In Vivo Methods to Assess Retinal Ganglion Cell and Optic Nerve Function and Structure in Large Animals
Summary
Here we demostrate several in vivo tests (flash visual evoked potential, pattern electroretinogram and optic coherence tomography) in goat and rhesus macaque to understand the structure and function of the optic nerve and its neurons.
Abstract
The optic nerve collects axons signals from the retinal ganglion cells and transmits visual signal to the brain. Large animal models of optic nerve injury are essential for translating novel therapeutic strategies from rodent models to clinical application due to their closer similarities to humans in size and anatomy. Here we describe some in vivo methods to evaluate the function and structure of the retinal ganglion cells (RGCs) and optic nerve (ON) in large animals, including visual evoked potential (VEP), pattern electroretinogram (PERG) and optical coherence tomography (OCT). Both goat and non-human primate were employed in this study. By presenting these in vivo methods step by step, we hope to increase experimental reproducibility among different labs and facilitate the usage of large animal models of optic neuropathies.
Introduction
The optic nerve (ON), which consists of axons from the retinal ganglion cells (RGC), transmits visual signal from the retina to the brain. ON diseases, such as glaucoma, traumatic or ischemic optic neuropathy, often caused irreversible ON/RGC degeneration and devastating visual loss. Although there are currently many breakthroughs in ON regeneration and RGC protection in rodent models1,2,3,4,5,6, clinical treatments for most of the ON diseases remained essentially the same over the last half century with unsatisfactory outcome7,8. To fill the gap between basic research and clinical practice, translational studies using large animal model of ON diseases are often necessary and beneficial because of their closer anatomical similarity to humans than rodent models.
Goat and rhesus macaques are two large animal species used in our lab to model human's ON disease. The size of a goat's eyeball, ON, and the adjacent structure (orbital and nasal cavity, skull base, etc.) is similar to that of a human based on skull CT scan9. As such, goat model provides an opportunity to evaluate and refine therapeutic devices or surgical procedures prior to use in humans. The rhesus macaque, as non-human primate (NHP), has human-like unique visual system that does not exist in other species10,11. In addition, pathophysiological responses to injuries and treatments in NHP is much similar to that in humans12.
In vivo tests to assess the ON and RGC's structure and function longitudinally are important in large animal studies. Pattern electroretinogram (PERG) has been used to evaluate the RGC function. Flash visual evoked potential (FVEP) reflects the integrity of retino-geniculo-cortical pathway in the visual system. Thus, PERG combined with FVEP can reflect the ON function9,13,14 . The retinal optic coherence tomography (OCT) imaging can show the retinal structure with high temporal and spatial resolution, which enables measurement of the thickness of the retinal ganglion complex (GCC)9,15. For electrophysiological examinations in this study, monitoring vital signs (heat rate, breach rate, blood pressure) and level of oxygen saturation (SpO2) before testing are crucial since these parameters have potent impacts on ocular blood flow and thus the function of the visual system. However, we didn't monitor the vital signs when carrying out OCT retinal imaging for the sake of simplicity. According to our previous study9, the GCC thickness measured by OCT retinal imaging is quite stable, with inter-session coefficient of variation close to 3%. These in vivo tests in goat and rhesus macaque have been described in detail in our previous study9. Here we present these methods to help increase experimental transparency and reproducibility.
Protocol
Experiments were conducted strictly in accordance with the ARRIVE guidelines and the National Institutes of Health guide for the care and use of Laboratory animals, and adhere to the protocols approved by the Institutional Animal Care and Use Committee in Wenzhou Medical University (WMU) and Joinn Laboratory (Suzhou). The male Saanen goats, aged from 4 to 6 months with weight of 19-23 kg, were housed in the WMU animal facility. The male Rhesus macaques, aged from 5 to 6 years with weight of 5-7 kg, were housed in the Joinn animal facility. All the animals were maintained in an air-conditioned room with controlled temperature (21 ± 2 °C) under a 12 h light/12 h dark cycle with food ad libitum.
1. Flash visual evoked potential (FVEP) in goat
- General anesthetization
- Shave the hock hair with an electronic razor.
- Prepare the skin by rubbing with 70% alcohol three times to clean the skin, and then expose the subcutaneous vein.
- Insert a peripheral venous catheter intravenously (0.9 mm x 25 mm), and then inject atropine (0.025 mg/kg) and propofol (5 mg/kg).
