249 (2007).
- Chun, Y. S. Role of Radical Antegrade Modular Pancreatosplenectomy (RAMPS) and Pancreatic Cancer. Annals of Surgical Oncology. 25 (1), 46-50 (2018).