- Intubate the goat with a 6 mm tracheal tube and connect it to an artificial respirator.
- Maintain anesthesia with 3.5% isoflurane in oxygen at a constant flow rate of 2 L/min.
NOTE: The goat recovers from the anesthesia induced by propofol within minutes, so be fast to intubate the goat.
- Cardiopulmonary monitoring
- Place the temperature sensor beneath the tongue.
- Connect the pulse oximeter to the proximal end of the ear.
- Tie the blood pressure cuff to the base of the thigh.
- Clamp the ECG clips onto the limbs accordingly.
NOTE: The normal heart rate of goats is 68-150 bpm. Due to the use of gas anesthesia, the heart rate of goats will increase. Therefore, our heart rate during inspection is 170 ± 30 bpm. The systolic blood pressure of goats under normal conditions is 110-130 mmHg, and the diastolic blood pressure is 50-60 mmHg. In the state of inhaling oxygen, the goat's blood oxygen saturation can always be maintained at 99%. The breathing rate of goats under anesthesia is synchronized with that of the ventilator, which is 10 breaths/min. Since the temperature was measured from under the tongue of the goat, not the core temperature, the temperature of the goat is generally 35 ± 2 °C.
- Skull screws implantation and electrodes placement
- Shave the hair with a clipper. Disinfect the skin on the center of the frontal bone by rubbing with a cotton ball soaked in betadine and 70% alcohol three times.
- Use sterilized screws and scissors.
NOTE: Autoclave all surgical instruments for sterilization (121 °C, 20 min). - Make a 5 mm skin incision to expose the frontal bone with an ophthalmic scissor, and then implant a sterilized screw at the center of the frontal bone using a screwdriver.
- Shave the hair and disinfect the skin on the central occipital bone between two ears with betadine and 70% alcohol, one followed by the other, three times.
- Make a 5 mm skin incision to expose the occipital bone with an ophthalmic scissor, and then implant a sterilized screw at the center of the occipital bone.
NOTE: The ground needle electrode is inserted subcutaneously beneath the frontal skull screw. The active and reference electrodes are connected to the occipital and frontal screws, respectively, with alligator clips to reduce the electrode impedance16.
- Animal preparation
- Use lightproof cloth to cover the eye and fix by the blindfold to patch one eye.
- Apply topical anesthetic eyedrops (proparacaine hydrochloride eyedrops) onto both eyes. Bilateral pupils are dilated by topical administration of mydriatic eyedrops with tropicamide (5%) and phenylephrine (5%).
- Place the head of the goat into the Ganzfeld stimulator and dim the ambient light.
NOTE: It was found that goats can maintain good eyeball fixation under anesthesia, so no extra eyeball fixation operation intervention is required. - Cover the stimulator and the goat's head with a black blanket for 5 min for adaptation.
- Use eyelid speculum to expose the bulbar conjunctiva. Fold the upper ring, pull the upper eyelid up, and insert the upper ring first into the conjunctival sac of the upper eyelid and then into the lower eyelid in a similar manner.
- Press the Impedance button to check the electrode-tissue contact impedance and the values of impedance will be shown in each channel.
- Ensure that the impedance is below 10 kΩ for each electrode to avoid electromagnetic interference from other electrical devices in the same room.
NOTE: If it is above 10 kΩ, reconnect or replace the electrode. The impedance may appear abnormally high if the electrical metal surgical bed, where the goat lies, is plugged. The impedance should differ by less than 1 kΩ between the active and reference electrodes to reduce electrical interference17. - Press the Oscillograph button to check the baseline noise without light stimulation.
NOTE: If there is a large baseline noise, unplug all other electrical devices in the same room and turn off cell phones. If the baseline problem persists, jump to step 1.3.10. to check if a typical FVEP waveform can be elicited. If not, reschedule the FVEP test at another time. - Start FVEP recording by choosing the light intensity of 0.025, 0.5, and 3.0 cd·s/m2, respectively in the white background box in the upper-right corner. Then, press the Examination button. Note that FVEP recording at each light intensity is performed twice.
NOTE: If the two waveforms appear obviously different, one more repeat is needed. - Moist the cornea with artificial tear eyedrops, if it appears dry on the infrared camera.
NOTE: Monitor the eye position from the incorporated infrared camera before recording to make sure that the visual gaze is correct and the pupil is fully exposed (so that the field size of a flash stimulus is 90°. The eye position of an anesthetized goat can be adjusted by turning its head accordingly. Based on our observation, gaze wandering rarely occurs during FVEP recording (~10 min) in goat under general anesthesia9. So there is no need to pause and re-adjust the gaze while recording. - Repeat the above steps for the contralateral eye.
- Cease isoflurane supply and increase the tidal volume slightly on the ventilator to help the goat recover from general anesthesia.
- After general anesthesia, treat the goat with gentamicin (4 mg/kg, IM) and ceftiofur sodium (a cephalosporin, 2 mg/kg, IM) to prevent infection.
- FVEP measurement and quantitative analysis
NOTE: As shown in Figure 1A, the first positive and negative peaks in the FVEP waveform are designated as P1 and N1, and the second positive peak as P2. The typical implicit time of P1, N1, and P2 are around 40, 60, and 120 ms, respectively. P1 and P2 amplitudes are measured from the trough of N1 waveform to the peaks of P1 and P2 waveforms, respectively.- In case of monocular injury, use interocular comparison of amplitude and implicit time to help reduce intersession variation and increase sensitivity17.
2. PVEP in rhesus macaque
NOTE: Pattern VEPs could be elicited in rhesus macaques9 and are more stable than Flash VEP in amplitude and implicit time17. Therefore, PVEP was used to detect the integrity of the retino-geniculo-cortical pathway in non-human primates.
- Animal preparation
- Anesthetize the monkey with isoflurane (1.5%-2%) after induction with Zoletil50 (4-8 mg/kg IM, tiletamine/zolazepam).
- Position the sterilized ground electrode at the earlobe. Insert the sterilized active and reference electrodes subcutaneously along the midline at the frontal and occipital bone, respectively.
- Apply eyelid speculum to expose the bulbar conjunctiva.
- Use adhesive opaque black tape to patch the contralateral eye.
- PVEP recording
- Press the Impedance button to check the electrode-tissue contact impedance and the values of impedance will be shown in each channel; ensure that it is below 10k Ω. If not, reconnect or replace the electrode.
- Check the values of impedance in Impedance Test Window and ensure that the impedance differ by less than 1 kΩ between the active and reference electrodes to reduce electrical interference17.
- Press the Oscillograph button to check the baseline noise without stimulation.
NOTE: If there is a large baseline noise, unplug all other electrical devices in the same room and turn off the cell phones. If the baseline problem persists, redo the PVEP test on another day. - Record PVEP responses of the unpatched eye by choosing the light intensity of 0.5 and 1.0 cycle/degree, respectively in the white background box in the upper-right corner, and then press the Examination button.
NOTE: For each recording, 64 traces are averaged to yield one waveform. For each frequency, a minimal of two recordings are acquired to verify the reproducibility of PVEP signals. - Repeat the procedure for the contralateral eye.
- Once done, cease isoflurane supply to awake the monkey.
- After general anesthesia, treat the monkey with gentamicin (4 mg/kg IM) and ceftiofur sodium (2 mg/kg IM) to prevent infection.
- PVEP measurement and quantitative analysis
- As shown in Figure 1B, the first negative and positive peaks in the PVEP waveform were designated as N1 and P1, which typically occur at around 50 and 90 ms. The P1 amplitude is measured from the trough of N1 to the peak of P1.
- In case of monocular injury, use interocular comparison of amplitude and implicit time to help reduce intersession variation and to increase sensitivity17.
3. Pattern ERG (PERG) in goat
NOTE: In the previous study, no interocular crosstalk of PERG signal was observed in goats, so PERG responses can be recorded simultaneously from both eyes9.
- Examination preparation
- Anesthetize the goat using xylazine (3 mg/kg, IM), and place on an exam table.
- Place a temperature sensor under the goat's tongue.
- Connect the pulse oximeter to the proximal end of the goat's ear.
- Tie the blood pressure cuff to the thigh.
- Clamp the ECG clips on the limbs accordingly.
- To reduce electrode impedance, place a sterilized skull screw on the frontal bone and connect to the ground electrode with an alligator clip.
- Place two sterilized needle reference electrodes subcutaneously 1 cm behind the lateral canthi on both sides.
- Use the eyelid speculum to expose the bulbar conjunctiva.
- Place two disinfected ERG-Jet recording electrodes at the center of bilateral corneas after topical application of artificial tear.
- Place two 47.6 cm x 26.8 cm LED monitors in front of both the eyes with a viewing distance of 50 cm.
- Adjust each monitor to be parallel to the pupil plane on the same side and align the center of the monitor to the pupil plane.
- Ensure that the contrast-reversing checkerboard (temporal frequency, 2.4 Hz) is displayed on both the monitors and has a maximal aspect ratio of 4:3, which is set by equipment settings.
- Ensure that the contrast between white and black checkers remains 96%, and the mean luminance is 200 cd/m2 (Candela per square metre), which is checked by the luminance meter.
NOTE: According to ISCEV, a mean photopic luminance of 40-60 cd/m2 is required in humans17. In another study using mice model, the pattern remained at a mean luminance of 800 cd/m2,18. A field size of at least 15° in its narrowest dimension is needed for a standard PERG test in humans17. Adjust the position of corneal electrode if it is not at the center of the corneal surface.
- PERG recording
- Dim the ambient light and press the Impedance button to check the electrode-tissue contact impedance. The values of impedance will be shown in each channel.
- Check the values of impedance in Impedance Test Window and ensure that impedance is below 10 kΩ. If not, reconnect or replace the electrode.
- Press the Oscillograph button to check the baseline noise without light stimulation.
NOTE: Pay attention to protect the fragile ERG-Jet recording electrodes. Baseline noise in PERG is usually smaller than that in FVEP in goat. - Start PERG recording from both eyes simultaneously at the spatial frequencies of 0.1, 0.3, 1.0, 3.0, and 12.6 cycles/degree consecutively. For each spatial frequency, 64 traces are averaged to yield one readout.
- Finally, turn off the monitor to record PERG without visual stimulus as a negative control.
NOTE: PERG signals are usually stable and don't need repeat. - Remove the front skull screw and awake the goat by injecting Idzoxan (1.5 mg/kg), which is a xylazine antagonist.
- After general anesthesia, treat the goat with gentamicin (4 mg/kg IM) and ceftiofur sodium (2 mg/kg IM) to prevent infection.
- PERG measurement and quantitative analysis
- Set the bandpass filter to range from 1 to 75 Hz. For 3.0 cpd PERG, the bandpass filter is set to be from 1 to 50 Hz to smoothen the trace without affecting its amplitude.
- As shown in Figure 1C, the first positive and negative peaks in the waveform are designated as P1 (typically around 25 ms) and N1 (typically around 55 ms). The PERG amplitude is measured from N1 to P1.
- In case of monocular injury, we use interocular comparison of amplitude and implicit time to help reduce intersession variation and reduce sensitivity17.
4. PERG in rhesus macaque
NOTE: It is unclear if there is interocular crosstalk of PERG signal in rhesus macaque, so PERG responses from both eyes are recorded separately.
- Examination preparation
- Anesthetize the monkey with isoflurane (1.5%-2%) after injection of Zoletil50 (4-8 mg/kg IM, tiletamine/zolazepam) and tracheal intubation.
- Place a sterilized ground electrode subcutaneously on the frontal bone. Insert a sterilized needle reference electrode subcutaneously, 1 cm behind the lateral canthus on the same side.
- Place a disinfected ERG-Jet recording electrode on the central cornea after topical application of artificial tear.
NOTE: Adjust the position of corneal electrode, if it is not at the center of the corneal surface. - Use adhesive opaque black tape to patch one eye.
- Place a monitor (47.6 x 26.8 cm) at a viewing distance of 50 cm.
- Ensure that the monitor is adjusted to be parallel to the pupil plane. Align the center of the monitor to the pupil plane.
- Ensure that the black and white checkerboard is reversing at a frequency of 2.4 Hz, and the aspect ratio is 4:3, which is set by equipment settings.
- Ensure that the contrast between the white and black checkers is 96%, and the averaged luminance remains 200 cd/m2, which is checked by luminance meter.
- PERG recording
- Dim the ambient light and check the electrode-tissue contact impedance.
- Ensure that impedance is below 10 kΩ. If not, reconnect or replace the electrode.
- Check the baseline noise without light stimulation.
- Patch one eye and start PERG recording from the other eye at spatial frequencies of 0.1, 0.3, 1.0, 3.0, and 12.6 cycles/degree consecutively.
- Repeat the steps 4.2.1-4.2.4 for the contralateral eye.
- Cease isoflurane supply to awaken the monkey.
- After general anesthesia, treat the monkey with gentamicin (4mg/kg IM) and ceftiofur sodium (2 mg/kg IM) to prevent infection.
- PERG measurement and quantitative analysis
- As shown in Figure 1D, the first positive and negative peaks in the waveform are designated as P1 (typically around 40ms) and N1 (typically around 85 ms). The PERG amplitude is measured from N1 to P1.
- In case of monocular injury, we use interocular comparison of amplitude and implicit time to help reduce intersession variation and increase sensitivity17.
5. OCT in goat
- Animal preparation
- Anesthetize the goat using xylazine (3mg/kg, IM), and then intubate.
- Dilate the pupil by topical administration of mydriatic eyedrops with tropicamide (5%) and phenylephrine (5%).
- Use the eyelid speculum to fully expose the pupil.
- Place the head of the goat on the chin rest.
NOTE: Although gas anesthetization is not performed, intubate the goat regularly to protect the airway from being compressed by the chin rest.
- OCT imaging
NOTE: The retinal OCT imaging is performed using the OCT system at a wavelength of 870 nm in this study. The optical axial resolution of the OCT scanner is 12 µm. Circular scan mode is used to scan the optic nerve head (ONH) with high-resolution mode. 100 frames are averaged to optimize the image quality. The detailed training guide is available online (see Table of Materials).- Initial OCT scanning (baseline exam)
- Click on the Start button to enter the detection interface. Wait for the machine to finish loading, and then press the yellow Start button to initiate imaging.
- Align the goat with the infrared camera to center the ONH in the confocal Scanning Laser Ophthalmoscopy (cSLO) image by modifying its head position.
- Adjust the joystick to evenly illuminate the entire infrared image to improve image quality.
- Move the joystick forward until an upright retinal OCT image is showed on the upright screen.
- Modify the joystick to have an evenly dense and horizontally placed retinal OCT image.
- Press the button on the joystick to catch the image automatically and hold the joystick to maintain the image quality on the live image screen until image acquisition is completed. Then, press Acquire.
- Awaken the goat by injecting Idzoxan (1.5 mg/kg), which is a xylazine antagonist.
NOTE: Centering the ONH in the baseline exam helps align the baseline scan and follow-up scan according to our experience.
- Follow-up OCT scanning
- Select a high-quality initial OCT image; right-click and select Set Reference.
- Initiate OCT imaging as mentioned above.
- Press the Follow-up button to allow automatic match of the current scan to the reference scan.
- Once matched (the circular scan ring turns green), press the button on the joystick to activate Automatic Real-time Tracking.
- Awaken the goat by injecting Idzoxan (1.5 mg/kg ), which is a xylazine antagonist.
NOTE: To facilitate the process of matching, (1) move the ONH in the live window by turning the head accordingly or (2) rotate the ONH in the live window by tilting the head to make the current cSLO image appear more similar to the baseline image. This vestibulo-ocular reflex works well under xylazine anesthesia19.
- Initial OCT scanning (baseline exam)
- OCT measurement
- Click on the Measurement button to enter the measurement window.
- Choose the eraser tool and wipe the RNFL line, which is automatically labeled by the program.
- Choose the Line Drawing tool to manually outline the boundary between IPL and INL (Figure 2).
NOTE: GCC thickness in six peripapillary regions (T, TS, TI, N, NS, NI) and averaged GCC thickness around the ONH (G) can be read on the screen (Figure 2). The student test, one-way ANOVA or two-way ANOVA can be used to quantify the OCT data in case of normal distribution.
6. OCT in rhesus macaque
- Perform retinal OCT imaging in rhesus macaque using the same equipment and procedure as done in the case of goat.
Representative Results
Figure 1A shows representative results of FVEP in goat. Although the waveforms in same flash intensity have relative similarity, we still recommend to examine the waveforms twice. Electromagnetic waves generated by electronic devices will interfere with the collected electrical signals, resulting in high baseline noise and poor repeatability of the waveform. Therefore, it is recommended to ensure that there are no redundant electronic devices plugged into the surrounding environment during electrophysiological examination to avoid such interference, and it is recommended to repeat at least two measurements to determine the stability and repeatability of experimental results. When patching both eyes, the waveforms are totally flat on both eyes, which shows that the waveforms are certainly generating from our flash stimulus. Figure 1B shows representative results of PERG in goat. Due to its stability and high signal, we acquire reliable waveform just by one time measurement at each spatial frequency. As the spatial frequency increases, the size of the checkerboard will gradually exceed the recognition of the goat's eyes. So we can see that at 12.7 cpd, the PVEP waveform has dropped a lot. Analogous to FVEP, the waveform disappears when we close the screen. Figure 1C shows representative results of PVEP in rhesus macaque. We repeat the measurement twice at each spatial frequency. As the spatial frequency increases, the amplitude will decrease. This is due to the fact that the spatial frequency exceeds the perception of the eye. Figure 1D shows representative results of PERG in rhesus macaque. The reason why the amplitude decreases with the spatial frequency is the same as described above. When analyzing these data, you can choose to analyze the amplitude between the peaks and troughs or the latency time of the peaks or troughs as the statistics.
Figure 2 shows the representative results of OCT in goat. The leftmost image shows a fundus photograph taken by infrared camera. Tightly to the right is a tomogram of the retina, showing the overall thickness of the retina around the ONH and the thickness of each layer. As shown in the picture, we can clearly see that goats have larger retinal blood vessels than monkeys. The green discs on the far right is a quantitative analysis of the thickness of the GCC around the ONH. G stands for general, T stands for temporal side, N stands for nasal side, S stands for superior, and I stands for inferior. The black font represents the GCC thickness measurement value in micrometers, and the green is the clinical reference measurement value for human, not as a reference for this experiment.
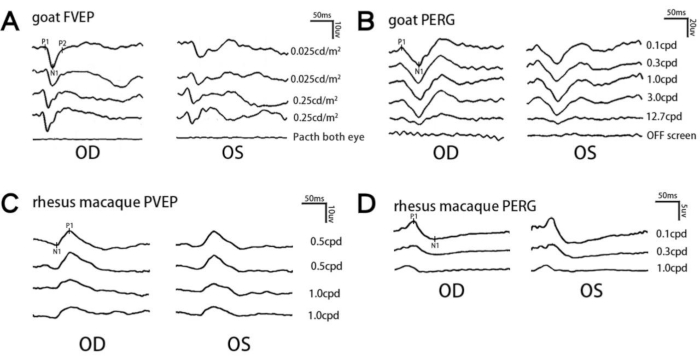
Figure 1: Representative electrophysiological waveforms in goat and rhesus macaques. (A) Representative FVEP waveforms at different light intensities from an individual goat within the same anesthetic session. (B,D) Representative PERG waveforms at different spatial frequencies from an individual goat (B) or rhesus macaque (D) within the same anesthetic session. (C) Representative PVEP waveforms at different spatial frequencies from an individual rhesus macaque within the same anesthetic session. The typical implicit time of each waveform is mentioned in the Protocol section. n = 1 subject for each test. Please click here to view a larger version of this figure.
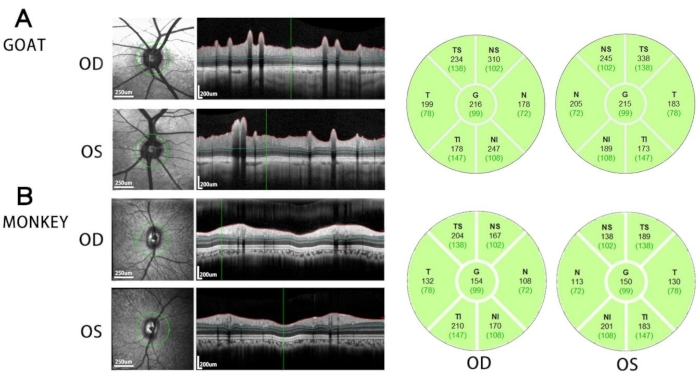
Figure 2: OCT results. Representative retinal OCT images around the optic nerve head (left panel) and the thickness of GCC in different peripapillary regions (right panel) in goat (A) and rhesus macaque (B). n = 1 subject for each test. Please click here to view a larger version of this figure.
Discussion
In this study, we present a protocol of VEP, PERG, and OCT in goat and rhesus macaque. These in vivo methods can be applied in large animal models of various optic neuropathies, such as glaucoma, ischemic, or traumatic optic neuropathy and optic neuritis9.
PVEP is more stable and sensitive than FVEP17; however, it can’t be elicited in goat9. As such, FVEP is performed in goat and PVEP is performed in rhesus macaque in our lab to evaluate the integrity of the retino-geniculo-cortical pathway. The mechanism underlying the observation that PERG, but not PVEP, can be induced in goat is still unclear according to our knowledge. It is possible that the optic nerve function and structure from goat might be different from human.
Since the amplitude of FVEP may be affected by pupillary size and ambient light, we dilate the pupil and place a black blanket over the goat’s head during FVEP recording. It should be noticed that pupil dilation is not needed for FVEP in clinics17. Similarly, although dark adaptation is not necessary for FVEP recording in clinics, we found that a 5 min adaption to the ambient light before FVEP recording may increase intrasession repeatability in amplitude.
PERG signal is thought to originated from the RGCs and thus its amplitude could be used to estimate the function of the RGCs13,20. Compared with some special flash ERG components, such as scotopic threshold response (STR) and photopic negative response (PhNR), PERG is more sensitive to RGC dysfunction13. Potential limitations of PERG test in this study are as follows. First, ISCEV recommends using classic CRT (cathode ray tube) stimulator for PERG recording to keep mean luminance constant. However, the classic CRT stimulator is less available than liquid crystal display (LCD). Although the screen of LCD usually presents transient luminance changes during pattern reversal, potentially causing luminance artifact17, it didn’t contribute to the amplitude of PERG in goat according to our previous finding: the amplitude of PERG at the spatial frequency of 12.6 cpd is usually neglectable compared with those at lower spatial frequencies9. Another limitation is that we didn’t correct refractive error before PERG test for the sake of simplicity. To make up for this limitation, baseline PERG amplitude should be recorded as a reference.
Our previous study had assessed and optimized the intra- and inter session variation of VEP and PERG9. We found that compared with xylazine, isoflurane resulted in more repeatable FVEP but more variable PERG waveforms in goats9. Therefore, we used isoflurane in FVEP test and xylazine in PERG and OCT test in goats. Additionally, compared with PERG, VEP recording is potentially more variable. As such, we regularly repeat VEP recording at each light intensity or spatial frequency to check the intrasession variation. On the contrast, PERG waveforms are much more stable. Therefore, we generally do not repeat PERG recording. Although repeated recording on a different day on the same subject is generally recommended, we do not regularly repeat VEP or PERG recording on the same subject on a different day for the sake of simplicity and animal ethics. Nevertheless, FVEP and PERG recordings without inter-session repeat is sensitive enough to detect optic nerve injury according to our previous study9.
Retinal OCT imaging is a convenient, reliable and non-invasive technique to longitudinally monitor and quantify dynamic changes in the retinal structure. Compared with PERG and VEP, OCT imaging has much better intersession repeatability9. Furthermore, OCT imaging can capture and quantify all the optic nerve fiber within minutes without sampling error, offering a much cheaper and effective opportunity to examine the retinal structure than traditional histological analysis. However, the spatial resolution of current OCT imaging is still too limited to tell individual RGC soma or optic nerve fiber. Additionally, it should be noticed that a thicker GCC measured by OCT does not necessarily mean a more intact inner retina because GCC thickening may be caused by retinal edema or hemorrhage.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was funded by the following grants: National Key R&D Program of China (2021YFA1101200); Medical Research Project of Wenzhou (Y20170188), National Key R&D Program of China (2016YFC1101200); National Natural Science Foundation of China (81770926;81800842); Key R&D Program of Zhejiang Province (2018C03G2090634); and Key R&D Program of Wenzhou Eye Hospital (YNZD1201902). The sponsor or funding organization had no role in the design or conduct of this research.
Materials
| 47.6 x 26.8 cm monitors | DELL Inc. | E2216HV | The visual stimuli of contrast-reversal black-white checkerboards were displayed on screens |
| 6.0 mm tracheal tube | Henan Tuoren Medical Device Co., Ltd | PVC 6.0 | ensure the airway |
| alligator clip | |||
| atropine | Guangdong Jieyang Longyang Animal pharmaceutical Co.,Ltd. | reduce bronchial secretion and protect heart from vagal nerve activation | |
| Carbomer Eye Gel | Fabrik GmbH Subsidiary of Bausch & Lomb | moisten the cornea and stabilize the recording electrodes | |
| ERG-Jet recording electrodes | Roland Consult Stasche&Finger GmbH | 2300 La Chaux-De-Fonds | ERG recording |
| eye speculum | Shanghai Jinzhong Medical Device Co., Ltd | ZYD020 | open palpebral fissure |
| Heidelberg Spectralis OCT system | Heidelberg Engineering | OCT system | |
| Imaging | (https://www.heidelbergengineering.com/media/e-learning/Totara-US/files/pdf-tutorials/2238-003_Spectralis-Training-Guide.pdf) | ||
| isoflurane | RWD Life Science Co., Ltd | R510-22 | isoflurane anesthesia |
| male Saanen goats | Caimu Livestock Company, country (Hangzhou, China) | The male Saanen goats, aged from 4 to 6 months with weight of 19–23 kg | |
| needle electrode | Roland Consult Stasche&Finger GmbH | U51-426-G-D | use for FVEP ground electrode and PERG reference electrodes |
| periphery venous catheter intravenously | BD shanghai Medical Device Co., Ltd | 383019 | intravenous access for atropine and propofol |
| propofol | Xian Lipont Enterprise Union Management Co.,Ltd. | induce Isoflurane anesthesia in goat | |
| Tropicamide Phenylephrine Eye Drops | SANTEN OY, Japan | 5% tropicamide and 5% phenylephrine hydrochloride | |
| visual electrophysiology device | Gotec Co., Ltd | GT-2008V-III | use for FVEP & PERG |
| xylazine | Huamu Animal Health Products Co., Ltd. | xylazine anesthesia: intramuscular injection of xylazine 3mg/kg | |
| zoletil50 | Virbac | induce Isoflurane anesthesia in monkey |
References
- Benowitz, L., Yin, Y. Rewiring the injured CNS: lessons from the optic nerve. Experimental Neurology. 209 (2), 389-398 (2008).
- Park, K. K., et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 322 (5903), 963-966 (2008).
- Duan, X., et al. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron. 85 (6), 1244-1256 (2015).
- Bei, F., et al. Restoration of visual function by enhancing conduction in regenerated axons. Cell. 164 (1-2), 219-232 (2016).
- He, Z., Jin, Y. Intrinsic control of axon regeneration. Neuron. 90 (3), 437-451 (2016).
- Yang, S. -. G., et al. Strategies to promote long-distance optic nerve regeneration. Frontiers in Cellular Neuroscience. 14, 119 (2020).
- Foroozan, R. New treatments for nonarteritic anterior ischemic optic neuropathy. Neurologic Clinics. 35 (1), 1-15 (2017).
- Singman, E. L., et al. Indirect traumatic optic neuropathy. Military Medical Research. 3, 2 (2016).
- Zhang, Y., et al. In vivo evaluation of retinal ganglion cells and optic nerve’s integrity in large animals by multi-modality analysis. Experimental Eye Research. 197, 108117 (2020).
- Tolbert, W. D., et al. From Rhesus macaque to human: structural evolutionary pathways for immunoglobulin G subclasses. mAbs. 11 (4), 709-724 (2019).
- Preuss, T., et al. . Specializations of the human visual system: the monkey model meets human reality. , 231-259 (2004).
- Friedli, L., et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Science Translational Medicine. 7 (302), (2015).
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Experimental Eye Research. 141, 164-170 (2015).
- Smith, C. A., Vianna, J. R., Chauhan, B. C. Assessing retinal ganglion cell damage. Eye. 31 (2), 209-217 (2017).
- Schuman, J. S., et al. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Investigative Ophthalmology & Visual Science. 48 (8), 3645-3654 (2007).
- You, Y., et al. Improving reproducibility of VEP recording in rats: electrodes, stimulus source and peak analysis. Documenta Ophthalmologica. 123 (2), 109-119 (2011).
- Odom, J. V., et al. ISCEV standard for clinical visual evoked potentials: (2016 update). Documenta Ophthalmologica. 133 (1), 1-9 (2016).
- Zhang, J., et al. Silicone oil-induced ocular hypertension and glaucomatous neurodegeneration in mouse. eLife. 8, 45881 (2019).
- Seidman, S. H., Telford, L., Paige, G. D. Vertical, horizontal, and torsional eye movement responses to head roll in the squirrel monkey. Experimental Brain Research. 104 (2), 218-226 (1995).
- Porciatti, V. The mouse pattern electroretinogram. Documenta Ophthalmologica. 115 (3), 145-153 (2007).

